Abstract
Objective
Obesity is associated with an increase in various pro-inflammatory and anti-inflammatory cytokines, but the interplay of these cytokines is incompletely understood. We conducted experiments to test a broader hypothesis that a dynamic interplay of pro-inflammatory and anti-inflammatory cytokines controls lipid storage in adipocytes.
Design
Three experiments were designed to test the overall hypothesis that pro-inflammatory cytokine (e.g. TNF-α) inhibits anti-inflammatory cytokine (e.g. adiponectin) activity in an attempt to limit excess lipid accumulation in adipocytes.
Results
Experiment 1 showed that in pro-inflammatory animal models (ap2-P65, ob/ob and high fat diet-induced obese mice), the increase in TNF-α expression was associated with a decrease in adiponectin expression. Experiment 2 showed that in 3T3-L1 adipocytes, TNF-α significantly reduced lipid accumulation and glucose uptake induced by adiponectin, and increased lipolysis. Experiment 3 showed that in 3T3-L1 adipocytes, TNF-α reduced mRNA and protein expression of adiponectin. Adiponectin gene transcription and mRNA stability were both reduced by TNF-α. The expression of PPAR-γ, an activator of adiponectin gene promoter, was reduced by TNF-α. The inhibitory activity of TNF-α was blocked by chemical inhibitors of NF-κB and super suppressor IκBα (ssIκBα).
Conclusion
TNF-α opposes the action of adiponectin in the regulation of lipid metabolism, and inhibits adiponectin expression at transcriptional and post-transcriptional levels. The results suggest that pro-inflammatory cytokine inhibit anti-inflammatory cytokine in adipocytes to reduce lipid storage. This suggests a potential role of anti-inflammatory cytokines in the control of adipose tissue expansion.
Keywords: Adiponectin, mRNA stability, transcription, TNF-α, NF-κB, PPARγ
INTRODUCTION
Evidence suggests that the physiology of human body evolved to maintain adipose tissue levels within a range that is optimum for survival and reproduction. Too low as well as excessive adipose tissue content are associated with impaired reproductive potential for women 1-3 and men4, 5 and survivability of progeny 6. Extremes of adiposity are also associated with greater comorbidities, which may influence lifespan. In addition, adipose tissue and its products play an important role in the control of immune function 7. These suggest that maintenance of optimum adiposity for energy store would be a high priority for body. Not surprisingly, adipose tissue products like adipokines appear to be engaged in maintenance of optimal adiposity as well as fertility 8.
The white adipose tissue as a primary energy store is regulated by pro-inflammatory and anti-inflammatory cytokines. Many pro-inflammatory cytokines (such as TNF-α and IL-1) inhibit adipogenesis and promote lipolysis. In contrast, anti-inflammatory cytokines (such as adiponectin) stimulate adipogenesis and lipid accumulation. We postulate that interplay between pro-inflammatory and anti-inflammatory cytokines is employed in the cellular mechanism to counter extremes of adiposity. Lower levels of adiposity is associated with preponderance of anti-inflammatory cytokines and less expression of pro-inflammatory cytokines 9, 10, which is in favor of fat accretion. Conversely, obesity is characterized by a state of chronic low-grade inflammation with pro-inflammatory cytokine elevation and anti-inflammatory cytokine reduction in circulation and adipose tissue 11, 12, which may collectively discourage further fat accumulation. This Yin and Yang effect of cytokines on adiposity may be illustrated with interaction of TNF-α and adiponectin, as representatives of the pro-inflammatory and anti-inflammatory cytokines, respectively.
Adiponectin is an example of anti-inflammatory adipokine expressed in mature adipocytes and is the most abundantly secreted protein from adipose tissue 13. Adiponectin inhibits lipolysis in adipocytes 14, stimulates fatty acid oxidation in muscle cells, suppresses gluconeogenesis in hepatocytes, increases systemic insulin sensitivity, protects body from chronic inflammation and regulates food intake and body weight 15. Although adipose tissue secretes adiponectin, its levels are reduced with excess adiposity, and increased with weight loss. The reduction of adiponectin is also associated with a suppression of insulin actions in the liver and muscle, known as insulin resistance in obesity.
On the other hand, TNF-α is a pro-inflammatory cytokine whose expression and circulating levels are increased with obesity and decreased with weight loss in obese mice 16, 17. TNF-α has numerous effects in adipose tissue, including the induction of apoptosis and lipolysis, inhibition of adipogenesis and insulin signaling 18, 19. TNF-α triggers a signaling cascade (IKKβ/NF-kB) to regulates adipose tissue mass 18, 20. In the control of adipogenesis, TNF-α regulates key transcription factors, such as peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT enhancer-binding protein-α (C/EBPα) 21. An increase in TNF-α promotes the expression of other pro-inflammatory cytokines and reduces expression of anti-inflammatory cytokines, resulting in an overall pro-inflammatory state 22. Thus, it can be conjectured that the preponderance of adiponectin and reduction of TNF-α activity may promote lipid accumulation in favor of adiposity. Whereas, an increase in TNF-α and a reduction in adiponectin will limit adipocyte expansion in favor of adipose tissue reduction.
The aim of this study was to use TNF-α and adiponectin as representatives to test the hypothesis that pro-inflammatory cytokines attempt to limit excess adipocyte expansion by reducing anti-inflammatory cytokine activity, which favors induction of energy expenditure in obesity. Specifically, we investigated TNF-regulation of adiponectin activity and expression in adipocytes.
METHODS
Each experiment is described in detail below, followed by detailed protocols in the Techniques and Assays (T&A) section.
Experiment 1: TNF-α and adiponectin mRNA expression in adipose tissue of pro-inflammatory mouse models
Male ob/ob mice (B6.V-Lepob/J, stock no. 000632), male C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The male C57BL/6 mice were fed a high-fat diet (HFD, 58% kcal from fat, D12331, Research Diets, New Brunswick, NJ) at 5 wk of age for diet-induced obesity (DIO). aP2-p65 transgenic mice were generated in a previous study 20. The ob/ob mice and aP2-p65 mice were maintained on normal chow diet (12.8% kcal from fat). All of the mice were group housed with free access to water and diet in the animal facility at the Pennington Biomedical Research Center, which is with a 12:12-h light-dark cycle and constant temperature (22–24°C). All procedures were performed in accordance with the National Institutes of Health guidelines for the care and use of animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at the Pennington Biomedical Research Center. At sacrifice, total RNA were isolated from mouse white adipose tissue samples as previously described 23, to determine TNF-α and adiponectin mRNA levels by quantitative real time PCR as described in T&A section.
Experiment 2: TNF-α inhibition of adiponectin metabolic activities in adipocytes
TNF-α was tested in the inhibition of adiponectin activities by examining lipid accumulation and glucose uptake in 3T3-L1 cells. The cells and differentiation were described elsewhere 18. Forty-eight hours after initiating adipogenic induction, the cells were treated with TNF-α (Sigma#T6674) (20 ng/ml) and/or adiponectin (Sigma#SRP4902) (20 ng/ml or 100 ng/ml) for 7 days. Media containing TNF-α and adiponectin was refreshed every two days. Prior to changing media, old media was collected to determine glycerol output during differentiation. At the end of 7 days, cells were fixed for Oil red O (ORO) staining to determine lipid accumulation. Cells were also processed for glucose uptake determination and collected for protein expression analysis of adipogenic and lipolytic genes by Western blotting (WB). All assays were performed with a minimum of three biological replicates. For all metabolic assays, 8-12 biological replicates were used, and normalized to protein content.
Experiment 3: Regulation of adiponectin expression by TNF-α
The luciferase reporter driven by adiponectin gene promoter (–1,300/+18) was kindly provided by Dr. Jianhua Shao (Graduate Center for Nutritional Sciences, University of Kentucky, Lexington, KY). The PPRE luciferase reporter was described elsewhere 18. The super suppressor IκBα (ssIκBα) expression vector was originally described elsewhere 18. For TNF-α and troglitazone treatment, the cells were kept in serum-free medium for 16 h and treated with TNF-α or troglitazone for 24 h before reporter assay. HEK 293 cells, 3T3-L1 fibroblasts transient transfection was conducted in triplicate in 12-well plates. The total DNA concentration was corrected in each well with a control plasmid, and the reporter activity was normalized by SV40-Renilla luciferase. Each experiment was repeated at least three times and triplicates were used at each point.
To determine signaling pathways of TNF-α, cells were also exposed to chemical inhibitor for JNK (SP600125, EI-305; Biomol, Plymouth Meeting, PA), the inhibitors for MEK (PD098059, p-215, Sigma, St. Louis, MO), and p38 (SB203580, s-8307, Sigma, St. Louis, MO), or IKK (15d-PGJ2, 538927, Calbiochem, San Diego, CA). Cells were pretreated with the inhibitor for 30-60 minutes and then followed by TNF-α treatment.
ssIκBα 3T3-L1 cell line is derived from 3T3-L1 cell line through stable transfection with a non-degradable mutant of NF-κB inhibitor IκBα (ssIκBα), in which S-A mutation was made at S32 and S36. This cell line was made and used in a previous study 18.
TECHNIQUES & ASSAYS
A. Western Blotting
Western blotting was conducted using a protocol described elsewhere 18. Adiponectin antibody (MAB3832) was from Chemicon International and PPAR-γ (sc-7373x) antibody was purchased from the Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to tubulin (ab7291) and β-Actin (ab6276) were obtained from Abcam (Cambridge, UK). All of the experiments were conducted three or more times. The intensity of the western blotting signal was quantified using a computer program, PDQuest 7.1 (Bio-Rad).
B. Quantitative Real Time RT-PCR
RNA was isolated and purified using Trizol (T9424), chloroform (C2432), Isoproponal (I9516) from Sigma (St. Louis, MO). Tagman or cyber green methods were used in the quantitative real time RT-PCR to determine mRNA levels for TNF-α (Mm00443258_m1, Applied Biosystems), adiponectin (Mm00456425_m1, Applied Biosystems), PPAR-γ (Mm00440945-m1, Applied Biosystems), P65 (Mm00501346_m1), Applied Biosystems), C/EBPα (5’-GCGAGCACGAGACGTCTATAGA-3’;5’-GCCAGGAACTCGTCGTTGAA-3’), C/EBPβ (5’-AGCGGCTGCAGAAGAAGGT-3’,5’-GGCAGCTGCTTGAACAAGTTC-3’), C/EBPδ (5’-CATCGACTTCAGCGCCTACAT-3’, 5’-TGAAGAGGTCGGCGAAGAGT-3’). Mouse 18s was used as an internal control to normalize the mRNA levels in each sample. The reaction was conducted as described elsewhere 20. The relative mRNA level was normalized over 18S ribosomal RNA gene.
C. Oil Red O Staining
At the end of 7 days post adipogenic induction, cells were fixed with 10% formalin for 1 hour and stained Oil red O dye (Fisher Biotech BP112-10) as described elsewhere 18. Three biological replicates were analyzed for each treatment group and 4 technical replicates averaged for each biological replicate.
D. Glycerol output determination
The endogenous glycerol released in the media as a result of lipolysis was measured using a free glycerol determination kit (Sigma#FG0100) according to the manufacturer's instructions with some modifications.
E. Glucose uptake assay
Glucose uptake was conducted as described previously in detail24, 25. Briefly, cells in 6 well plates were serum starved for 2 hours, and then washed 2X with PBS before adding 900 μl KRP (136 mM NaCl, 4.7 mM KCl, 10 mM NaPO4, 0.9 mM CaCl2, 0.9 mM MgSO4) for 15 minutes. One well was treated with 100 nM cytochalasin B (Sigma Aldrich, #C6762) for subtraction of nonspecific glucose uptake. 100 μl of 10X isotope solution was then added to each well for a final concentration of 100 nM cold 2-deoxy glucose and 0.5 μCi/ml 26-2-Deoxyglucose (PerkinElmer #NEC720A250UC) for 5 minutes. Immediately following the incubation, cells were washed in ice-cold PBS. 1 ml of 0.05% SDS was then added to each well, after incubating for 30 min at 37°C, 900 μL of cell lysate was added to a scintillation vial by combining 4 wells and the remaining 50 μl was used for protein determination via BCA (bicinchoninic acid) assay. The scintillation values were normalized to protein content of each well.
F. Adiponectin ELISA
Mouse adiponectin ELISA kit (EZMADP-60K) from EMD Millipore (Billerica, Massachusetts 01821, USA) was used to detect adiponectin in the supernatant of 3T3-L1 adipocytes. The results were normalized to protein content in the supernatant.
Statistical Analysis
The data are presented as a mean ± SEM. The data were analyzed using two directional Student's t test with statistical significance considered at p < 0.05.
RESULTS
Experiment 1: TNFα and adiponectin mRNA in mice adipose tissue are negatively correlated
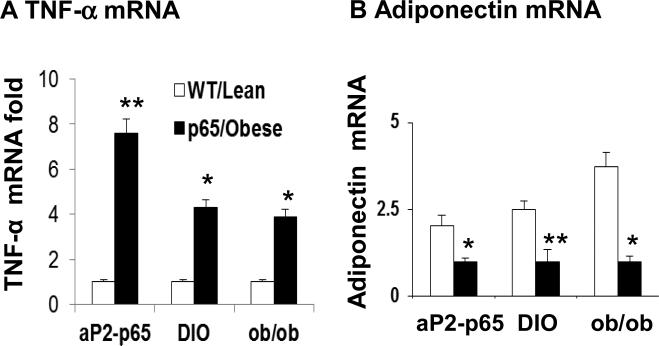
To gain insight into the relationship between pro-inflammatory and anti-inflammatory cytokines in adipose tissue, we investigated TNF-α and adiponectin expression in three mouse models of chronic inflammation including aP2-p65 mice, DIO mice, and ob/ob mice. aP2-p65 mouse is a transgenic mouse in which NF-kB subunit p65 (RelA) gene is overexpressed in adipose tissue under aP2-gene promoter 20. The overexpression induced a high level of chronic inflammation in the adipose tissue of aP2-p65 mice compared to the wild type littermate. As shown in Figure 1, TNF-α mRNA was increased in the adipose tissue of transgenic mice (Fig. 1A). In the metabolic inflammatory models, the increase was also observed in the DIO mice and ob/ob mice relative to their lean controls (Fig. 1A). While, adiponectin mRNA was significantly reduced in the adipose tissue in all of those models (Fig. 1B). The results consistently suggest that TNF-α elevation is associated with adiponectin reduction in adipose tissue. The in vivo data provide physiological significance to the study of adiponectin regulation by TNF-α.
Figure 1. Adiponectin and TNF-α expression in white adipose tissue.
Epididymal fat tissues were collected at the age of 20 weeks from aP2-P65 mice, DIO mice and ob/ob mice. mRNA was determined in qRT-PCR. (A) TNF-α mRNA. (B) Adiponectin mRNA.
Experiment 2: TNF-α and adiponectin have opposing effects in 3T3-L1 adipocytes
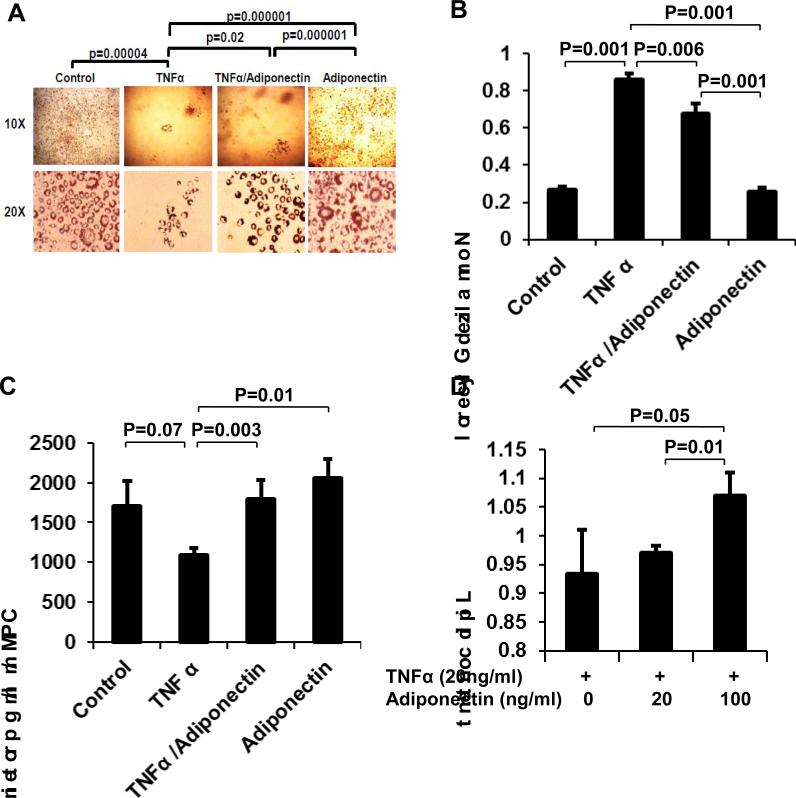
We used adipogenesis to determine the interplay of TNF-α and adiponectin in regulation of lipid accumulation. 3T3-L1 preadipocytes were treated with TNF-α (20 ng/ml) during induction of adipogenesis for 7 days. Adipogenesis was measured by lipid accumulation in cells using oil red O (ORO) staining. TNF-α treatment blocked the cell differentiation (Fig. 2A) and resulted in significant reduction in lipid accumulation in these cells (p<0.001). An increase in lipolysis often leads to reduction in lipid accumulation in adipocyte. Glycerol concentration was measured in the cell culture supernatant and normalized to the cellular lipid content to determine the lipolysis activity in the cells. In TNF-treated cells, we observed increased glycerol content in the media (Fig. 2B), suggesting that lipolysis is induced by TNF-α to contribute to the inhibition of adipogenesis. TNF-α treatment also reduced glucose uptake in these cells (p=0.07; Fig. 2 C).
Figure 2. Dynamic balance between TNF-α and Adiponectin on adipogenesis.
3T3-L1 cells were treated with TNF-α (20 ng/ml) and/or adiponectin after adipogenic induction for 7 days. TNF-α and adiponectin showed opposite activities in following aspects. (A) Adipocyte differentiation and lipid accumulation. Lipid is stained by oil red O. (B) Glycerol output by adipocytes. Glycerol was normalized by lipid content in cells. (C) Glucose uptake. Insulin-induced glucose uptake was measured in differentiated 3T3-L1 adipocytes with radiolabeled glucose. (D) Lipid content by oil red O staining and quantification. Adiponectin exhibited dose-dependent activity in antagonizing TNF-activity for lipid accumulation. The results are presented as mean ± SEM.
In this adipogenesis system, the anti-inflammatory cytokine adiponectin was tested in the regulation of lipid accumulation. Adiponectin treatment rescued the cells from TNF-induced block in adipogenesis and lipid accumulation. Adiponectin (20 ng/ml) significantly increased lipid content in the TNF-treated cells (Fig. 2A). Adiponectin treatment also reduced lipolysis (Fig. 2B) and significantly increased glucose uptake in the TNF-treated cells (Fig. 2C). In the presence of TNF-α, adiponectin dose-dependently increased lipid accumulation (p=0.05 and 0.01) (Fig. 2D). These data suggest that TNF-α and adiponectin antagonize each other in the regulation of lipid and glucose metabolism in adipocytes.
Experiment 3: Regulation of adiponectin expression by TNF-α
TNF-α inhibits adiponectin gene expression in mature 3T3-L1 adipocytes
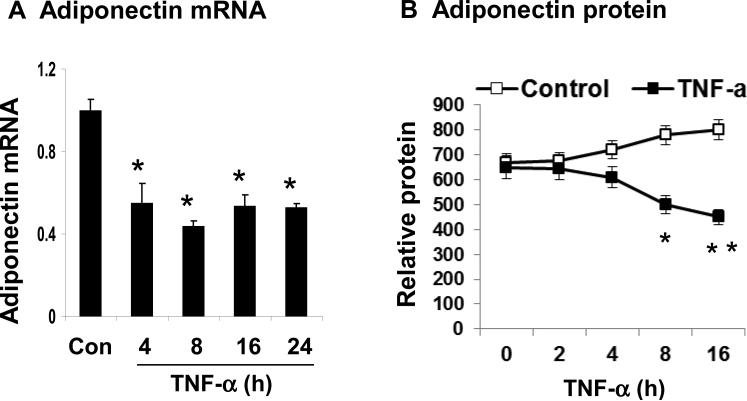
To understand the mechanism of adiponectin suppression by TNF-α, we used the cellular model system to study adiponectin expression. Adiponectin mRNA was reduced by TNF-α (20 ng/ml) in the 3T3-L1 adipocytes (Fig. 3A). At the four time points with TNF-treatment, the adiponectin mRNA was reduced by 50% (p<0.05) at 4 h, and the reduction was maintained at all of other time points thereafter (8, 16 and 24 h). Adiponectin protein was determined with ELISA in the supernatant and a reduction was observed at 8 h and thereafter in the presence of TNF-α (Fig. 3B, p<0.05). These data suggest that adiponectin expression is suppressed by TNF-α in 3T3-L1 adipocytes at mRNA and protein levels. The protein reduction is about 4 h late relative to the mRNA change.
Figure 3. Inhibition of adiponectin expression by TNF-α in 3T3-L1 adipocytes.
(A) Inhibition of adiponectin mRNA by TNF-α. The cells were serum-starved in DMEM containing 0.25% BSA overnight and then treated with TNF-α (20 ng/ml) for different times as indicated. The total RNA was extracted and subjected to qRT-PCR analysis for adiponectin mRNA. (B) Inhibition of adiponectin secretion by TNF-α. The cells were serum-starved overnight and treated with TNF-α for different times as indicated. Adiponectin in the culture medium of 3T3-L1 adipocytes was quantified by ELISA and the result was normalized with the supernatant protein content. In this figure, each bar represents mean ± SEM (n=3). *, P< 0.05. **, P<0.001.
TNF-α inhibits the adiponectin transcription and mRNA stability
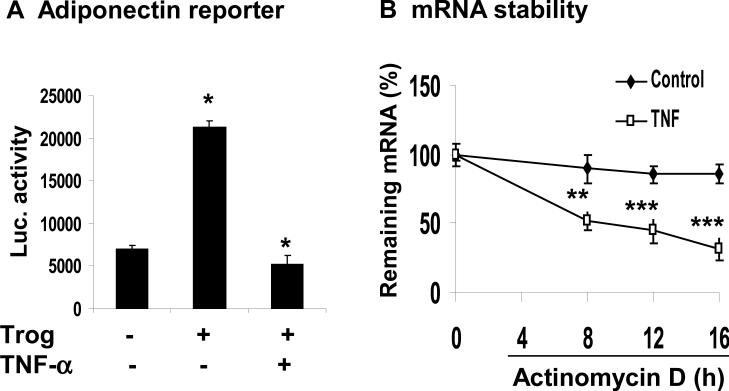
Since both transcriptional and post-transcriptional events are able to influence mRNA level, we would like to determine which event is more important in the mechanism of adiponectin inhibition by TNF-α. We examined adiponectin gene promoter activity using a luciferase reporter, which contains -2.0 kb human adiponectin promoter. In the transient transfection, the reporter activity was enhanced 4.5 fold by troglitazone in 3T3-L1 adipocytes (Fig. 4A). The induction was completely blocked by TNF-α (20 ng/ml) (Fig. 4A). The data suggest that TNF-α inhibits the adiponectin promoter activity. For this inhibition, TNFα probably targets PPARγ.
Figure 4. Adiponectin transcription and mRNA stability.
(A) Inhibition of adiponectin gene promoter by TNF-α. The transcriptional activity of mouse adiponectin promoter (–1,300/+18) was analyzed in 3T3-L1 adipocytes in a transient transfection. After 24 h transfection, the cells were serum-starved overnight and treated with troglitazone (10 μM) and TNF-α (20 ng/ml) for 16 h. (B) Influence of TNF-α on the adiponectin mRNA stability. De novo mRNA transcription was inhibited by actinomycin D (5 μg/ml) in 3T3-L1 adipocytes. The residual mRNA of adiponectin was quantified by qRT-PCR. Values represent percentage of residual mRNA versus mRNA level at time 0. Results are means ± SEM (n=3). *, P< 0.05. ***, P< 0.001.
To determine whether the posttranscriptional events are involved in adiponectin inhibition by TNF-α, we examined the stability of adiponectin mRNA. In the study, mRNA synthesis was blocked by actinomycin D (5 μg/ml) and the remaining adiponectin mRNA was measured at multiple time points (4, 8, 12 and 16 h) to determine the mRNA stability, which is indicated by the rate of mRNA reduction. The adiponectin mRNA had a long half-life over 16 h in the absence of TNF-treatment (Fig. 4B). The mRNA was reduced by less than 10% at 16 h, suggesting a long half-life (>16 h) of adiponectin mRNA. In the presence of TNF-α, the mRNA degradation was accelerated at all of the time points, and the mRNA half-life was reduced to 8 h (Fig. 4B). At 16 h, the mRNA was reduced by 70% (Fig. 4B). The data suggests that TNF-α induces degradation in adiponectin mRNA and shortens the mRNA stability. These results provide a new mechanism for adiponectin inhibition by TNF-α, which inhibits the adiponectin expression at both transcriptional and post-transcriptional levels.
TNF-α inhibits PPARγ gene expression through NF-κB pathway
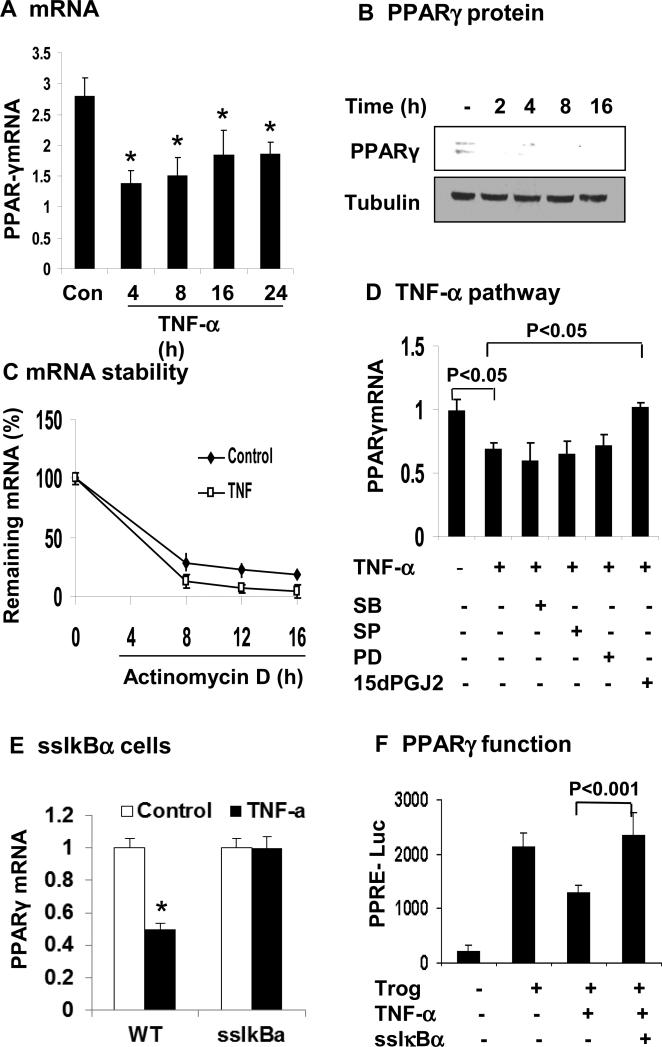
PPARγ is an activator for adiponectin gene transcription, and likely a target of TNF-α in the inhibition of adiponectin expression. To test the possibility, we examined PPARγ expression in 3T3-L1 adipocytes after TNF-treatment. PPARγ mRNA was significantly decreased by TNF-α (20 ng/ml). The inhibition was observed at 4 h and maintained throughout the experiment of 24 h (Fig. 5A). PPARγ protein was also decreased by TNF-α (Fig. 5B). These data suggest that TNF-α suppresses PPARγ in mRNA and protein in adipocytes. In the study, we also examined the PPARγ mRNA stability. As shown in Figure 5C, the half-life of PPARγ mRNA was about 4 h and was not changed by TNF-α.
Figure 5. TNF-α inhibits PPARγ expression through NF-κB pathway.
(A) Inhibition of PPARγ mRNA expression by TNF-α in 3T3-L1 adipocytes. The cells were serum-starved overnight and treated with TNF-α (20 ng/ml) for hours as indicated. PPARγ mRNA was determined in qRT-PCR. (B) Inhibition of PPARγ protein expression by TNF-α. PPARγ protein was determined in the whole cell lysate in a Western blot. (C) PPARγ mRNA stability. De novo mRNA transcription was inhibited by the addition of actinomycin D (5 μg/ml). The residual mRNA of PPARγ was quantified by qRT-PCR. Values represent percentage of residual mRNA versus mRNA level at time 0. (D) Blocking of TNF-α activity by the NF-κB inhibitor. The 3T3-L1 adipocytes were pretreated with the pharmacological inhibitors for 30 min before addition of TNF-α. The inhibitors are SB203580 (SB, 10 μM), SP600125 (SP, 25 μM), PD98059 (PD, 40 μM), and 15dPGJ2 (15 μM). mRNA was determined in the cells after 16 h. (E) PPARγ mRNA was quantified in ssIκBα 3T3-L1 adipocytes after TNF-α treatment. (F) The transcriptional activity of PPARγ was analyzed in 3T3-L1 fibroblasts using the PPRE (3X)-luciferase reporter system in the transient transfection. Expression vector of ssIκBα was co-transfected to block NF-κB activation. At 24 h after transfection, the cells were serum-starved overnight and treated with troglitazone (10 μM) and TNF-α (20 ng/ml). In this figure, each bar represents mean ± SEM (n=3). *, P< 0.05.
Several signaling pathways are activated by TNF-α, such as IKKβ/NF-κB pathway and MAPKs (ERK, JNK, and p38) pathways. To determine which pathway is required for the TNF-α activity, kinase-specific chemical inhibitors were used. These inhibitors include 15dPGJ2 (IKK2/IKKβ), SP600125 (JNK), SB203580 (p38), and PD98059 (MEK/ERK). 3T3-L1 adipocytes were pretreated with the inhibitors and then treated with TNF-α. IKKβ inhibitor (15dPGJ2) was able to block the TNF-α activity (Fig. 5D). In addition to inhibition of IKKβ, 15dPGJ2 has an activity in the activation of PPARγ. It is possible that the effect of 15dPGJ2 is a result of PPARγ activation. To exclude this possibility, the experiment was repeated in ssIκBα adipocytes, in which IKKβ activity in the activation of NF-kB is blocked by supper suppressor IκBα 18. In this cell line, the ability of TNF-α to inhibit PPARγ was blocked (Fig. 5E). The inhibitors to MAPKs were unable to block the TNF-activity (Fig. 5D). To ascertain the IKK/NF-κB activity in the regulation of PPARγ, we examined the PPARγ function using a luciferase reporter in 3T3-L1 cells. The reporter activity was induced by troglitazone (Fig. 5F). In the presence of TNF-α, the reporter response was reduced to 40% and the TNF-activity was completely blocked by the NF-kB inhibitor (ssIκBα) in a transient transfection. The data suggests that NF-κB mediates the TNF-α activity to inhibit PPARγ.
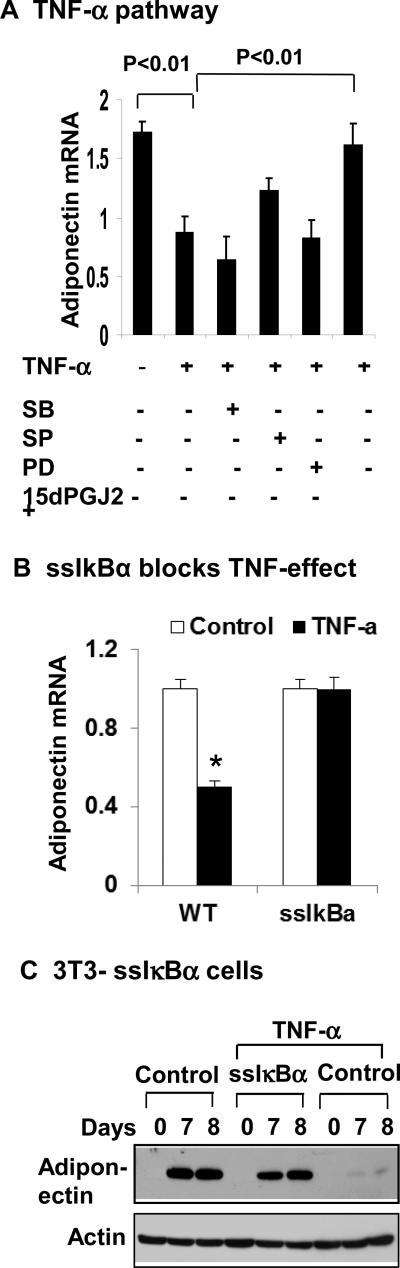
Adiponectin inhibition by IKK/NF-κB pathway
To determine the role of IKKβ/NF-κB pathway in the inhibition of adiponectin expression, we examined adiponectin mRNA in the same systems with NF-kB inhibition. The TNF-activity was blocked when IKK/NF-κB pathway, but not MAPK pathway was inhibited (Fig. 6A). The signaling pathways were suppressed by chemical inhibitors to IKK or MAPK (Fig. 6A). To ascertain the role of IKK/NF-κB pathway, we used the ssIκBα 3T3-L1 cells to determine the role of NF-kB in the adiponectin inhibition by TNF-α. Adiponectin was resistant to TNF-α treatment in ssIκBα 3T3-L1 adipocytes (Fig. 6B). Adiponectin was also examined during the adipogenesis of ssIκBα 3T3-L1 cells. Expression of adiponectin protein was completed blocked by TNF-α in the control cells, but not in ssIκBα 3T3-L1 cells during adipogenesis. These results suggest that IKKβ/NF-κB pathway is required by TNF-α to inhibit adiponectin gene expression.
Figure 6. IKK/NF-κB pathway in adiponectin inhibition.
(A) Blocking of TNF-α activity by the NF- κB inhibitor. 3T3-L1 adipocytes were pretreated with the pharmacological inhibitors for 30 min before addition of TNF-α. The inhibitors are SB203580 (SB, 10 μM), SP600125 (SP, 25 μM), PD98059 (PD, 40 μM), and 15dPGJ2 (15 μM). mRNA was determined in the cells 16 h later. (B) Inhibition of TNF-α activity in 3T3-L1 ssIκBα stable cells. Wild type and ssIκBα 3T3-L1 adipocytes were serum starved overnight and treated with TNF-α (20 ng/ml) for 16 h. Adiponectin mRNA was determined by qRT-PCR. (C) Adiponectin expression during adipogenesis. The wild type and ssIαBa 3T3-L1 cells were treated with TNF-α (20 ng/ml) during adipogenesis. Adiponectin was determined in the whole cell lysate at days 7 and 8 of differentiation. In this figure, each bar represents mean ± SEM (n=3).
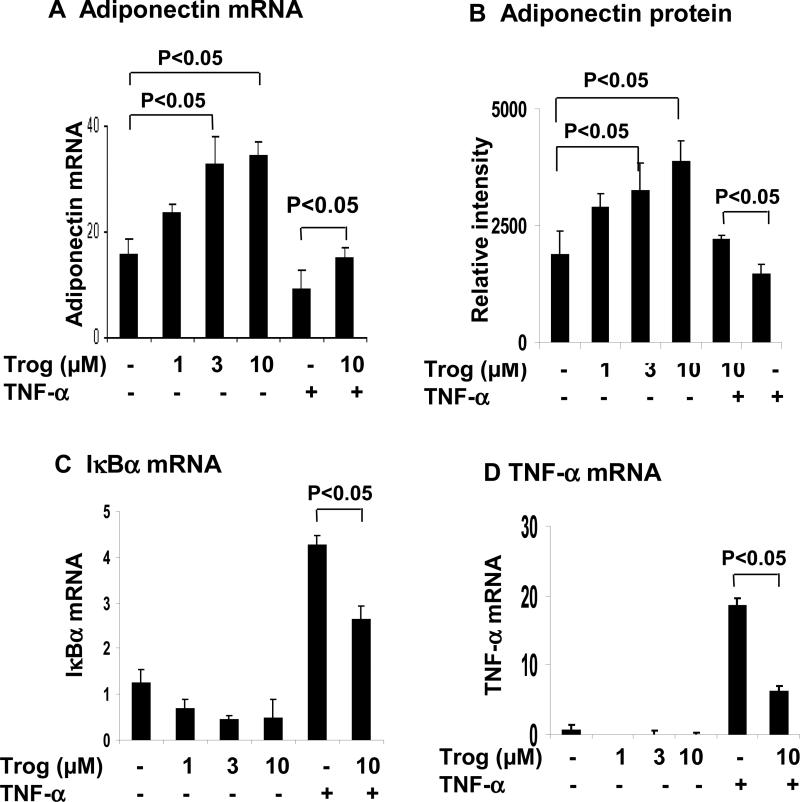
Troglitazone antagonizes TNF-α activity by inhibition of NF-κB
We investigated the effect of troglitazone in antagonizing TNF-α activity. Troglitazone (3μM for 24 h) increased adiponectin expression in mRNA (Fig. 7A) and protein in the cell culture media (Fig. 7B). The troglitazone effect was in a dose-dependent manner at concentrations between 1-10 μM. The TNF-α activity was partly blocked by troglitazone (Fig. 7A). To determine whether troglitazone acts through inhibition of NF-κB activity, we examined NF-κB target genes (TNF-α and IκBα). Expression of IκBα and TNF-α was highly induced by NF-kB activation from TNF-treatment (Fig. 7, C and D). The induction was reduced by troglitazone. Troglitazone alone inhibited the basal level of gene expression as well. The data suggest that troglitazone antagonizes the TNF-α action through inhibition of NF-κB pathway.
Figure 7. Troglitazone increases adiponectin mRNA and protein through antagonizing NF-κB pathway.
(A) and (B) Regulation of adiponectin expression by troglitazone in 3T3-L1 adipocytes. After serum-starved for overnight, the cells were treated with troglitazone (0, 1, 3, 10 μM) and TNF-α (20 ng/ml) for 24 h. Adiponectin mRNA was determined in qRT-PCR. The amount of adiponectin secreted into the medium was measured in Western blot. The adiponectin protein signal was determined by densitometry. (C) and (D) NF-κB target genes (IκBα, TNF-α) were measured after troglitazone and TNF-α treatment. mRNA was quantified in qRT-PCR. In this figure, each bar represents mean ± SEM (n=3). *, P<0.05.
DISCUSSION
In this study, we investigated the interplay of pro-inflammatory and anti-inflammatory cytokines in the regulation of adipocyte function. TNF-α and adiponectin were selected as representatives in each class of cytokines. We observed that in adipose tissue of mice with chronic inflammation, TNF-α induction was associated with adiponectin reduction in aP2-p65 mice, DIO mice and ob/ob mice. The results support the negative association of the expression of the two cytokine in human obesity 27. The two cytokines, adiponectin and TNF-α, exhibited opposite metabolic activities in the regulation of adipogenesis, lipolysis and glucose uptake in adipocytes, which is consistent with observations in other studies 18, 28. Adiponectin increased lipid accumulation, reduces lipolysis and promotes insulin-induced glucose uptake. In contract, TNF-α decreased lipid accumulation, induced lipolysis, and attenuated insulin-induced glucose uptake. In addition, we observed that TNF-α repressed the adiponectin gene promoter activity, which is in line with observations in other reports 29, 30. More importantly, we found that TNF-α promoted degradation of adiponectin mRNA, which was not reported in other studies 29, 30.
Inhibition of adiponectin expression by TNF-α was reported 31, 32. However, post-transcriptional regulation of adiponectin mRNA was not examined in these studies. We observed that TNF-α inhibited adiponectin mRNA stability at the post-transcriptional level. Adiponectin mRNA exhibited a long half-life of more than 16 h. In response to TNF-α, the half-life was reduced to 8 h. The mRNA degradation was enhanced in response to TNF-α treatment. The observation provides a new mechanism for adiponectin regulation by inflammation. Adiponectin mRNA does not contain a classical AU-rich motif and poly (A) tail structure that are required for mRNA stability. Further studies are needed to explore the mechanism underlying the adiponectin mRNA stability.
At the transcriptional level, TNF-α inhibits adiponectin expression by targeting PPARγ, which is a transcriptional activator of adiponectin gene33 and a master regulator of adipogenesis 35. In this study, we observed that adiponectin gene promoter activity was enhanced by PPARγ ligand troglitazone, which is consistent with adiponectin mRNA expression in 3T3-L1 adipocytes in the response to troglitazone. The data suggests a critical role of PPARγ in the regulation of adiponectin transcription. A functional PPARγ response element (PPRE) was reported in the adiponectin gene promoter 33. Our data suggests that PPRE is likely a target of TNF-α in the transcriptional suppression of adiponectin. In the mechanism, PPARγ expression was reduced and the mRNA stability was not altered by TNF-α. The PPARγ inhibition was dependent on activation of IKKβ/NF-kB pathway in our study. The NF-kB signaling pathway was reported to inhibit ligand-induced PPARγ activity in adipocytes through activation corepressor component histone deacetylase 3 34.
Adiponectin may antagonize TNF-α activity by activating AMPK pathway. Adiponectin is able to attenuate the metabolic activities of TNF-α. The signaling pathway of adiponectin action was not investigated in this study. Adiponectin activates AMPK kinase through its cell membrane receptor 36. This signaling pathway is likely involved in the inhibition of TNF-α activity. Specifically, AMPK inhibits mTOR/S6K pathway 37 that is required for activation of IKKβ/NF-kB signaling pathway by TNF-α 38. Adiponectin was reported to inhibit NF-kB activity in endothelial cells 39. Inhibition of IKKβ/NF-kB activity by adiponectin is a potential mechanism by which adiponectin antagonize the TNF-α activities.
Although cytokines are generally grouped as pro- or anti-inflammatory cytokines, the function and characteristics of individual cytokines may vary. Adiponectin expression pattern suggests that in general, preponderance of anti-inflammatory cytokines is dominant in adipose tissue at lower levels of adiposity. The biological significance is likely to promote energy accumulation and storage in adipose tissue. This point is supported by the phenotype of adiponectin over-expressing mice 40, which exhibit super obesity without adipose chronic inflammation. Pro-inflammatory cytokines are increased in adipose tissue by obesity through a local hypoxia response 23. The inflammation reflects a compensatory response in an effort to limit energy storage and adipose tissue expansion in obesity to maintain body fat within an optimal range 41. Pro-inflammatory cytokine deficiency impairs this feedback response and leads to adulthood obesity. Phenotypes of many transgenic models suggest that when pro-inflammation is reduced by gene knockout, energy accumulation is enhanced in adipose tissue and the risk for obesity is increased. The phenotype has been reported in mice with global knockout for IL-1β 42, 43, IL-6 44, and IL-18 45. In contract, an elevation in pro-inflammation in adipose tissue prevents energy accumulation and blocks diet-induced adipose tissue expansion. The conclusion is supported by the phenotypes of aP2-p65 mice or aP2-IKKβ mice 20, 34, 46, in which NF-κB-induced chronic inflammation represses adipocyte differentiation and adipose tissue expansion. The mice are resistant to diet-induced obesity and insulin resistance 20, 46. These studies suggest that the interplay of pro-inflammatory and anti-inflammatory cytokines is important in the control of energy homeostasis and adipose tissue size.
In summary, we used TNF-α and adiponectin to demonstrate an interplay of pro-inflammatory and anti-inflammatory cytokines in the regulation of adipocyte activities. The result suggests that the interplay is important in the control of lipid accumulation in adipocytes. This study provides a new support to our hypothesis that in obesity, pro-inflammatory response serves as a feedback control of energy storage in adipose tissue 41, 47. It opposes lipid accumulation by stimulating lipolysis and inhibiting adipogenesis as well as adiponectin expression. The chronic inflammation may represent a body's attempt to stimulate energy expenditure in the control of adiposity. As a result, inhibition of the inflammatory response by anti-inflammation agents may not yield a strong beneficial effect in the control of obesity and obesity-associated disorders. Although nearly two dozens of clinical trials have been conducted to test anti-inflammatory agents in the control of insulin resistance in type 2 diabetes patients, the results are not promising as most studies failed to yield the beneficial effects at the magnitude expected 48. A better understanding of the physiological interplay of pro-inflammatory and anti-inflammatory cytokines may provide an explanation to the limited efficacy of anti-inflammatory therapies.
Acknowledgments
We appreciate the technical support from Ms. Xin Ye. qRT-PCR assay was conducted in the genomics core of the Pennington Biomedical Research Center. The core is supported by the NIH grants (P30DK072476 and P20 GM103528).
Funding: National Institute of Health research projects (DK085495; DK068036) to Ye, J.
Footnotes
Competing Interests: The authors have declared no potential conflicts of interest relevant to this article.
Author contributions
Y.W., H.W., V.H, and O.D., performed the experiments. V.H., N.D, G.Z, and J.Y analyzed the data and wrote the manuscript. J.Y is fully responsible for this article. All authors read and approved the final manuscript.
References
- 1.Chavarro JE, Ehrlich S, Colaci DS, Wright DL, Toth TL, Petrozza JC, et al. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertility and sterility. 2012 doi: 10.1016/j.fertnstert.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frisch RE. Population, food intake, and fertility. There is historical evidence for a direct effect of nutrition on reproductive ability. Science. 1978;199(4324):22–30. doi: 10.1126/science.199.4324.22. [DOI] [PubMed] [Google Scholar]
- 3.Scott EC, Johnston FE. Critical fat, menarche, and the maintenance of menstrual cycles: a critical review. Journal of adolescent health care : official publication of the Society for Adolescent Medicine. 1982;2(4):249–60. doi: 10.1016/s0197-0070(82)80059-4. [DOI] [PubMed] [Google Scholar]
- 4.Jensen TK, Andersson AM, Jorgensen N, Andersen AG, Carlsen E, Petersen JH, et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertility and sterility. 2004;82(4):863–70. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 5.Shayeb AG, Harrild K, Mathers E, Bhattacharya S. An exploration of the association between male body mass index and semen quality. Reproductive biomedicine online. 2011;23(6):717–23. doi: 10.1016/j.rbmo.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Veleva Z, Tiitinen A, Vilska S, Hyden-Granskog C, Tomas C, Martikainen H, et al. High and low BMI increase the risk of miscarriage after IVF/ICSI and FET. Human reproduction. 2008;23(4):878–84. doi: 10.1093/humrep/den017. [DOI] [PubMed] [Google Scholar]
- 7.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 8.Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertility and sterility. 2002;77(3):433–44. doi: 10.1016/s0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- 9.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147(2):173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 10.Stryjecki C, Mutch DM. Fatty acid-gene interactions, adipokines and obesity. Eur J Clin Nutr. 2011;65(3):285–97. doi: 10.1038/ejcn.2010.277. [DOI] [PubMed] [Google Scholar]
- 11.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 12.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 13.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13(2):84–9. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 14.Qiao L, Kinney B, Schaack J, Shao J. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes. 2011;60(5):1519–27. doi: 10.2337/db10-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 16.Mauer J, Chaurasia B, Plum L, Quast T, Hampel B, Bluher M, et al. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS genetics. 2010;6(5):e1000938. doi: 10.1371/journal.pgen.1000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 18.Gao Z, He Q, Peng B, Chiao PJ, Ye J. Regulation of Nuclear Translocation of HDAC3 by IkBa Is Required for Tumor Necrosis Factor Inhibition of Peroxisome Proliferator-activated Receptor {gamma} Function. J Biol Chem. 2006;281(7):4540–7. doi: 10.1074/jbc.M507784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor KappaB kinase complex. J Biol Chem. 2002;277(Dec 13):48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 20.Tang T, Zhang J, Yin J, Staszkiewicz J, Gawronska-Kozak B, Mynatt R, et al. Uncoupling of Inflammation and Insulin Resistance by NF-kB in Transgenic Mice through Induction of Energy Expenditure. J Biol Chem. 2010;285:4637–4644. doi: 10.1074/jbc.M109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye J. Regulation of PPARg function by TNF-a. Biochem Biophys Res Commun. 2008;374:405–408. doi: 10.1016/j.bbrc.2008.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker RG, Hayden MS, Ghosh S. NF-kappaB, Inflammation, and Metabolic Disease. Cell Metab. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye J, Gao Z, Yin J, He H. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 24.Rogers PM, Mashtalir N, Rathod MA, Dubuisson O, Wang Z, Dasuri K, et al. Metabolically favorable remodeling of human adipose tissue by human adenovirus type 36. Diabetes. 2008;57(9):2321–31. doi: 10.2337/db07-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang ZQ, Cefalu WT, Zhang XH, Yu Y, Qin J, Son L, et al. Human adenovirus type 36 enhances glucose uptake in diabetic and nondiabetic human skeletal muscle cells independent of insulin signaling. Diabetes. 2008;57(7):1805–13. doi: 10.2337/db07-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adachi H, Kurachi H, Homma H, Adachi K, Imai T, Sakata M, et al. Involvement of epidermal growth factor in inducing adiposity of age female mice. Journal of endocrinology. 1995;146(3):381–93. doi: 10.1677/joe.0.1460381. [DOI] [PubMed] [Google Scholar]
- 27.Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144(9):3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 28.Cawthorn WP, Heyd F, Hegyi K, Sethi JK. Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 2007;14(7):1361–73. doi: 10.1038/sj.cdd.4402127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hector J, Schwarzloh B, Goehring J, Strate TG, Hess UF, Deuretzbacher G, et al. TNF-alpha alters visfatin and adiponectin levels in human fat. Horm Metab Res. 2007;39(4):250–5. doi: 10.1055/s-2007-973075. [DOI] [PubMed] [Google Scholar]
- 30.Zappala G, Rechler MM. IGFBP-3, hypoxia and TNF-alpha inhibit adiponectin transcription. Biochemical and biophysical research communications. 2009;382(4):785–9. doi: 10.1016/j.bbrc.2009.03.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50(9):2094–9. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 32.Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, et al. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. American Journal of Physiology - Endocrinology And Metabolism. 2003;285(3):E527–E533. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- 33.Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, et al. Induction of Adiponectin, a Fat-Derived Antidiabetic and Antiatherogenic Factor, by Nuclear Receptors. Diabetes. 2003;52(7):1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Henagan TM, Gao Z, Ye J. Inhibition of Glyceroneogenesis by Histone Deacetylase 3 Contributes to Lipodystrophy in Mice with Adipose Tissue Inflammation. Endocrinology. 2011;152(5):1829–1838. doi: 10.1210/en.2010-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14(11):1293–307. [PubMed] [Google Scholar]
- 36.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 37.Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279(16):15719–22. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Gao Z, Yin J, Quon MJ, Ye J. S6K Directly Phosphorylates IRS-1 on Ser-270 to Promote Insulin Resistance in Response to TNF-α Signaling Through IKK2. J Biol Chem. 2008 Dec 19th;283:35375–35382. doi: 10.1074/jbc.M806480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 40.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117(9):2621–37. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye J. Emerging Role of Adipose Tissue Hypoxia in Obesity and Insulin Resistance. Int J Obes. 2009;33(1):54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MohanKumar SM, Smith CL, MohanKumar PS. Central adaptation to chronic administration of interleukin-1beta (IL-1beta) in rats. Brain Res Bull. 2003;62(1):71–6. doi: 10.1016/j.brainresbull.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Matsuki T, Horai R, Sudo K, Iwakura Y. IL-1 Plays an Important Role in Lipid Metabolism by Regulating Insulin Levels under Physiological Conditions. J. Exp. Med. 2003;198(6):877–888. doi: 10.1084/jem.20030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8(1):75–9. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 45.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12(6):650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 46.Jiao P, Feng B, Ma J, Nie Y, Paul E, Li Y, et al. Constitutive Activation of IKKβ in Adipose Tissue Prevents Diet-Induced Obesity in Mice. Endocrinology. 2012;153(1):154–165. doi: 10.1210/en.2011-1346. [DOI] [PubMed] [Google Scholar]
- 47.Ye J, Keller J. Regulation of energy metabolism by inflammation: A feedback response in obesity and calorie restriction. Aging. 2010;2(6):361–368. doi: 10.18632/aging.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nature reviews. Immunology. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]