Abstract
Objective
Acute HIV infection often causes influenza-like illness (ILI) and is associated with high infectivity. We estimated the effectiveness and cost-effectiveness of strategies to identify and treat acute HIV infection in men who have sex with men (MSM) in the US.
Design
Dynamic model of HIV transmission and progression.
Interventions
We evaluated three testing approaches: viral load (VL) testing for individuals with ILI, expanded screening with antibody testing, and expanded screening with antibody and VL testing. We included treatment with antiretroviral therapy for individuals identified as acutely infected.
Main Outcome Measures
New HIV infections, discounted QALYs and costs, and incremental cost-effectiveness ratios.
Results
At the present rate of HIV-antibody testing, we estimated that 538,000 new infections will occur among MSM over the next 20 years. Expanding antibody screening coverage to 90% of MSM annually reduces new infections by 2.8% and costs $12,582 per QALY gained. Symptom-based VL testing with ILI is more expensive than expanded antibody screening, but is more effective and costs $22,786 per QALY gained. Combining expanded antibody screening with symptom-based VL testing prevents twice as many infections compared to expanded antibody screening alone, and costs $29,923 per QALY gained. Adding VL testing to all annual HIV tests costs more than $100,000 per QALY gained.
Conclusions
Use of HIV VL testing in MSM with ILI prevents more infections than does expanded annual antibody screening alone and is inexpensive relative to other screening interventions. Clinicians should consider symptom-based VL testing in MSM, in addition to encouraging annual antibody screening.
Keywords: acute infection, antiretroviral therapy, cost effectiveness studies, HIV diagnostic tests, HIV prevention, men who have sex with men
INTRODUCTION
In the United States, more than 1.1 million people are living with the human immunodeficiency virus (HIV), and an estimated 56,000 people are infected with HIV annually [1, 2]. Men who have sex with men (MSM) account for 53% of new HIV infections in the US and are an important target group for treatment and prevention programs [1].
Currently, approximately two-thirds of MSM receive HIV testing at least annually, as recommended by the US Centers for Disease Control and Prevention (CDC) [3-5]. Most screening programs use antibody tests to detect HIV infection. However, current antibody tests fail to detect HIV infection in the first few weeks after infection [6, 7]. During the acute infection phase, viral load (VL) is very high, and infectivity is much greater than during chronic infection [6, 8]. Infection can be detected during this phase with VL tests. Early identification could reduce disease transmission through interventions to limit risky sexual behavior and early initiation of antiretroviral therapy (ART).
Approximately 70% of people with acute HIV infection develop symptoms of influenza-like illness (ILI), which can sometimes facilitate the early identification of new infections [9-11]. The CDC currently recommends an HIV VL test in addition to an antibody test for patients with an ILI and recent high-risk behavior [5]. However, decisions about VL testing are complicated by the lack of sensitivity and specificity of ILI symptoms for acute HIV.
Initiating ART during the acute phase may offer substantial benefits. ART effectively suppresses viral replication during acute infection, suggesting that treatment could be an effective method of reducing transmission [12, 13].
Prior studies have assessed the diagnostic yield, costs, and cost-effectiveness of screening for acute infection [7, 14-18]. However, no studies included treatment with ART and the associated benefits from reduced transmission. We examined the effectiveness and cost-effectiveness of strategies for expanded testing of MSM, with an emphasis on identifying acutely infected individuals and providing them ART.
METHODS
Overview and model structure
We developed a dynamic compartmental model of HIV transmission and progression to compare the effectiveness and cost-effectiveness of alternative testing strategies (additional model details in the Appendix). We instantiated the model for MSM aged 13-64 in the US, consistent with CDC recommendations of routine HIV screening [5]. We implemented the model using weekly time steps and calibrated to estimates of HIV incidence among MSM [1]. We estimated HIV prevalence, incidence, quality-adjusted life years (QALYs), and healthcare costs over a 20-year time horizon. All costs (in 2009 US dollars) were assessed from a societal perspective, and costs and QALYs were discounted at 3% annually [19]. Table 1 summarizes key model parameters.
Table 1.
Summary of Key Model Parameters
| Parameter* | Value | Range |
|---|---|---|
| Demographic Parameters | ||
| Total MSM population age 13-64 | 6,435,210 | 5.5-7.5 million |
| HIV prevalence in MSM | 8.5% | 1-17% |
| Male mortality rate | 0.0043 | 0.003-0.005 |
| Male maturation rate | 0.0106 | 0.005-0.02 |
| Male entry rate | 0.022 | 0.01-0.04 |
| Disease Parameters | ||
| Average disease duration (years) | ||
| Acute HIV | 0.25 | 0.08-0.40 |
| Asymptomatic HIV | 7 | 6-10 |
| Symptomatic HIV | 3 | 1-4 |
| Symptomatic HIV – Treated with ART | 18 | 12-30 |
| AIDS | 2 | 1-3 |
| AIDS – Treated with ART | 5 | 2-15 |
| Sexual Behavior Parameters | ||
| Annual transmission probability per MSM partnership (MHIV+→MHIV−) |
||
| Acute HIV | 0.210 | 0.10-0.40 |
| Asymptomatic HIV | 0.039 | 0.02-0.08 |
| Symptomatic HIV | 0.039 | 0.02-0.08 |
| AIDS | 0.160 | 0.08-0.30 |
| Annual number of male partners | 3.0 | 2.0-5.0 |
| Condom usage with male partners | 40% | 30-60% |
| Treatment Parameters | ||
| Fraction of acutely infected starting ART after diagnosis | 50% | 0-100% |
| Fraction starting ART at CD4=350 cells/mm3 | 50% | 25-75% |
| Rate of initiating ART at CD4<350 cells/mm3 | 0.05 | 0-0.10 |
| Reduction in sexual infectivity due to ART | 90% | 50-99% |
| Screening Parameters | ||
| Fraction of population tested annually | 67% | 30-90% |
| Fraction of acutely infected who develop symptoms | 70% | 40-90% |
| Fraction of patients with influenza-like symptoms who seek medical attention |
35% | 10-100% |
| Identification duration if uninfected (years) | 1 | 0.5-3 |
| Reduction in sexual behavior due to testing and counseling | 20% | 0-50% |
| Cost Parameters (2009 US $) | ||
| Annual HIV-related healthcare costs | ||
| Acute HIV | 30 | 10-500 |
| Asymptomatic HIV – Untreated | 4,100 | 3,000-6,000 |
| Symptomatic HIV – Untreated | 6,883 | 5,000-9,000 |
| Symptomatic HIV – Treated with ART (excludes ART costs) | 6,136 | 5,000-7,000 |
| AIDS – Untreated | 21,700 | 15,000-25,000 |
| AIDS – Treated with ART (excludes ART costs) | 9,877 | 6,000-17,000 |
| Annual non-HIV-related healthcare costs | 4,028 | 3,000-6,000 |
| Annual cost of ART | 15,475 | 12,500-19,000 |
| Cost of HIV testing – VL test | ||
| Uninfected | 124 | 51-248 |
| HIV-Infected | 277 | 102-344 |
| Cost of HIV testing – antibody test | ||
| Uninfected | 13 | 5-25 |
| HIV-Infected | 67 | 50-100 |
| Cost of counseling | ||
| Pre-test counseling | 13 | 0-100 |
| Post-test counseling for HIV-negative persons | 7 | 0-50 |
| Post-test linkage/counseling for HIV-positive persons | 14 | 0-100 |
| Cost of HIV diagnosis | 500 | 125-1,200 |
| Discount Rate | 3% | 0-5% |
All rates are annual. See Appendix for sources. ART = antiretroviral treatment, MSM = men who have sex with men, VL = viral load
The population was segmented by HIV infection status, screening status, HIV disease stage, and treatment status if infected. Initial HIV prevalence in the MSM population was 8.5%, with undetected prevalence of 3.2%, representing an average across the US [1, 2, 20, 21]. Mortality was decomposed into HIV-related and non-HIV-related death rates.
Testing strategies
We estimated that 67% of MSM were screened annually using antibody tests [3, 4]. Pre- and post-test counseling resulted in a 20% reduction in risky behavior for both infected and uninfected individuals [22-24]. Uninfected tested individuals were eligible for retesting after one year. Our antibody screening test had characteristics similar to a rapid-test protocol in which positive results are confirmed by a Western blot test, and newly identified individuals received results and counseling at the point of care.
We evaluated testing for acute infection with an individual VL test following a negative antibody test [7, 14]. Costs of testing also included a confirmatory VL test and Western blot at a follow-up visit.
We considered two approaches for acute infection testing: (1) symptom-based VL testing and (2) adding VL testing to the annual screening protocol. We also considered expanding screening coverage, alone and in combination with symptom-based testing. We compared alternative strategies to the status quo of 67% of MSM screened annually with antibody tests.
We first evaluated expanding annual antibody screening coverage to 90%, without VL testing, and then we considered symptom-based testing for acute infection with 67% and 90% screening coverage. Approximately 70% of people with acute infection are expected to develop ILI [9-11], and we estimated that 35% of those seek care, based on the percentage of people in the US who seek healthcare for ILI [10, 25]. Our symptom-based strategies assumed that every MSM presenting with febrile ILI received an antibody test followed by a VL test if the antibody test was negative. We also examined strategies with routine VL testing for screened individuals whose antibody test was negative, in combination with symptom-based testing.
Antiretroviral therapy
Individuals identified as chronically HIV-infected with a CD4 cell count of 350 cells/mm3 or lower were offered ART [26]. We assumed a 90% reduction in sexual infectivity due to ART in our base case and varied this in sensitivity analysis [8, 22, 27]. We incorporated the benefits of ART during chronic infection, including improved quality of life and reduced disease progression and mortality [22, 23]. In sensitivity analysis, we examined the effects of individuals initiating ART at a CD4 cell count of 500 cells/mm3 or higher, given recent guidelines recommending earlier ART initiation [28].
We assumed ART reduces infectivity of acutely infected individuals by 90% as well [12, 13]. We assumed ART was initiated immediately following diagnosis and continued for 3 months. We estimated that half of those identified as acutely infected would accept ART using data on willingness of chronically infected patients to start ART [22, 29]. Thus, in our base case symptom-based strategy, we estimated that 70% of acutely infected individuals develop symptoms, 35% of those seek care and receive VL testing, and 50% of those identified receive ART, for a total of 12% treated during acute infection.
HIV transmission and progression
We modeled HIV transmission via homosexual contact. The probability of HIV transmission between sero-discordant homosexual partners depended on the number of sexual partners [3, 30], average condom use [31], condom effectiveness [32], and the transmission probability per partnership [8, 33]. The transmission probability per partnership depended on the HIV disease stage and ART status of the infected individual.
HIV-infected individuals progressed through the four modeled disease stages: acute infection, asymptomatic HIV, symptomatic HIV, and AIDS. Progression rates were based on models of HIV natural history [22, 23].
Health outcomes and costs
We calculated discounted costs and QALYs for each strategy. We estimated quality of life for each health state from published literature and adjusted the utilities based on the average age of the modeled population [22, 23, 34].
Total health-related costs for individuals during the 20-year time frame were calculated from the costs associated with each health state and the costs of HIV testing, counseling, and diagnosis. Baseline medical costs, HIV-related healthcare costs, cost of ART, and costs of counseling were estimated from the published literature [22, 35]. Costs of HIV testing protocols were obtained from the Centers for Medicare and Medicaid Services 2009 fee schedules [36]. Discounted lifetime health-related costs and QALYs were also incorporated for the population remaining at the end of the time horizon and for individuals who matured out of the model.
RESULTS
Health outcomes
At current testing and treatment levels, we estimate that 538,371 new HIV infections will occur among MSM in the US in the next 20 years (Table 2). This incidence can be reduced with the testing and treatment strategies we evaluated. Expanding antibody screening coverage to 90% annually will reduce the number of infections over 20 years by 14,923 (2.8% of the projected total) and yield 183,535 incremental QALYs (Table 2). Symptom-based VL testing yields greater health benefits. Over 20 years, adding symptom-based testing to current annual screening rates leads to 22,446 fewer new infections and 218,085 more QALYs compared to the status quo. Combining symptom-based VL testing with expanded antibody screening of MSM averts 30,780 new infections (5.7%) and adds 321,164 QALYs compared to the status quo. Finally, expanded screening with both antibody and VL tests, in combination with symptom-based VL testing, provides the most health benefits, with 38,995 (7.2%) infections averted.
Table 2.
Benefits and Costs of Acute HIV Testing and Treatment Strategies Over 20 Years – Base Case
| HIV Ab | HIV VL | ICER relative to§ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strategy* | New HIV Infections ¶ |
HIV Infections Prevented † |
Tests Admini- stered (millions) |
Tests Admin- istered (millions) |
Total Costs (billions) ¥ |
Total QALYs (millions) ¥ |
Incremental Costs‡ (billions) |
Incremental QALYs‡ |
Status Quo |
Next Best Strategy |
| 90% Annually, Ab+VL + Symptom-based |
499,376 | 38,995 (7.2%) |
118.28 | 120.19 | $1,283 | 176.39 | $13.65 | 389,711 | $35,032 | $105,398 |
| 90% Annually, Ab + Symptom-based |
507,591 | 30,780 (5.7%) |
118.31 | 38.88 | $1,276 | 176.32 | $6.43 | 321,164 | $20,013 | $29,923 |
| 67% Annually, Ab+VL + Symptom-based |
510,651 | 27,720 (5.1%) |
95.43 | 96.85 | $1,280 | 176.27 | $10.23 | 263,663 | $38,783 | Dominated |
| 67% Annually, Ab + Symptom-based |
515,925 | 22,446 (4.2%) |
95.44 | 38.85 | $1,275 | 176.22 | $4.97 | 218,085 | $22,786 | Dominated |
| 90% Annually, Ab | 523,448 | 14,923 (2.8%) |
87.68 | - | $1,272 | 176.19 | $2.31 | 183,535 | $12,582 | $12,582 |
| Status Quo (67% Annually, Ab) |
538,371 | 65.46 | - | $1,270 | 176.00 | |||||
Ab = antibody testing, VL = viral load testing.
New HIV infections and HIV infections prevented are undiscounted totals. Discounting infections at 3% annually reduces the number of infections averted for each strategy by approximately 25%.
The values in parentheses are the fraction of total HIV infections prevented.
Costs and quality-adjusted life years (QALYs) are net present values (3% discount rate) over 20 years.
Incremental costs and QALYs are relative to the status quo.
ICER = Incremental cost-effectiveness ratio, relative to the status quo or the next-best strategy. Strategies that are dominated yield fewer QALYs at higher cost than the comparator.
Cost-effectiveness
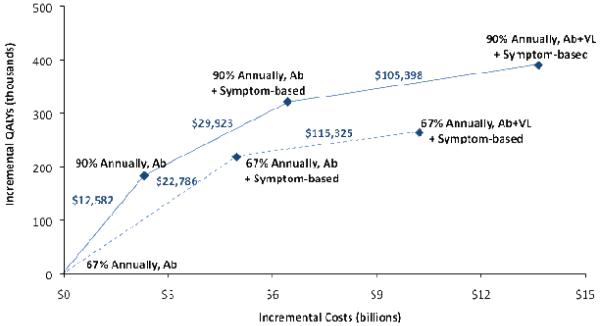
Expanding annual antibody screening coverage to 90% is cost-effective by conventional standards, with an incremental cost-effectiveness ratio (ICER) of $12,582 per QALY gained compared to the status quo (Table 2, Figure 1). This expanded screening generates an incremental $2.31 billion in healthcare-related costs compared to the status quo, or $200,000 per infection averted.
Figure 1. Cost-Effectiveness of Testing for & Treating Acute HIV Infection.
Incremental costs and quality-adjusted life years (QALYs) are plotted for each strategy of testing for HIV infection, with the origin corresponding to the status quo. Under each strategy, 50% of individuals identified as acutely infected receive antiretroviral therapy (ART) for the duration of their acute infection. The solid lines show the incremental cost-effectiveness ratio (ICER) relative to the next-best alternative. The dashed lines show the ICER relative to the next-best alternative if increasing annual screening coverage is infeasible. Although these strategies are dominated by similar strategies with expanded annual screening coverage, they are relevant if increasing screening coverage is infeasible. Incremental costs and QALYs are calculated over a 20-year time horizon and are discounted to the present at 3% annually.
Note: Ab = antibody, VL = viral load, Symptom-based = 35% of symptomatic acutely infected MSM receive Ab & VL testing.
Adding symptom-based testing for acute infection to current antibody screening rates is also cost-effective under our base case assumptions, costing $22,786 per QALY gained relative to the status quo (Table 2, Figure 1). Combining symptom-based testing with expanded antibody screening provides greater health benefits than does either strategy separately and costs $29,923 per QALY gained compared to expanded antibody screening alone. Symptom-based testing with current or expanded levels of screening is associated with higher healthcare-related costs incremental to the status quo than expanded screening alone, with total costs over 20 years of $4.97 billion at current screening rates and $6.43 billion with expanding screening. These costs include all medical costs (HIV- and non-HIV-related) over the lifetime of the cohort; testing costs comprise 76-86% of these totals (Table 3).
Table 3.
Costs of Acute HIV Testing and Treatment Strategies, Incremental to Status Quo – Base Case
| Strategy* | Incremental Non- HIV Healthcare Costs† (millions) |
Incremental HIV Healthcare Costs†¶ (millions) |
Incremental ART Costs† (millions) |
Incremental Screening & Testing Costs† (millions) |
Total Incremental Costs¥ (millions) |
|---|---|---|---|---|---|
| 90% Annually, Ab+VL + Symptom-based |
$302 | $214 | $435 | $12,304 | $13,652 |
| 90% Annually, Ab + Symptom-based |
$272 | $334 | $459 | $4,868 | $6,428 |
| 67% Annually, Ab+VL + Symptom-based |
$179 | $54 | $238 | $9,589 | $10,226 |
| 67% Annually, Ab + Symptom-based |
$156 | $89 | $235 | $4,294 | $4,969 |
| 90% Annually, Ab | $200 | $477 | $459 | $560 | $2,309 |
Ab = antibody testing, VL = viral load testing.
Costs are net present values (3% discount rate) over 20 years.
HIV healthcare costs do not include costs of ART.
Costs are net present values (3% discount rate) over 20 years and include future lifetime costs (those incurred after individuals age out of the model upon turning 65 until death, and those incurred by the population alive in the model at the end of 20-year time horizon until that population dies out). This column does not equal the sum of the other four columns because of the inclusion of future lifetime costs.
In general, we find that symptom-based testing offers substantial gains in health benefits with favorable cost-effectiveness ratios. However, VL screening of all MSM is substantially more expensive. In particular, annual antibody screening with VL testing costs $115,325 per QALY gained at current screening rates, or $105,398 per QALY gained with expanded screening coverage (Figure 1). Routine VL testing costs more than $10 billion over 20 years incremental to the status quo.
The budgetary consequences of the alternative strategies vary by an order of magnitude. This has important implications because short-term programmatic costs, such as testing costs and costs of ART during acute infection, may have different relevance than long-term costs of providing healthcare to the overall MSM population. In our analysis, the largest cost increment comes with adding VL testing, since HIV-negative MSM with ILI are also tested due to the non-specific symptoms (Table 3). Less than 1% of symptom-based VL tests detect acute HIV infection. Expanded annual antibody screening costs approximately $28 million more per year in testing costs than the status quo, or $560 million over 20 years, whereas symptom-based VL testing at current screening rates costs $215 million more per year in testing costs than the status quo, or $4.29 billion over 20 years.
Sensitivity analysis
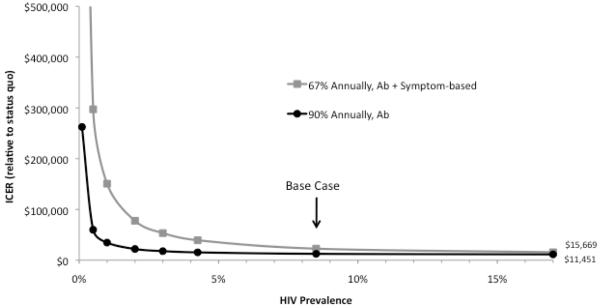
We performed sensitivity analysis on all model parameters (ranges in Table 1) and found that the results were stable to nearly all assumptions. Parameters to which results were sensitive are described below (probabilistic sensitivity analysis is presented in the Appendix). When initial HIV prevalence in the overall MSM population drops below 3% (compared to our base-case estimate of 8.5%), symptom-based testing costs more than $50,000 per QALY gained (Figure 2). If infectivity during acute infection is at least twice as high as in the base case or if MSM have at least 50% more sex partners, symptom-based VL testing becomes more favorable than expanded antibody screening, with an ICER of $10,000 or less compared to the status quo.
Figure 2. ICER of Testing for & Treating Acute HIV Infection by HIV Prevalence.
The horizontal axis displays the initial HIV prevalence in the total modeled population, and the vertical axis shows the incremental cost-effectiveness ratio (ICER) relative to the status quo. Under each strategy, 50% of individuals identified as acutely infected receive antiretroviral therapy (ART) for the duration of their acute infection. Incremental costs and quality-adjusted life years (QALYs) used to calculate the ICER are calculated over a 20-year time horizon and are discounted to the present at 3% annually.
Note: Ab = antibody, VL = viral load, Symptom-based = 35% of symptomatic acutely infected MSM receive Ab & VL testing.
The percentage of symptomatic MSM identified and treated could vary across cities and regions as a result of public health campaigns, and would depend on the proportion visiting a healthcare setting, consenting to or requesting treatment, and lost to follow up. If none of those identified as acutely infected receive ART, the cost-effectiveness of symptom-based VL testing decreases to approximately $60,000 per QALY gained. If, on the other hand, every symptomatic infected individual could be identified and put on ART, so that 70% of acutely infected individuals receive treatment, symptom-based VL testing is more effective than with base case assumptions and similarly cost-effective.
We tested the possibility that promoting symptom-based VL testing would lead to higher rates of uninfected MSM seeking symptom-based testing, as with minor cold symptoms. This would make symptom-based testing less attractive, as the case-finding rate of symptom-based testing would decline. We find that symptom-based testing remains cost-effective at less than $50,000 per QALY gained if up to four times as many uninfected individuals present for VL testing as in the base case. This could also occur, for example, during a heavy influenza season.
There is not yet conclusive data on long-term effects of early treatment [37], so we assumed in our base case that early treatment has no effect on disease progression or future health outcomes. In sensitivity analysis, we examined the possible contribution of increased long-term adverse events due to early treatment. Our results were insensitive to modest increases in HIV-related mortality as a possible consequence of early ART. We also examined the effects of individuals treated during the acute phase remaining on ART for their entire life, incurring costs of treatment but not receiving any benefit beyond the benefits others obtain when starting ART during chronic infection. This results in more favorable cost-effectiveness for all VL testing strategies, as the additional infections averted and QALYs gained from guaranteed treatment in later disease stages outweigh the costs of earlier treatment. The results are similar if individuals treated during acute infection remain on ART for six months or a year but not indefinitely, as the reduced infectivity from ART produces additional transmission benefits.
DISCUSSION
Our analysis provides novel insights into the clinical impact and cost-effectiveness of HIV testing and treatment strategies among MSM in the US. We show that in this population, where HIV prevalence often exceeds 10%, expanded annual screening with HIV-antibody tests alone can reduce the number of new infections over the next 20 years by 15,000 and improve quality-adjusted life expectancy. However, we also show that symptom-based VL testing alone, without expanding antibody testing, can prevent more infections and provide more QALYs than expanded antibody screening, and may be an attractive strategy where expanded annual screening is not feasible. This underscores the importance of curtailing HIV transmission during acute infection, when high infectivity facilitates HIV transmission, and of the use of ART in preventing transmission.
Among the various preventive measures being considered to reduce the HIV epidemic, our analysis illustrates the importance of testing interventions for HIV prevention among MSM. Our demonstration of the efficacy of expanded testing alone provides an important addition to our current understanding of HIV testing.
Although expanding antibody screening to 90% annually represents the best value among our strategies, implementation may be challenging. Current estimated rates of annual testing – 67% – are high, and MSM who do not already test annually may be difficult to reach. Alternatively, we show that symptom-based VL testing, while more expensive per QALY gained than expanded antibody testing, is also more effective and is very cost-effective by conventional criteria when compared with the status quo. This suggests a unique opportunity for substantial improvements in health outcomes at an acceptable cost through a strategy that can be implemented by physicians who come in contact with MSM.
A key parameter in our analysis is that only 35% of MSM with symptoms of ILI seek medical attention [25]. A recommendation for MSM to get an HIV VL test when experiencing influenza-like symptoms may lead some MSM to seek medical attention with mild cold symptoms, and physicians to subsequently recommend HIV VL testing. We show that even if four times as many uninfected individuals seek HIV testing due to symptoms, symptom-based testing remains cost-effective by conventional criteria.
The budget implications of symptom-based testing and treatment are important. Implementing symptom-based testing would result in an 8% increase in spending on HIV testing and treatment in the MSM population. This is largely due to the high cost of VL testing, which accounts for 86% of the incremental costs of symptom-based testing. Because of the short duration of acute HIV infection and the non-specific symptoms, less than 1% of VL tests detect a case of HIV infection. Increasing the yield of symptom-based VL testing, for example by encouraging MSM to watch for symptoms after high-risk behaviors, could make the intervention more efficient.
Prior studies assessing the cost-effectiveness of VL testing for acute infection are limited. Coco [17] assessed the cost-effectiveness of symptom-based testing, and Hutchinson et al. [18] evaluated the cost-effectiveness of pooled VL screening. Coco found symptom-based testing cost approximately $30,000 per QALY gained in a general population with viral symptoms. The analysis did not include the secondary effects of testing on transmission. Hutchinson et al. found that pooled VL testing after a negative antibody test during routine screening was only likely to cost less than $100,000 per QALY gained in settings with very high HIV incidence, such as a community clinic serving MSM. Our study builds on the findings of each of these studies and illustrates the health benefits that can be achieved in the MSM population with symptom-based VL testing and use of ART during acute infection.
Our study has several limitations. First, we assumed that treatment with ART during acute infection provides no benefits to the treated individual. Observational studies suggest that ART during acute infection may delay CD4 decline, increase the probability of low plasma viral load after treatment discontinuation, and delay immunological decline[37, 38]. Incorporating such benefits would only improve cost-effectiveness estimates and the case for early identification. Second, we assumed that HIV antibody tests are completely insensitive during acute infection. However, the point at which antibodies become detectable varies. A fourth-generation enzyme immunoassay (EIA) that detects infection earlier was approved for use in the US in June 2010 [6, 39]. Standard VL tests are more sensitive to acute infection, however, and the new fourth-generation EIA does not distinguish between the detection of acute infection or HIV antibodies. Since acutely infected patients must be identified as such in order to receive ART during the acute phase, strategies using fourth-generation EIAs to detect acute infection would require confirmatory testing to identify infections as acute, complicating the testing algorithm and reducing the cost savings from avoiding VL tests. Thus, VL tests may be more appropriate for symptom-based testing. Third, we assumed a homogeneous population of MSM, while in reality MSM fall along a spectrum of risky behavior. If high-risk MSM are less likely than low-risk men to present to a healthcare setting when they have ILI, the impact of symptom-based VL testing may be overestimated here; if the converse is true, the impact of symptom-based testing may be underestimated. Fourth, we did not consider the possibility of increased drug resistance, which could be a concern with increased ART use. However, the effects of resistance could be approximated by lower ART efficacy and higher ART cost, to which our results were not sensitive. Fifth, we did not explicitly model non-AIDS defining events, such as neurocognitive decline, cardiovascular events, renal disease, and cancers, which factor into the life expectancy and quality of life of AIDS patients. However, we account for these in the mortality rates and quality-of-life weights that we use for HIV patients. Lastly, we assumed individual VL tests. While this is necessary for symptom-based testing and critical for short turnaround times in reporting results and initiating ART, annual VL screening could make use of pooling schemes to reduce cost. We examine the implications of this in more depth in the Appendix.
Our analysis did not consider some factors specific to the strategies themselves that could be key to their implementation. The clinical and ethical ramifications of discontinuing treatment after the acute infection phase is over should be examined in more detail. Additionally, symptom-based testing may depend on patient and physician education, as a large proportion of MSM do not disclose same-sex activities to their primary care provider [40]. Convincing MSM to seek HIV testing upon onset of viral symptoms, and to comply with an ART regimen, may require evidence of individual health benefits, as transmission benefits alone may not be viewed as an impetus for treatment.
Our study indicates that augmenting annual HIV-antibody testing of MSM with VL testing in patients with influenza-like symptoms could prevent more than 30,000 new HIV infections over 20 years, while costing less than many interventions accepted as cost-effective. Targeted VL testing of symptomatic MSM provides approximately 80% of the benefit of universal VL testing at less than half the cost. Identifying persons with acute HIV can prevent future new infections through behavior modification as well as early initiation of ART, and testing only symptomatic patients considerably narrows the pool of eligible MSM to test, although this strategy will invariably miss detecting persons with acute HIV who are asymptomatic. These findings can assist clinicians and MSM in making decisions about the value of testing and can inform policymakers’ decisions about how to allocate limited HIV screening resources.
Supplementary Material
ACKNOWLEDGEMENTS
Sponsorship: This work was supported by Grant Number R01-DA15612 from the National Institute on Drug Abuse. Dr. Owens is supported by the Department of Veterans Affairs. Dr. Bendavid is supported by the National Institute of Allergy and Infectious Diseases (K01-AI084582).
Footnotes
Author contributions: Conception and design: J.L. Juusola, M.L. Brandeau, D.K. Owens, E. Bendavid
Analysis and interpretation of the data: J.L. Juusola, D.K. Owens, E. Bendavid
Drafting of the article: J.L. Juusola, M.L. Brandeau, D.K. Owens, E. Bendavid
Final approval of the article: J.L. Juusola, M.L. Brandeau, E.F. Long, D.K. Owens, E. Bendavid
Modeling expertise: J.L. Juusola, M.L. Brandeau, E.F. Long
Collection and assembly of data: J.L. Juusola
Obtaining of funding: M.L. Brandeau, D.K. Owens
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) [Accessed September 30, 2009];Estimates of New HIV Infections in the United States. 2008 http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/incidence.htm.
- 2.Centers for Disease Control and Prevention (CDC) [Accessed September 30, 2009];HIV Prevalence Estimates -- United States, 2006. 2008 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5739a2.htm.
- 3.Centers for Disease Control and Prevention (CDC) [Accessed January 27, 2010];HIV/AIDS Surveillance Special Report, Number 5 - HIV Testing Survey, 2002. 2006 http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2004spec_no5/default.htm.
- 4.Centers for Disease Control and Prevention (CDC) Human immunodeficiency virus (HIV) risk, prevention, and testing behaviors -- United States, National HIV Behavioral Surveillance System: Men who have sex with men, November 2003-April 2005. MMWR Morbid Mortal Wkly Rep. 2006;55:1–16. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. quiz CE11-14. [PubMed] [Google Scholar]
- 6.Pilcher CD, Christopoulos KA, Golden M. Public health rationale for rapid nucleic acid or p24 antigen tests for HIV. J Infect Dis. 2010;201(Suppl 1):S7–15. doi: 10.1086/650393. [DOI] [PubMed] [Google Scholar]
- 7.Patel P, Klausner JD, Bacon OM, Liska S, Taylor M, Gonzalez A, et al. Detection of acute HIV infections in high-risk patients in California. J Acquir Immune Defic Syndr. 2006;42:75–79. doi: 10.1097/01.qai.0000218363.21088.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormick AW, Walensky RP, Lipsitch M, Losina E, Hsu H, Weinstein MC, et al. The effect of antiretroviral therapy on secondary transmission of HIV among men who have sex with men. Clin Infect Dis. 2007;44:1115–1122. doi: 10.1086/512816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergis EN, Mellors JW. Natural history of HIV-1 infection. Infect Dis Clin North Am. 2000;14:809–825. v–vi. doi: 10.1016/s0891-5520(05)70135-5. [DOI] [PubMed] [Google Scholar]
- 10.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Daar ES, Little S, Pitt J, Santangelo J, Ho P, Harawa N, et al. Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network. Ann Intern Med. 2001;134:25–29. doi: 10.7326/0003-4819-134-1-200101020-00010. [DOI] [PubMed] [Google Scholar]
- 12.Pantazis N, Touloumi G, Vanhems P, Gill J, Bucher HC, Porter K. The effect of antiretroviral treatment of different durations in primary HIV infection. AIDS. 2008;22:2441–2450. doi: 10.1097/QAD.0b013e328319ea4e. [DOI] [PubMed] [Google Scholar]
- 13.Hoen B, Dumon B, Harzic M, Venet A, Dubeaux B, Lascoux C, et al. Highly active antiretroviral treatment initiated early in the course of symptomatic primary HIV-1 infection: results of the ANRS 053 trial. J Infect Dis. 1999;180:1342–1346. doi: 10.1086/315002. [DOI] [PubMed] [Google Scholar]
- 14.Pilcher CD, Fiscus SA, Nguyen TQ, Foust E, Wolf L, Williams D, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med. 2005;352:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 15.Priddy FH, Pilcher CD, Moore RH, Tambe P, Park MN, Fiscus SA, et al. Detection of acute HIV infections in an urban HIV counseling and testing population in the United States. J Acquir Immune Defic Syndr. 2007;44:196–202. doi: 10.1097/01.qai.0000254323.86897.36. [DOI] [PubMed] [Google Scholar]
- 16.Stekler J, Swenson PD, Wood RW, Handsfield HH, Golden MR. Targeted screening for primary HIV infection through pooled HIV-RNA testing in men who have sex with men. AIDS. 2005;19:1323–1325. doi: 10.1097/01.aids.0000180105.73264.81. [DOI] [PubMed] [Google Scholar]
- 17.Coco A. The cost-effectiveness of expanded testing for primary HIV infection. Ann Fam Med. 2005;3:391–399. doi: 10.1370/afm.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchinson AB, Patel P, Sansom SL, Farnham PG, Sullivan TJ, Bennett B, et al. Cost-effectiveness of pooled nucleic acid amplification testing for acute HIV infection after third-generation HIV antibody screening and rapid testing in the United States: a comparison of three public health settings. PLoS Med. 2010;7:e1000342. doi: 10.1371/journal.pmed.1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) [Accessed September 30, 2009];HIV/AIDS Surveillance Report. 2006 Volume 18 http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2006report/default.htm. [Google Scholar]
- 21.U.S. Census Bureau PD [Accessed March 25, 2010];U.S. Interim Projections by Age, Sex, Race, and Hispanic Origin: 2000-2050. 2001 http://www.census.gov/population/www/projections/usinterimproj/
- 22.Long EF, Brandeau ML, Owens DK. Health outcomes and costs of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med. 2010;153 doi: 10.1059/0003-4819-153-12-201012210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders GD, Bayoumi AM, Sundaram V, Bilir SP, Neukermans CP, Rydzak CE, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 24.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 25.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 26.Hammer SM, Eron JJ, Jr., Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 27.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372:314–320. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents [Accessed May 11, 2011];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2011 http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 29.Teshale EH, Kamimoto L, Harris N, Li J, Wang H, McKenna MT. Estimated Number of HIV-infected Persons Eligible for and Receiving HIV Antiretroviral Therapy, 2003--United States (Abstract #167). 12th Conference on Retroviruses and Opportunistic Infections.2005. [Google Scholar]
- 30.Koblin BA, Chesney MA, Husnik MJ, Bozeman S, Celum CL, Buchbinder S, et al. High-risk behaviors among men who have sex with men in 6 US cities: baseline data from the EXPLORE Study. Am J Public Health. 2003;93:926–932. doi: 10.2105/ajph.93.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med. 2006;145:416–425. doi: 10.7326/0003-4819-145-6-200609190-00005. [DOI] [PubMed] [Google Scholar]
- 32.Long EF, Brandeau ML, Galvin CM, Vinichenko T, Tole SP, Schwartz A, et al. Effectiveness and cost-effectiveness of strategies to expand antiretroviral therapy in St. Petersburg, Russia. Aids. 2006;20:2207–2215. doi: 10.1097/QAD.0b013e328010c7d0. [DOI] [PubMed] [Google Scholar]
- 33.Xiridou M, Geskus R, De Wit J, Coutinho R, Kretzschmar M. The contribution of steady and casual partnerships to the incidence of HIV infection among homosexual men in Amsterdam. AIDS. 2003;17:1029–1038. doi: 10.1097/00002030-200305020-00012. [DOI] [PubMed] [Google Scholar]
- 34.Honiden S, Sundaram V, Nease RF, Holodniy M, Lazzeroni LC, Zolopa A, et al. The effect of diagnosis with HIV infection on health-related quality of Life. Qual Life Res. 2006;15:69–82. doi: 10.1007/s11136-005-8485-x. [DOI] [PubMed] [Google Scholar]
- 35.Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Medicare & Medicaid Services [Accessed April 9, 2010];2009 http://www.cms.hhs.gov/home/medicare.asp. [PubMed]
- 37.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fidler S, Fox J, Porter K, Weber J. Primary HIV infection: to treat or not to treat? Curr Opin Infect Dis. 2008;21:4–10. doi: 10.1097/QCO.0b013e3282f428bf. [DOI] [PubMed] [Google Scholar]
- 39.FDA Approves First-of-Its-Kind HIV Test Which Can Detect HIV Days Earlier Than Current U.S. Tests. Abbott Press; 2010. Release. [Google Scholar]
- 40.Daskalakis D, Silvera R, Bernstein K, Stein D, Hagerty R, Hutt R, et al. Implementation of HIV testing at 2 New York City bathhouses: from pilot to clinical service. Clin Infect Dis. 2009;48:1609–1616. doi: 10.1086/598979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.