Abstract
The brain is highly accessible for nutrients and oxygen, however delivery of drugs to malignant brain tumors is a very challenging task. Convection-enhanced delivery (CED) has been designed to overcome some of the difficulties so that pharmacological agents that would not normally cross the BBB can be used for treatment. Drugs are delivered through one to several catheters placed stereotactically directly within the tumor mass or around the tumor or the resection cavity. Several classes of drugs are amenable to this technology including standard chemotherapeutics or novel experimental targeted drugs. The first Phase III trial for CED-delivered, molecularly targeted cytotoxin in the treatment of recurrent glioblastoma multiforme has been accomplished and demonstrated objective clinical efficacy. The lessons learned from more than a decade of attempts at exploiting CED for brain cancer treatment weigh critically for its future clinical applications. The main issues center around the type of catheters used, number of catheters and their exact placement; pharmacological formulation of drugs, prescreening patients undergoing treatment and monitoring the distribution of drugs in tumors and the tumor-infiltrated brain. It is expected that optimizing CED will make this technology a permanent addition to clinical management of brain malignancies.
Keywords: catheter, CNS, convection-enhanced delivery, cytotoxin, gene therapy, glioblastoma multiforme, imaging, infusate, liposomes, molecular targets, real-time MRI
Convection-enhanced delivery to brain parenchyma
Accessibility of pharmacological agents from the bloodstream to the CNS meets with a formidable obstacle in the form of the BBB [1]. There is an absence of direct intracranial administration-only agents that are actively transported through the BBB or that are otherwise permeable through the barrier, which can directly kill tumor cells. Even for drugs that are permeable through the BBB it is difficult to obtain effective pharmacological concentrations through simple diffusion from blood to CNS [1]. Moreover, most drugs having an effect on the CNS are inherently not sufficiently permeable for the BBB. Another difficulty of delivering drugs to the CNS is how to direct them preferably to the discrete anatomic regions or diseased parts of brain. Another related question is how to maintain relatively constant and pharmacologically effective concentrations of the drugs in the diseased brain. These questions are particularly relevant to the delivery of antineoplastic drugs [2–4].
A translational research group directed by Edward Oldfield at the National Institute of Neurological Disorders developed a very innovative idea of bypassing the BBB by infusing drugs directly into the CNS parenchyma. In their landmark initial work, they tested two molecules of different biochemical/biophysical characteristics and sizes (molecular weights): transferrin (Tf) and sucrose [5]. Tf and sucrose were radiolabeled and delivered through a needle installed intraparenchymally in cats and attached to a pump providing positive pressure and constant flow of the infusates, a technique named convection-enhanced delivery (CED). They found significantly enhanced distribution of these varied-size molecules and an increase in the infused compounds’ locoregional concentration. For agents that do not cross the BBB the concentration gradient at the surface of maximum distribution is steep, a potentially large benefit in the distribution of antineoplastic agents. This work initiated almost 15 years of experimentation with CED, leading to multiple clinical trials in various CNS diseases [4,6–20]. Nanoparticles, toxins, chemotherapeutics, oligonucleotides, liposomes, nanolipoparticles, antibodies, viruses, growth factors, radioisotopes, peptides, proteins, contrast agents and even hypothermia were all found to be deliverable through CED [6–25]. In this review we will discuss the aspects of CED that we believe are crucial for its further successful clinical development for the treatment of brain tumors, especially in the treatment of high-grade astrocytomas, such as glioblastoma multiforme (GBM). Astrocytomas are the most prevalent primary brain tumors and GBM is the most prevalent form of astrocytoma [26]. They represent an unmet need in medicine, because only months have been added to the lives of patients with GBM since statistics have been kept [27,28].
Catheters for CED
Reflux-preventing catheters
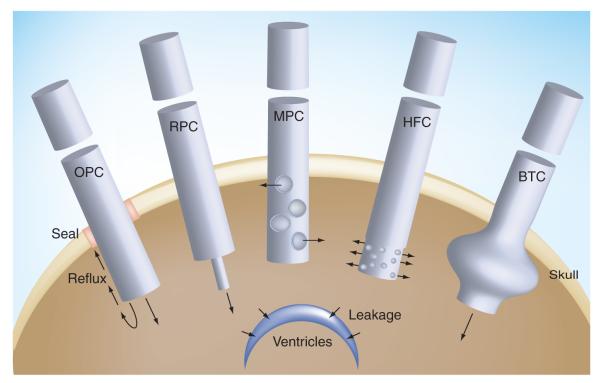
The first infusion tool for CED was a needle implanted in the CNS of experimental animals [5]. For clinical application, a more practical and flexible method was to employ a catheter instead of a needle, which could be stereotactically placed in the CNS and deliver the infusates through a port at the distal end. The barium-impregnated Medtronic® PS Medical (Goleta, CA, USA; Catalog number 43209) and Vygon US LLC (Valley Forge, PA, USA) one port catheters have been frequently utilized in the most recent clinical trials (FIGURE 1) [8]. One interesting observation made recently by Fiandaca et al. was that extending the end of the tip of a lead catheter with a smaller gauge catheter apparently has a positive effect on the prevention of infusate reflux, which is one of the undesirable events that can alter successful CED and, unfortunately, can occur in an unacceptable number of cases [29,30].
Figure 1. Schema of several catheters tested for convection-enhanced delivery.
Arrows symbolize the flow of infusate during convection-enhanced delivery. BTC: Balloon-tipped catheter; HFC: Hollow fiber catheter; MPC: Multiple port catheter; OPC: One port catheter; RPC: Reflux-preventing catheter.
The presence of reflux negates the very reason that CED was designed for, that is, an interrupted homogenous delivery of drugs into the extracellular space by bulk fluid flow away from catheter replacing the extracellular fluid with the infusate. In the presence of reflux, any further increase in the infusion volume is not accompanied by an increase in the distribution volume of the drug and, thus, the CED may become a futile exercise with the potential for appearance of toxic effects owing to possible ventricular or subacrachnoid space leakage at rates above 3 μl/min [29]. While a reduction in CED infusion rate may reduce the chance of reflux, it would be ideal to have the option of delivering drugs at varying flow rates, including 5 μl/min or more if possible, to maximize the volume of tissue to which the drug is delivered. Experimental results also demonstrate that the larger the diameter of catheters used for CED, the greater the chance of reflux taking place during infusion [31].
Thus, Fiandaca et al. constructed a step design cannula that allows CED at flow rates as high as 5.0 μl/min without an accompanying reflux (Figure 1) [29]. The cannula is composed of a 0.2-mm needle with a glued-in silica tubing (0.168-mm external diameter) that extends beyond the end of the needle by 5–10 mm. Even with this technical improvement, the new catheter is not reflux-free and real-time MRI documented that in up to 20% of catheter placements reflux can be seen along the insertion tract, but at higher achievable infusion flow rates. There might be a threshold for the performance of a reflux-preventing catheter related to the level of the infusates’ flow rate. Nevertheless, the improved catheter offers an attractive alternative to those traditionally used and is currently under clinical investigation. Interestingly, a simple maneuver of thorough sealing of the entry of the catheter through the skull also had a preventive effect on the extent of reflux during CED (Figure 1) [32].
Hollow fiber or multi-tipped catheters
Another type of catheter has been developed to improve the distribution of drugs using CED. Standard catheters release drugs at just one site, at their tips with one opening (port) including the one with reflux-preventing properties (Figure 1) [29]. This output must provide sufficient flow of infusates in all the desired directions and with sufficient pressure/force to displace the extracellular fluid farther away from the catheter. Conceptually, it is hard to demand such a performance from a catheter with one opening taking into account that the infusates will follow the path of the lowest interstitial pressure met at the tip (just one) of the catheter. Better success with the CED could be envisioned with the use of catheters with multiple openings. However, previous studies have shown that a multiport catheter delivered infusates efficiently only through the proximal port and, thus, resembled a one-port catheter in terms of performance (Figure 1) [33].
A porous hollow fiber catheter has been constructed in order to increase the surface area of the brain in immediate contact with the released drugs and the volume of tissue exposed to which drugs can be delivered (Figure 1) [34]. The hollow fiber has millions of nano-openings (0.45 μm) along its wall. The hollow-fiber catheter offers up to a threefold increase in the distribution volume of the drug into normal mouse brain when compared with a needle-mediated (one larger drug-releasing port) infusion [34]. It should be noted, however, that a short, 3-mm long, hollow fiber catheter was used by Oh et al., which may not be generalizable to longer catheter lengths required for human applications [34]. Thus, studies in larger experimental animals with hollow fiber catheters longer than 3 mm are warranted in order to verify whether the effect seen with multiport catheter also applies to hollow fibers. For example, theoretically, the infusate could leave the hollow fiber catheters only at its proximal portion leaving the remaining, distal part useless for CED. However, this does not have to happen, since the tiny openings in the hollow fiber may not play the role of a large port in reducing pressure, but rather maintaining relative resistance and a more even release of the infusate from the catheter. On the other hand, hollow fibers might also be used to ‘cap’ the tip of traditional catheters. It remains to be studied whether hollow-fiber caps that are smaller in diameter could also provide additional anti-reflux protection.
A hypothetical option would be to construct a multiport catheter functioning so that each port is a continuation of a mini-catheter within a lead catheter (multicannula catheter). Such a catheter would be both multiport and antireflux by design and allow more extensive planning of the infusate’s delivery into various targeted regions of brain tumor via a single initial trajectory. We feel that considerably more engineering and experimentation needs to be dedicated to the construction and testing of CED catheters for the specific purpose of delivering antineoplastic drugs to malignant CNS lesions.
Balloon-tipped catheter
A hopeful catheter design to enhance efficiency of CED for the treatment of brain tumors has recently been examined in the form of a balloon-tipped catheter (Figure 1). Different from standard one-port-at-the-tip catheters, this catheter has a balloon proximal to the catheter tip that can be inflated to fill a resection cavity, limiting reflux and forcing infusate into the tumor parenchyma. Olson et al. examined this catheter in normal canine CNS [35]. A similar balloon catheter without a distal opening was originally made to deliver brachytherapy through GliaSite radiation therapy system [36] using organically bound 125I Iotrex radiotherapy solution in recurrent glioma requiring resection. In both applications, the balloon physically fills the resection cavity. In the case of brachytherapy the radioactive solution is infused through a catheter and contained within the balloon. In the case of balloon-catheter-based CED, the infusate is delivered through a single distal opening into the parenchyma of the post-resection cavity. Extensive delivery of infusate was demonstrated with an inflated balloon with continuous CED in the canine model [35]. The infusate penetrated the brain parenchyma all around the balloon up to a depth of 25 mm, which speculatively covered enough volume to reach 90% of the suspected infiltrative glioma cells. The ventricular or subarachnoid space leakage was observed, as expected. The results are very encouraging and this approach needs to be examined in a preclinical brain tumor model, preferentially in canine gliomas for the reasons discussed later. The balloon-tipped catheter highlights a fundamental question about the best approach to CED after resection: is it better to use multiple catheters around the resection cavity or a balloon-tipped catheter inside the cavity, or to use both and in what sequence?
Leakage of infusates during CED
In the ideal scenario, agents delivered through CED should be contained within the targeted area of the CNS. Studies both in humans and experimental animals have shown widespread distribution of the infusate, such as labeled liposomes into various regions of the brain. Infused agents may leave targeted areas, such as tumors, and leak into either the ventricles or sulci [37]. This is undesirable because it results in waste in terms of the therapeutic agent and an inadvertent opportunity for more intense, direct and potentially adverse interactions of drugs with the normal the CNS. The frequency of the leakage is substantial, since it may affect more than 20% of CED attempts. It becomes critical to eventually understand and measure what happens to the efficiency of the targeted CED in the presence of the leakage. Varenika et al. made one of the first attempts in this direction generating volume-distributed versus volume-infused graphs during CED in dogs and monkeys using real-time MRI. Quite predictably, the presence of leakage prevented further increase in the concentration of the infused agent in the targeted area of CNS; instead, the concentration of infusate in the target actually diminished once leakage begins to occur. It remains to be seen if the phenomenon of inadvertent leakage is irreversible, that is, whether stopping and subsequent restarting of the infusion would potentially eliminate leakage routes. It would also be interesting to analyze how repositioning of catheters might avoid unwanted leakage in the same study or treatment subject. This would provide an argument for not abandoning CED in a patient in whom leakage takes place and who could still be offered effective CED following changes in stereotactic placement of catheters. One important conclusion that can be derived from the type of studies conducted by Veranika et al. [37] is that CED must be supported by effective imaging of the fate of the infusates, which has already been investigated extensively [38–41]. This is compatible with the need for personalized targeted therapy approaches and personalized medicine.
Pharmacologic formulation of CED infusates
The initial purpose of CED was to deliver pharmacologically active, soluble compounds of varying molecular weights into the parenchyma of tumors and also of normal brain [5]. The pressurized delivery of such solutes encounters physico–chemical forces in the extracellular space of either malignant tissue or normal CNS and the two differ to a significant degree. One important difference is the level of interstitial fluid pressure, which is high in brain tumors [42]. These physico–chemical forces theoretically and practically represent obstacles to effective CED and inevitably affect the possibility of delivering infusates to the entire desired target volume. Intuitively, less viscous solutes might have a better chance of penetrating the extracellular space of tissues, and thus deliver soluble drugs deeper and perhaps with more uniform and higher concentrations locally into malignant tumors of the brain. However, some evidence suggests that increasing the viscosity of infusates actually improves the volume of distribution significantly [43]. Similar results were obtained in a rat model of normal brain CED documented by MRI and spectroscopic measurements [44]. The infusates were formulated as monodispersed maghemite nanoparticles in solutions of 3% sucrose or 3–6% polyethene glycol and the nanoparticles were also dextran coated. In addition, liposomes and their contents have been successfully delivered through CED, which further supports the notion that the formulation of infusates is an important parameter to consider in CED [45]. More studies are needed in preclinical tumor models to document the utility of an increased viscosity as a tool to improve controlled delivery of anticancer agents through CED.
Another, no less important, factor determining the extent of infusate distribution is the surface properties of the infusates [46–48]. Several careful studies have documented that surface properties are even more important than, for example, the size of particles infused with the CED [47]. All of these studies point to the need for a much better understanding of the combination of variables affecting distribution of solutes of varying physico–chemical compositions within the parenchyma of brain tumors and what changes in the surface properties/pharmacological formulation would bring optimal results for drug distribution.
Predicting individualized CED drug distributions using computer software
As for any invasive procedure, it would be desirable to base decisions on how to plan CED on standardized parameters customized to suit individual patients. Commercially available, US FDA-approved software has been developed by BrainLAB AG (Feldkirchen, Germany). The software requires input of data obtained by MRI regarding brain tissue characteristics of an individual patient. The target of calculations is desired drug distribution volume and the plan for treatment can be visualized in 3D, including the number and position of catheters [11]. The software was examined in practice, clinically, in a retrospective manner based on data obtained with magnetic resonance diffusion tensor imaging. A drug, targeted recombinant cytotoxin, hIL13-PE38QQR (see later), was coinfused with iodo-labeled human serum albumin (HSA). The distribution of HSA was predicted to mimic the actual distribution volume and pattern of the cytotoxin’s distribution by serving as a measurable tracer. The studies demonstrated that the software was sensitive and specific for the prediction of infusion reflux and leakage. The concordance between the plan of infusion and actual distribution of HSA was statistically significant, but achieved a mean accuracy of only approximately 65% overall. The software simulation was considered clinically confirmed for more than 80% of individual catheters used for CED. It should be kept in mind that HSA has no known affinity towards malignant tissue or normal brain parenchyma, opposite to the properties of a targeted cytotoxin. The patients were not examined for the status (e.g., high-, moderate-, low- or non-expressors) of the targeted receptor either. Nevertheless, software taking into account individual characteristics of a patient’s anatomy and pathophysiology for the initial plan of CED is likely to be a helpful addition to personalized surgical management.
Canine model for CED
The introduction of CED into the clinic was preceded by experimentation, primarily in rodents or nonhuman primates [45,49–58]. The problem with these models is that they are only partially clinically relevant with respect to human brain tumors. A collaboration between the University of California Davis and University of California at San Francisco has pioneered the use of a canine model for studying the intricacies of CED in spontaneous brain tumors, which are of size and clinical history similar to those in human patients. Dickinson et al. exploited real-time MRI for monitoring and follow-up of CED of nanoparticles containing gadolinium and/or irinotecan (CPT-11), initially in the brain of nondiseased dogs [59]. CED using one catheter for pumping the infusate resulted in significant volumes of distribution in both white and gray matters. Imaging offered precise measurements of volumes and routes of infusate distribution. Ventricular leakage was observed in some cases again with little serious adverse effects attributable to CED. The study by Dickinson et al. has paved the way for the use of CED in tumor-bearing dogs and testing of the technology and novel experimental drugs in more clinically relevant set ups. Representing a unique pathobiological phenomenon, canine gliomas exhibit essentially the same properties as human gliomas, including pathology (markers), genetics, behavior (invasiveness), lack of metastases and a similar clinical course of the disease [60].
Anti-GBM cytotoxins for CED
An extremely promising approach in anti-GBM therapy uses molecularly targeted recombinant chimera cytotoxic fusion proteins [2,61–63]. Recombinant cytotoxins are composed of a targeting ligand/vector and an effector in the form of derivatives of various bacterial toxins [2,62]. Bacterial toxins that are frequently utilized in the design of cytotoxins are Pseudomonas exotoxin A (PE) and Diphtheria toxin (DT). PE and DT are extremely potent killing agents for eukaryotic cells [64] and they have been genetically modified for the purpose of attachment to cell type-specific ligands/carriers [65]. Several targeted cytotoxins delivered through CED have reached the clinic. Clinical trials with the cytotoxins provide invaluable information on the applicability of this new technology.
Cytotoxins exhibit attractive pharmacological features for clinical GBM therapy. They are relatively small, highly soluble proteinaceous compounds and extremely potent at killing GBM cells [2]. It is difficult to produce resistance against the cytotoxins because they work through irreversible de novo protein synthesis. Targeted cytotoxins are an example of experimental drugs traveling the translational path from bench to the clinic quite quickly. The first cytotoxin that entered clinical trials was DT.CRM107–Tf, a conjugate between Tf and a DT derivative [63]. The compound was designed and generated by the group at the NIH/National Institute of Neurological Disorders and Stroke (Bethesda MD, USA) and most recently commercially developed as TransMID™ by Celtic Pharma (HM, USA). The cytotoxin was delivered directly into the tumor bed by CED [63,66]. Numerous significant clinical responses were observed in Phase I and II clinical trials [63]. Unfortunately, the possibility of normal tissue toxicity due to DT.CRM107–Tf killing nonmalignant cells materialized in the clinical setting and the Phase III efficacy trial was stopped [67].
Another cytotoxin that was clinically examined was based on IL-4 [68]. Phase I and II clinical trials demonstrated a lesser number of objective clinical responses than that seen with DT.CRM107–Tf [69]. Another cytotoxin that entered the clinic, TGF-α-PE38 (TP-38), targets the receptor (EGF receptor) that is abundantly overexpressed in less than 30% of patients with GBM and is naturally present in many normal organs [66]. Thus far, moderate responses were recorded in several patients [70], but more recent data demonstrated toxic events at very low doses of TP-38. Only 20% of patients retained the cytotoxin within the tumors by imaging, which correlates with the overall number of GBM patients overexpressing EGF receptor [9].
Almost 15 years ago, Debinski’s laboratory generated a cytotoxin consisting of IL-13 and a truncated form of PE, PE38QQR [71]. The cytotoxin was made for comparison with a previously produced IL-4-based cytotoxin, since IL-13 and IL-4 are homologous cytokines. Only later was it found that the wild-type IL-13-based cytotoxin has a very potent and specific killing effect on GBM cells and tumors [72]. hIL13-PE38QQR demonstrated efficacy in several preclinical GBM models that were strongly supportive of further clinical translation. The compound was licensed by NeoPharm, Inc. based on interinstitutional agreement between the US Public Health Service and University of Montreal and moved to the clinic as cintredekin besudotox (CB). The CB cytotoxin went from several multicenter Phase I and II trials to a Phase III clinical trial in a relatively short period of time [13,73]. This took place because of a significant number of responses were seen in early-phase trials, even though patients were enrolled without taking into account the status of the IL-13Rα2 receptor expression.
Early trials were to determine the maximum tolerated dose at increasing flow rates of the infusate and intracranial distribution of the cytotoxin. The maximum tolerated dose of 0.5 μg/ml of infusate via CED performed up to 94 h was not associated with unexpected adverse events. The responses in patients with recurrent GBM reflect the drug’s anti-tumor efficacy. In the early-phase trial the overall mean survival for GBM patients was 55.6 weeks in patients with optimized catheter placement. A number of patients survived beyond 2 years. These trials demonstrated that most effective drug delivery was achieved into the parenchyma surrounding gross-total tumor resection cavities rather than by delivery into tumors in situ. They also showed that the chances of successful delivery without reflux or leakage was enhanced if the catheter tip was at least 2 cm deep from the last traverses pial surface and at least 5 mm from the nearest nontraversed pial or ependymal surface [13].
The results of early clinical trials led to a randomized Phase III efficacy trial (Phase III Randomized Evaluation of CED of IL13-PE38QQR Compared to Gliadel Wafer with Survival End Point in GBM Patients at First Recurrence [PRECISE]) conducted worldwide in more than 52 clinical centers powered to show superiority in comparison with active control using FDA-approved Gliadel wafers [28]. Patients in the experimental arm of the trial received CB (huIL13-PE38QQR) [68], while the control arm consisted of the standard care, that is, the delivery of carmustine (bis-chloroethyl-nitrosourea [BCNU]) in Gliadel wafers. The median survival of the 184 patients in the CB arm was 36.4 weeks compared with 35.3 weeks for the 92 patients in the control arm (p = 0.476). While this result did not meet expectations of superiority of the CB, the results were much more encouraging once the dataset was restricted to sites having enrolled more than six patients progressing to drug delivery. In this case, the CB arm had an overall survival of 46.8 weeks versus 41.6 in the control arm (p = 0.288) and a hazard ratio of 0.77 (p = 0.163). Most significant was the finding that progression-free survival was 17.7 versus 11.4 weeks in favor of CB (p = 0.008). The trial implies that a uniform method must be applied in different centers to ensure exact and reproducible drug delivery. Future trials will probably benefit from determination of targeted factors, level of expression for enrollment and data analysis. The next generations of rationally designed cytotoxins that bind specifically to IL-13Rα2, but not the IL-13 physiological receptor, are available for subsequent trials [61,74].
Locoregional chemotherapy with CED
The application of CED in the treatment of brain tumors found its way in using standard chemotherapeutics, such as taxol [75]. Such studies have demonstrated that small molecule delivery, which cannot penetrate BBB, is feasible and that the antitumor responses could be seen with the procedure [75]. Interestingly, the anti-tumor responses were vigorous, but associated with neurological complications, either as a result of the treatment or drug-induced. [18]. This observation gave impetus to the development of nanochemotherapy where the drug is delivered in a more biologically accessible and tolerable manner [45,47]. This still preliminary experience with CED chemotherapy of brain tumors is encouraging, and delivering potent chemotherapeutics that do not cross the BBB should be applicable on a larger scale in the future.
Future strategies for using CED in brain tumor treatment
For almost 15 years CED has been exploited for locoregional interstitial delivery of drugs to the CNS. It met with success in many instances, but challenges in achieving consistently accurate delivery to the desired volume remain to be overcome. Several factors have been shown to be detrimental to the efficient delivery of drugs, especially to malignant brain neoplasms. The most significant lesson learned thus far is that the placement of a tube-like catheter containing one port and originally produced for a release of soluble compounds into a space with no counter pressure to the flow of an infusate cannot efficiently perform all the functions expected to be offered through the CED. Other major issues, which are probably related to the type of catheters used in CED thus far, are leakage of the infusate into the ventricles or sulci and/or reflux along the catheter tract. The potential for these events to result in failure of delivery of the drug to the target in a substantial fraction of cases makes initial verification of the infusate path through intra-CED imaging obligatory. Unfortunately, there is perhaps small economic incentive to develop improved catheters for CED into malignant CNS tumors. However, it is the opinion of the authors that optimized CED will be needed even if systemically delivered drugs show substantially improved efficacy in the future. At some stage of brain cancer clinical history, tumors resistant to these drugs will probably develop and locoregional treatment, such as with cytotoxins, will become the best or only option. Notwithstanding this, the best clinical results obtained so far in a randomized efficacy trial in patients with recurrent GBM were with an anti-GBM recombinant cytotoxin administered by CED [73].
Expert commentary
As for the near future, we propose the following developmental steps for making CED an effective way of delivering antineoplastic drugs, and recombinant cytotoxins in particular, to brain tumors. Patients’ tumors should be prescreened for the target the drug is engineered against, unless the infusate is a combination of drugs that makes all patients likely responders [76]. Moreover, there is a need for more optimal catheters and more precise stereotactic placement directly into the tumor parenchyma and into the surrounding tumor-infiltrated brain. There are likely to be roles for multiport/multicannula or hollow-fiber catheters, which would provide more uniform distribution to larger effective volumes and antireflux protection at the same time. Balloon-tipped catheters await more experimental verification when tumors are resected. Perhaps most importantly, imaging must accompany CED in order to prove an effective distribution of drugs within tumors and tumor-infiltrated brain in each individual patient. The use of computerized algorithms may be helpful for catheter placement, but cannot and will not predict leakage and a subsequent futility of CED in every case. More attention should be paid to the development of pharmacologic formulations of drugs and carriers in preclinical models. Repetition with more than one cycle of CED is also suggested as it is difficult to expect optimal therapeutic results from a single application. These aggressive strategies may initially limit the number of centers capable of performing optimized CED; however, the benefit to patients is likely to be sufficiently high to justify this limitation.
Five-year view
It is not unreasonable to expect that more optimal catheters for CED will be identified among those currently being tested or that are newly engineered. This will have a great impact on the confidence with which the investigators/physicians will be using CED for the treatment of GBM. Another major development will be related to the imaging of both distribution and residence of the infusates in GBM patients. Hence, the drugs will not be delivered blindly, but actually this will be monitored with a possibility to correct whenever needed. Computer software will be perfected and aid in optimizing the distribution of the infusates. More drugs in more optimal pharmacological formulations will also be available for CED. It is also expected that longer CED deliveries, lasting up to 2 weeks, will be implemented and the cycles of treatment will be repeated at least once. All these factors should have a decisive impact on the efficacy of various non-BBB penetrating potent anti-GBM drugs.
Key issues.
Most anti-glioblastoma multiforme (GBM) drugs effective in vitro do not cross the BBB.
Convection-enhanced delivery (CED) enables administration of anti-GBM drugs directly to the tumor and thus circumvents the problem with BBB penetration.
Drugs of various natures can be delivered locoregionally using CED, including chemotherapeutics and recombinant proteins.
Catheters with one opening at their tips have primarily been used with CED to the CNS. This type of catheter poses multiple problems with homogenous and reproducible distribution of the drugs and is prone to reflux of the infusates.
The science on CED to CNS tumors is still in its infancy.
New or modified catheters suitable for CED have been tested, offering improvement in drugs’ distribution.
Imaging of the infusates becomes of intense interest, since monitoring of CED is believed to be of crucial importance in determining a drug’s efficacy.
The physico–chemical properties of the infusates have been found to affect the distribution of CED-administered drugs.
Large animal models have proven to be a valuable tool in improving CED.
A large number of patients with GBM responding to drugs given by CED warrants further systematic development of this locoregional method of drug administration.
Acknowledgments
Waldemar Debinski is a consulting scientific advisor and a shareholder in Targepeutics Inc. and NIH grant RO1 CA 74145 to Waldemar Debinski supported this work.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Waldemar Debinski, Director of Brain Tumor Center of Excellence, Comprehensive Cancer Center of Wake Forest University, Departments of Neurosurgery, Radiation Oncology and Cancer Biology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA Tel.: +1 336 716 9712 Fax: +1 336 713 7639 debinski@wfubmc.edu.
Stephen B Tatter, Liang Yee and Dixie Soo Professor in Neurosurgery, Co-director, Gamma Knife Radiosurgery Center Attending Neurosurgeon, Wake Forest University School of Medicine, Medical Center Boulevard Winston-Salem, NC 27157, USA Tel.: +1 336 716 4047 Fax: +1 336 716 3065 statter@wfubmc.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Pardridge WM. Blood–brain barrier delivery. Drug Discov. Today. 2007;12(1–2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Debinski W. Local treatment of brain tumors with targeted chimera cytotoxic proteins. Cancer Invest. 2002;20(5–6):801–809. doi: 10.1081/cnv-120003545. [DOI] [PubMed] [Google Scholar]
- 3.Groothuis DR. The blood–brain and blood–tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol. 2000;2(1):45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelbaum MA. Convection enhanced delivery for treating brain tumors and selected neurological disorders: symposium review. J. Neurooncol. 2007;83(1):97–109. doi: 10.1007/s11060-006-9308-9. [DOI] [PubMed] [Google Scholar]
- 5.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl Acad. Sci. USA. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. •• This work marked the beginning of studies on the use of convection-enhanced delivery (CED) for the treatment of CNS disorders.
- 6.Song DK, Lonser RR. Convection-enhanced delivery for the treatment of pediatric neurologic disorders. J. Child Neurol. 2008;23(10):1231–1237. doi: 10.1177/0883073808321064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogelbaum MA, Sampson JH, Kunwar S, et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: Phase 1 study of final safety results. Neurosurgery. 2007;61(5):1031–1037. doi: 10.1227/01.neu.0000303199.77370.9e. [DOI] [PubMed] [Google Scholar]
- 8.Mut M, Sherman JH, Shaffrey ME, Schiff D. Cintredekin besudotox in treatment of malignant glioma. Expert Opin. Biol. Ther. 2008;8(6):805–812. doi: 10.1517/14712598.8.6.805. [DOI] [PubMed] [Google Scholar]
- 9.Sampson JH, Akabani G, Archer GE, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neurooncol. 2008;10(3):320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson S, Lesniak MS. Convection enhanced drug delivery of novel therapeutic agents to malignant brain tumors. Curr. Drug Deliv. 2007;4(2):169–180. doi: 10.2174/156720107780362302. [DOI] [PubMed] [Google Scholar]
- 11.Sampson JH, Raghavan R, Brady ML, et al. Clinical utility of a patient-specific algorithm for simulating intracerebral drug infusions. Neurooncol. 2007;9(3):343–353. doi: 10.1215/15228517-2007-007. • Clinical verification of software individualizing CED clinical performance.
- 12.Slevin JT, Gash DM, Smith CD, et al. Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J. Neurosurg. 2007;106(4):614–620. doi: 10.3171/jns.2007.106.4.614. [DOI] [PubMed] [Google Scholar]
- 13.Kunwar S, Prados MD, Chang SM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J. Clin. Oncol. 2007;25(7):837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 14.Murad GJ, Walbridge S, Morrison PF, et al. Real-time, image-guided, convection-enhanced delivery of interleukin-13 bound to Pseudomonas exotoxin. Clin. Cancer Res. 2006;12(10):3145–3151. doi: 10.1158/1078-0432.CCR-05-2583. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro WR, Carpenter SP, Roberts K, Shan JS. (131)I-chTNT-1/B mAb: tumour necrosis therapy for malignant astrocytic glioma. Expert Opin. Biol. Ther. 2006;6(5):539–545. doi: 10.1517/14712598.6.5.539. [DOI] [PubMed] [Google Scholar]
- 16.Carpentier A, Laigle-Donadey F, Zohar S, et al. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro Oncol. 2006;8(1):60–66. doi: 10.1215/S1522851705000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonser RR, Walbridge S, Murray GJ, et al. Convection perfusion of glucocerebrosidase for neuronopathic Gaucher’s disease. Ann. Neurol. 2005;57(4):542–548. doi: 10.1002/ana.20444. [DOI] [PubMed] [Google Scholar]
- 18.Lidar Z, Mardor Y, Jonas T, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a Phase I/II clinical study. J. Neurosurg. 2004;100(3):472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 19.Weber F, Asher A, Bucholz R, et al. Safety, tolerability, and tumor response of IL-4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J. Neurooncol. 2003;64(1–2):125–137. doi: 10.1007/BF02700027. [DOI] [PubMed] [Google Scholar]
- 20.Broaddus WC, Gillies GT, Kucharczyk J. Minimally invasive procedures. Advances in image-guided delivery of drug and cell therapies into the central nervous system. Neuroimaging Clin. N. Am. 2001;11(4):727–735. [PubMed] [Google Scholar]
- 21.Wersall P, Ohlsson I, Biberfeld P, et al. Intratumoral infusion of the monoclonal antibody, mAb 425, against the epidermal-growth-factor receptor in patients with advanced malignant glioma. Cancer Immunol. Immunother. 1997;44:157–164. doi: 10.1007/s002620050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack WJ, Huang J, Winfree C, et al. Ultrarapid, convection-enhanced intravascular hypothermia: a feasibility study in nonhuman primate stroke. Stroke. 2003;34(8):1994–1999. doi: 10.1161/01.STR.0000079813.31539.6D. [DOI] [PubMed] [Google Scholar]
- 23.Rousseau J, Boudou C, Estève F, Elleaume H. Convection-enhanced delivery of an iodine tracer into rat brain for synchrotron stereotactic radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007;68(3):943–951. doi: 10.1016/j.ijrobp.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 24.Gasior M, White NA, Rogawski MA. Prolonged attenuation of amygdala-kindled seizure measures in rats by convection-enhanced delivery of the N-type calcium channel antagonists omega-conotoxin GVIA and omega-conotoxin MVIIA. J. Pharmacol. Exp. Ther. 2007;323(2):458–468. doi: 10.1124/jpet.107.125047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacKay JA, Li W, Huang Z, et al. HIV TAT peptide modifies the distribution of DNA nanolipoparticles following convection-enhanced delivery. Mol. Ther. 2008;16(5):893–900. doi: 10.1038/mt.2008.36. [DOI] [PubMed] [Google Scholar]
- 26.CBTRUS . Statistical report: primary brain tumors in the United States, 1998–2002. Central Brain Tumor Registry of the United States; IL, USA: 2005. 2005. [Google Scholar]
- 27.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 28.Gallia GL, Brem S, Brem H. Local treatment of malignant brain tumors using implantable chemotherapeutic polymers. J. Natl Compr. Cancer Netw. 2005;3:721–728. doi: 10.6004/jnccn.2005.0042. • Review of a clinically successful approach of local delivery of an antineoplastic drug to the CNS.
- 29.Fiandaca MS, Forsayeth JR, Dickinson PJ, Bankiewicz KS. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics. 2008;5(1):123–127. doi: 10.1016/j.nurt.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krauze MT, Saito R, Noble C, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J. Neurosurg. 2005;103(5):923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J. Neurosurg. 1999;90(2):315–320. doi: 10.3171/jns.1999.90.2.0315. [DOI] [PubMed] [Google Scholar]
- 32.Tanner PG, Holtmannspotter M, Ton JC, Goldbrunner R. Effects of drug efflux on convection-enhanced paclitaxel delivery to malignant gliomas: technical note. Neurosurgery. 2007;61(4):E880–E882. doi: 10.1227/01.NEU.0000298922.77921.F2. [DOI] [PubMed] [Google Scholar]
- 33.Raghavan R, Brady ML, Rodríguez-Ponce MI, Hartlep A, Pedain C, Sampson JH. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg. Focus. 2006;20(4):E12. doi: 10.3171/foc.2006.20.4.7. [DOI] [PubMed] [Google Scholar]
- 34.Oh S, Odland R, Wilson SR, et al. Improved distribution of small molecules and viral vectors in the murine brain using a hollow fiber catheter. J. Neurosurg. 2007;107(3):568–577. doi: 10.3171/JNS-07/09/0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson JJ, Zhang Z, Dillehay D, Stubbs J. Assessment of a balloon-tipped catheter modified for intracerebral convection-enhanced delivery. J. Neurooncol. 2008;89(2):159–168. doi: 10.1007/s11060-008-9612-7. • The first attempt at using a balloon-tipped catheter for CED.
- 36.Tatter SB, Shaw EG, Rosenblum ML, et al. New approaches to brain tumor therapy central nervous system Consortium. An inflatable balloon catheter and liquid 125I radiation source (GliaSite radiation therapy system) for treatment of recurrent malignant glioma: multicenter safety and feasibility trial. J. Neurosurg. 2003;99(2):297–303. doi: 10.3171/jns.2003.99.2.0297. [DOI] [PubMed] [Google Scholar]
- 37.Varenika V, Dickinson P, Bringas J, et al. Detection of infusate leakage in the brain using real-time imaging of convection-enhanced delivery. J. Neurosurg. 2008;109(5):874–880. doi: 10.3171/JNS/2008/109/11/0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voges J, Reszka R, Gossmann A, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann. Neurol. 2003;54(4):479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 39.Pöpperl G, Goldbrunner R, Gildehaus FJ, et al. O-(2-[18F]fluoroethyl)-l-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging. 2005;32(9):1018–1025. doi: 10.1007/s00259-005-1819-7. [DOI] [PubMed] [Google Scholar]
- 40.Lonser RR, Schiffman R, Robison RA, et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology. 2007;68(4):254–261. doi: 10.1212/01.wnl.0000247744.10990.e6. [DOI] [PubMed] [Google Scholar]
- 41.Rueger MA, Winkeler A, Thomas AV, Kracht LW, Jacobs AH. Molecular imaging-guided gene therapy of gliomas. Handb. Exp. Pharmacol. 2008;185(Pt 2):341–345. doi: 10.1007/978-3-540-77496-9_15. [DOI] [PubMed] [Google Scholar]
- 42.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67(6):2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mardor Y, Rahav O, Zauberman Y, et al. Convection-enhanced drug delivery: increased efficacy and magnetic resonance image monitoring. Cancer Res. 2005;65(15):6858–6863. doi: 10.1158/0008-5472.CAN-05-0161. [DOI] [PubMed] [Google Scholar]
- 44.Perlstein B, Ram Z, Daniels D, et al. Convection-enhanced delivery of maghemite nanoparticles: increased efficacy and MRI monitoring. Neuro Oncol. 2008;10(2):153–161. doi: 10.1215/15228517-2008-002. • A study supporting the notion of better drug distribution with an increased viscosity of infusate.
- 45.Krauze MT, Noble CO, Kawaguchi T, et al. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neuro Oncol. 2007;9(4):393–403. doi: 10.1215/15228517-2007-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito R, Krauze MT, Noble CO, et al. Tissue affinity of the infusate affects the distribution volume during convection-enhanced delivery into rodent brains: implications for local drug delivery. J. Neurosci. Methods. 2006;154(1–2):225–232. doi: 10.1016/j.jneumeth.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 47.Chen MY, Hoffer A, Morrison PF, et al. Surface properties, more than size, limiting convective distribution of virus-sized particles and viruses in the central nervous system. J. Neurosurg. 2005;103(2):311–319. doi: 10.3171/jns.2005.103.2.0311. [DOI] [PubMed] [Google Scholar]
- 48.MacKay JA, Deen DF, Szoka FC., Jr. Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res. 2005;1035(2):139–153. doi: 10.1016/j.brainres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg L, Ocherashvilli A, Daniels D, et al. Salirasib (farnesyl thiosalicylic acid) for brain tumor treatment: a convection-enhanced drug delivery study in rats. Mol. Cancer Ther. 2008;7(11):3609–3616. doi: 10.1158/1535-7163.MCT-08-0488. [DOI] [PubMed] [Google Scholar]
- 50.Kikuchi T, Saito R, Sugiyama S, et al. Convection-enhanced delivery of polyethylene glycol-coated liposomal doxorubicin: characterization and efficacy in rat intracranial glioma models. J. Neurosurg. 2008;109(5):867–873. doi: 10.3171/JNS/2008/109/11/0867. [DOI] [PubMed] [Google Scholar]
- 51.Jagannathan J, Walbridge S, Butman JA, Oldfield EH, Lonser RR. Effect of ependymal and pial surfaces on convection-enhanced delivery. J. Neurosurg. 2008;109(3):547–552. doi: 10.3171/JNS/2008/109/9/0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hadjipanayis CG, Fellows-Mayle W, Deluca NA. Therapeutic efficacy of a herpes simplex virus with radiation or temozolomide for intracranial glioblastoma after convection-enhanced delivery. Mol. Ther. 2008;16(11):1783–1788. doi: 10.1038/mt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cunningham J, Pivirotto P, Bringas J, et al. Biodistribution of adeno-associated virus type-2 in nonhuman primates after convection-enhanced delivery to brain. Mol. Ther. 2008;16(7):1267–1275. doi: 10.1038/mt.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grondin R, Zhang Z, Ai Y, et al. Intraputamenal infusion of exogenous neurturin protein restores motor and dopaminergic function in the globus pallidus of MPTP-lesioned rhesus monkeys. Cell Transplant. 2008;17(4):373–381. [PMC free article] [PubMed] [Google Scholar]
- 55.Rousseau J, Boudou C, Barth RF, Balosso J, Estève F, Elleaume H. Enhanced survival and cure of F98 glioma-bearing rats following intracerebral delivery of carboplatin in combination with photon irradiation. Clin. Cancer Res. 2007;13(17):5195–5201. doi: 10.1158/1078-0432.CCR-07-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saito R, Krauze MT, Noble CO, et al. Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neuro Oncol. 2006;8(3):205–214. doi: 10.1215/15228517-2006-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saito R, Krauze MT, Noble CO, et al. Tissue affinity of the infusate affects the distribution volume during convection-enhanced delivery into rodent brains: implications for local drug delivery. J. Neurosci. Methods. 2006;154(1–2):225–232. doi: 10.1016/j.jneumeth.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen TT, Pannu YS, Sung C, et al. Convective distribution of macromolecules in the primate brain demonstrated using computerized tomography and magnetic resonance imaging. J. Neurosurg. 2003;98(3):584–590. doi: 10.3171/jns.2003.98.3.0584. [DOI] [PubMed] [Google Scholar]
- 59.Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: laboratory investigation. J. Neurosurg. 2008;108(5):989–998. doi: 10.3171/JNS/2008/108/5/0989. •• One of the first examples of utilizing canine model for CED plus confirmation of the necessity of drug distribution imaging and the benefit of using modified catheters.
- 60.Debinski W, Gibo DM, Wykosky J, Stanton C, Rosmeissl J, Robertson J. Canine brain tumors over-express common molecular denominators of human high-grade astrocytomas. Neurooncol. 2007;9(4):535–536. [Google Scholar]
- 61.Debinski W, Gibo DM, Kealiher A, et al. Novel anti-brain tumor cytotoxins specific for cancer cells. Nature Biotech. 1998;16:449–453. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- 62.Debinski W. Molecular targeting of brain tumors with cytotoxins. In: Lorberboum-Galski H, Lazarovici P, editors. Chimeric Toxins. Harwood Academic Publishers; NJ, USA: 2002. pp. 222–246. [Google Scholar]
- 63.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nature Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. •• First ever clinical trial performed with brain tumor-targeted cytotoxin using CED.
- 64.Iglewski BH, Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc. Natl Acad. Sci. USA. 1975;72:2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pastan I, Chaudhary VK, Fitzgerald D. Recombinant toxins as novel therapeutic agents. Ann. Rev. Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- 66.Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf–CRM107) for therapy of malignant gliomas. J. Neurooncol. 2003;65:3–13. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- 67.Celtic Pharma press release . Celtic Pharma terminates Transmid™ trial KSB311R/CIII/001. Bermuda; London, NY: Feb 7, 2007. [Google Scholar]
- 68.Debinski W, Puri RK, Kreitman RJ, et al. A wide range of human cancers express interleukin-4 receptors that can be targeted with a chimeric toxin containing Pseudomonas exotoxin A. J. Biol. Chem. 1993;268:14065–14070. [PubMed] [Google Scholar]
- 69.Nutt CL, Mani DR, Betensky RA, et al. Gene expression-based classification of malignant gliomas correlated better with survival than histological classification. Cancer Res. 2003;63:1602–1607. [PubMed] [Google Scholar]
- 70.Sampson JH, Akabani G, Archer GE, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant protein composed of transforming growth factor TGF-α and a mutated form of Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J. Neurooncol. 2003;65:27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 71.Debinski W, Obiri NI, Pastan I, et al. A novel chimeric protein composed of interleukin-13 (IL-13) and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for IL-13 and IL-4. J. Biol. Chem. 1995;270:16775–16780. doi: 10.1074/jbc.270.28.16775. [DOI] [PubMed] [Google Scholar]
- 72.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells over-express receptor for IL-13 and are extremely sensitive to a novel chimeric protein composed of IL-13 and Pseudomonas exotoxin. Clin. Cancer Res. 1995;1:1253–1258. [PubMed] [Google Scholar]
- 73.Kunwar S, Westphal M, Medhorn M, et al. Results from PRECISE: a randomized Phase 3 study in patients with first recurrent GBM comparing cintredekin besudotox administered via convection-enhanced delivery with Gliadel wafers. Neurooncol. 2007;9:531. •• Study demonstrating the best efficacy seen for any drug used in patients with recurrent glioblastoma multiforme.
- 74.Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. IL-13Rα2 is a glioma-restricted receptor for Interleukin-13. Neoplasia. 2002;4:388–399. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mardor Y, Roth Y, Lidar Z, et al. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res. 2001;61(13):4971–4973. [PubMed] [Google Scholar]
- 76.Wykosky J, Gibo DM, Stanton C, Debinski W. IL-13Rα-2, EphA2, and Fra-1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin. Cancer Res. 2008;14:199–208. doi: 10.1158/1078-0432.CCR-07-1990. [DOI] [PubMed] [Google Scholar]