Abstract
Malignant mesothelioma (MM) is a relatively rare but devastating tumor that is increasing worldwide. Yet, because of difficulties in early diagnosis and resistance to conventional therapies, MM remains a challenge for pathologists and clinicians to treat. In recent years, much has been revealed regarding the mechanisms of interactions of pathogenic fibers with mesothelial cells, crucial signaling pathways, and genetic and epigenetic events that may occur during the pathogenesis of these unusual, pleiomorphic tumors. These observations support a scenario whereby mesothelial cells undergo a series of chronic injury, inflammation, and proliferation in the long latency period of MM development that may be perpetuated by durable fibers, the tumor microenvironment, and inflammatory stimuli. One culprit in sustained inflammation is the activated inflammasome, a component of macrophages or mesothelial cells that leads to production of chemotactic, growth-promoting, and angiogenic cytokines. This information has been vital to designing novel therapeutic approaches for patients with MM that focus on immunotherapy, targeting growth factor receptors and pathways, overcoming resistance to apoptosis, and modifying epigenetic changes.
CME Accreditation Statement: This activity (“ASIP 2013 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2013 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Malignant mesotheliomas (MMs), among the most aggressive tumors, arise most often from the mesothelial cells that line the pleura, peritoneum, and, occasionally, the pericardium. Because of the multifaceted properties of mesothelium that maintain a protective barrier but also produce components of the extracellular matrix, hyaluronan and other lubricants, chemokines and cytokines, and fibrinolytic and procoagulant factors, understanding its complex biology is a challenge. The intermediate filament pattern of mesothelial cells, suggesting an epithelial–mesodermal hybrid morphology, and their several patterns of differentiation during the neoplastic process suggest their transformation to malignancy is complicated and raises the question of whether one is studying a single tumor type or multiple subgroups of tumors.
MMs are most commonly attributed to occupational exposures to asbestos, a regulatory term for a group of fibrous silicates that occur as needle-like amphiboles (crocidolite, amosite tremolite, anthophyllite, and antigorite) or curly serpentine (chrysotile) fibers. Although each of these fibers has its own distinctive properties, the fibrous nature and biopersistance of these inhaled fibers may be key to carcinogenic events that occur during the long latency periods (mean, 30 to 45 years) of most MMs. Most intensely investigated are chrysotile, the most commonly used type of asbestos historically (>90% use worldwide), and crocidolite, the asbestos type associated most often with MMs in humans1,2 (Figures 1 and 2). The morphology of crocidolite asbestos is similar to nonasbestos fibers of erionite or Libby amphibole, other naturally occurring minerals associated with the development of MMs.5,6 However, 20% to 25% of individuals with MM have no documented exposure to asbestos or other fibers, suggesting familial susceptibility (sporadic or idiopathic MM), unknown exposure to in-place or naturally occurring asbestos, or other causative agents, such as chemicals, radiation, and viruses.7
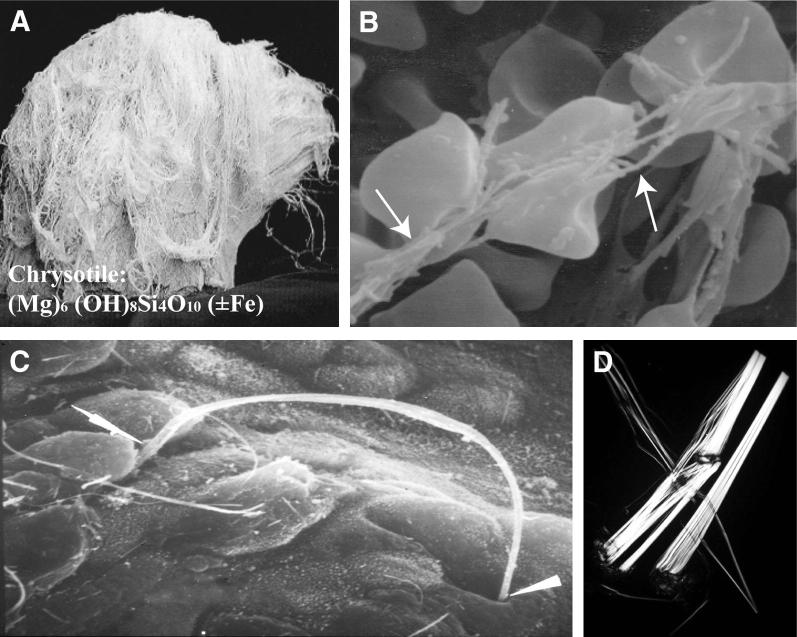
Figure 1.
Properties of chrysotile (white) asbestos. A: Image of bundle of curly chrysotile fibers before processing. B: Scanning electron micrograph of chrysotile fibers (arrows) causing deformation of red blood cells. Chrysotile is positively charged, hemolytic, and cytolytic, primarily due to its magnesium content. Leaching of magnesium renders chrysotile less toxic and also results in chrysotile fiber dissolution over time. C: Scanning electron micrograph of interaction of long chrysotile fiber with the respiratory epithelium of the alveolar duct junction after inhalation by rats. Arrowheads show points of contact with and between epithelial cells. Subsequent penetration into and between cells leads to fiber deposition in the lung interstitum and access to the visceral pleura and pleural space. D: Polarized microscopy showing chrysotile fibers and fibrils.
Photomicrograph is a courtesy of Lee Poye (J3 Resources, Inc., Houston, TX) Original magnification, ×100.
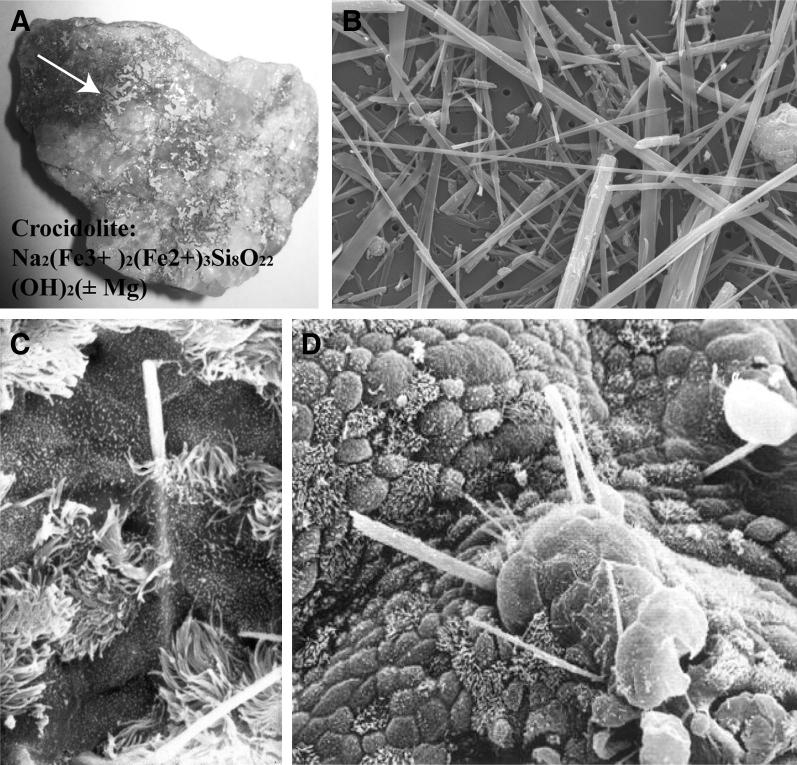
Figure 2.
Properties of crocidolite, or blue, asbestos. A: Riebeckito ore showing veins of crocidolite asbestos fibers (arrow) before processing. B: Scanning electron micrograph showing morphology of needle-like fibers. C: Early penetration of a crocidolite fiber into the differentiated tracheobronchial epithelium in tracheal organ culture. D: Growth of metaplastic cells over long fibers of crocidolite observed at 1 month in this model.3 These events have not been captured in the pleura in animal inhalation models or in clinical specimens in humans, but mesothelial cells undergo proliferation, as measured by cell counts, or immunochemical markers have been observed in response to crocidolite asbestos in vitro and after inhalation by rats.4
Because asbestos fibers neither appear to be metabolized nor directly interact with DNA, they are unlike most chemical carcinogens. The sensitivity of human mesothelial cells to fibers of high aspect (length to diameter) ratio is also perplexing, as are the phenomena governing fiber transport to the parietal pleura where most MMs are thought to develop. Although much insight exists on understanding how fibers (particularly high iron-containing amphibole asbestos types) generate reactive oxygen and nitrogen species to induce inflammation and cell signaling pathways important in proliferation and transformation, how these cellular events converge in the pathogenesis of MM remains enigmatic. This review amalgamates current observations in the field and their implications in strategies to prevent and manage MMs in patients.
Diagnosis of MMs
Detection of MMs is historically difficult and often occurs at a late stage, in part accounting for the poor prognosis of patients. A panel of staining approaches is necessary to ascertain MMs and discriminate pleural MMs from lung carcinomas or peritoneal MMs from ovarian and peritoneal adenocarcinomas.8,9 Several major histologic types (eg, epithelioid, sarcomatoid, biphasic, desmoplastic, or fibrotic) and more than a dozen subtypes of MMs exist, further complicating diagnosis using pathologic analysis. Moreover, no specific associations between exposure to different fiber types and the pathogenesis of distinct MM tumor subtypes have been reported. Since phenotypic heterogeneity within a tumor type arises by two principal mechanisms—reproducible genetic or epigenetic events that produce distinct patterns of gene expression from the same precursor cell or tumors arising from different subsets of cells within a tissue—diagnostic tests based on epigenetic profiles10,11 or down-regulated miRNAs12 have been suggested, particularly because these may be used on small amounts of cells in pleural or peritoneal effusions. However, these new diagnostic tests need further substantiation, and histopathologic analysis is the accepted diagnostic tool for MMs.13
Plasticity of Mesotheliomas
The histologic features of normal mesothelium from different body cavities are generally indistinguishable, although global gene expression studies suggest that there may be phenotypic differences between the mesothelium of the pleural and peritoneal cavities.14 Mature mesothelial cells are commonly flat and thin. However, at some anatomical sites and in response to injury, cuboidal or columnar mesothelial cells are observed, and these cells differ from squamous-like mesothelial cells in organelle distribution and nuclear ultrastructure.15 The precise relationship between these cellular variants is unknown, but studies on the regeneration of mesothelium in response to injury suggest they do not arise from a subserosal progenitor cell.16 Rather, in response to pleural injury, surface mesothelial cells at the edge of a wound increase their proliferation rate, as do mesothelial cells distant to the injury. In the pleural cavity, even free-floating mesothelial cells contribute to regeneration of mesothelium after injury.16 The fact that phenotypic variations in MMs are influenced by growth factors in vitro suggests that both mesothelial and MM cells have autocrine and paracrine cytokine pathways that contribute to plasticity of neighboring cells.
Mature, adult mesothelial cells may or may not have an innate capacity for phenotypic change or adaptation, but clearly mesothelial cells contribute to developmental processes in unexpected ways. In the mouse lung, only mesothelial cells express the WT1 gene, and by using this locus for lineage tracing, it has been shown that smooth muscle cells are derived from surface mesothelium that populate the walls of pulmonary blood vessels.17 Using the same WT1 locus for lineage tracing, others demonstrate that during development of mouse liver, both mesothelial cells and submesothelial cells contribute to the generation of hepatic stellate cells and perivascular mesenchymal cells.18 Analysis of populations of human pleural MM cells suggests that these tumors may also contain subpopulations of precursor cells with cancer stem cell properties.19 Given that mesothelial cells have stem cell–like properties during development and there appear to be distinct subpopulations,16 it seems unlikely that the variations in MM tumor pathology are simply a consequence of unique patterns of genetic events arising in the same precursor cell. Most likely, transformation occurs in distinct, vulnerable precursor cell populations. For example, most pleural MMs appear to arise from the parietal pleura, and longer, more pathogenic asbestos fibers may be trapped during drainage of fluid through stomata on the parietal surface, leading to preferential development of neoplasms at these sites.20 This hypothesis raises the possibility that a unique target population of mesothelial cells is located in the parietal pleura near or within stomata.
Uniqueness of Mesotheliomas
Like other solid tumors, the pathogenesis of mesothelioma is thought to occur in a stepwise fashion with cells progressively acquiring traits, including self-sufficiency for mitogenic signaling, suppression of apoptosis, unlimited capacity for cell replication, genetic instability, tissue invasion, and metastasis. Because tissue specimens representing reactive or intermediate stages of MM are not generally available, information on the pathogenesis of human MMs has been acquired from studying patient specimens, human MM cell lines, and animal models. Functional and molecular analyses indicate that MMs display some of the hallmarks of most cancers, but many oncogenic events typical to other tumor types are uncommon in MM. For example, activation of the Ras oncogene is common in pancreatic, lung, and other solid tumors but rarely observed in mesothelioma.21–23 Similarly, inactivation of the tumor suppressor genes TP53 or RB1 does not occur in MMs at the frequency observed in many other solid tumors. For some malignant tumors, such as colorectal cancer, stepwise genetic perturbations in oncogenes and tumor suppressor genes during the progression to malignancy have been defined. This is not the case with MMs, although enhanced mesothelial cell proliferation (reviewed in Heintz et al24) and suppression of apoptosis are assumed to represent early steps in tumor initiation. How asbestos contributes to these events is not well understood, although long amphibole fibers may act as stimulatory platforms for cell growth and metaplasia3 or interact with growth factor receptors on mesothelial cells.
Molecular Mechanisms in the Pathogenesis of Mesotheliomas
MMs display a wide array of defects in mitogenic signaling pathways and disruption of cell cycle control. In addition, like other solid tumors, persistent activation of the canonical receptor tyrosine kinase (RTK)/Ras/ERK1/2 and phosphatidylinositol 3-kinase/Akt pathways are common features of MM cells.25–29 RTK/SOS/Ras/ERK and phosphatidylinositol 3-kinase/Akt link mitogenic signaling to cell cycle progression. These signaling pathways can be dysregulated by i) aberrant activity of RTKs; ii) alterations in signaling adaptor proteins; iii) constitutive activation of GTPases, such as Ras and Raf; and iv) overexpression of target transcription factors or inactivation of negative regulators. Mitogenic signaling pathways converge on core cell cycle genes and loss of cell cycle checkpoints cooperates with dysregulation of signaling to promote cell proliferation and survival.
Mesothelial cells also respond to an unusually broad array of growth factors, including epidermal growth factor (EGF), keratinocyte growth factor, hepatocyte growth factor, tumor necrosis factor (TNF)-α, IL-8, fibroblast growth factors, and insulin-like growth factor 1) (reviewed in Heintz et al24 and Sekido30). MM cell lines commonly display phosphorylation of multiple RTKs with phosphorylation of epidermal growth factor receptor (EGFR) and MET being the most prominent among 42 RTKs studied.30 Combinatorial inhibition of MET and EGFR has stronger effects on inhibition of MM cell proliferation than either factor alone,30 a rationale for combination targeted therapy in patients.
Tumor suppressor proteins that negatively regulate the cyclin D1 regulatory axis are also common in MMs. For example, inactivation of the NF2 gene, either by homozygous deletion or mutation, is observed in 40% to 50% of mesotheliomas.31 NF2 encodes Merlin, a membrane-cytoskeleton protein regulated by Rac/PAK signaling, and recent work indicates that it suppresses mitogenic signaling by sequestering growth factor receptors.32 In tumor cells, NF2 inhibits cell cycle progression by repressing expression of cyclin D1,33 which controls S phase entry by phosphorylation of the retinoblastoma tumor suppressor protein and activation of the transcription factor E2F1. A second regulator of cyclin D1 kinase activity, the tumor suppressor p16(INK4a), is also deleted with high frequency in mesothelioma, and inactivation of both p16 and p19 (Arf) cooperate to accelerate asbestos-induced MMs.34 In a mouse model of asbestos carcinogenesis, heterozygous NF2+/ mice develop peritoneal MMs more quickly and with a higher frequency than the wild-type mice.35 Interestingly, tumors from heterozygous NF2 mice also showed frequent homozygous deletion of the p16Ink4a/p19Arf gene locus. Consistent with these observations, global gene expression studies indicate that regulation of E2F1 may represent a central control node in MM cell proliferation,36 and aberrant expression of cell cycle regulatory genes may predict survival in MM patients.37
Asbestos fibers induce dose-related proliferation and cell death in mesothelial cells, responses dependent on fiber type, size, duration of exposure, and the cell cycle phase of the target cell. At low doses, asbestos induces some production of reactive oxygen species (ROS) and mitogenic signaling, whereas cytotoxic doses of asbestos induce massive oxidant release, depletion of glutathione,38 mitochondrial dysfunction,39 and both apoptotic and necrotic cell death.40 Asbestos fibers dimerize and activate EGFR,41,42 and both EGFR and β1-integrin may up-regulate signaling through activation of the AKT and ERK pathways.43,44 Activation of ERK culminates in induction of AP-1, a heterodimeric transcription factor composed of members of the c-Fos and c-Jun proto-oncogene families. The c-Fos family member Fra-1 is the primary component of AP-1 required for MM cell growth.45 Moreover, the JUN gene and transcription factor are amplified in some human MMs.46 The strength and duration of ERK1/2 phosphorylation may be important in governing cell proliferation or death because persistent activation of nuclear ERK1/2 by crocidolite asbestos results in down-regulation of cyclin D1 and apoptotic cell death.47 Recently, individual members of the ERK family have been implicated in chemoresistance of MMs.48 The enhanced ability of mesothelial cells to respond to asbestos fibers, oxidants, and a wide array of growth factors that induce dysregulation of mitogenic signaling in MMs and loss of tumor suppressor proteins may govern the pathogenesis of MMs (Figure 3). For example, redox-regulated transcription factors (ie, FOXM150), redox-sensitive proteins (ie, thioredoxins51), and antioxidants52 have recently been used successfully as biomarkers,51 targets,50 and inhibitors52 of MM.
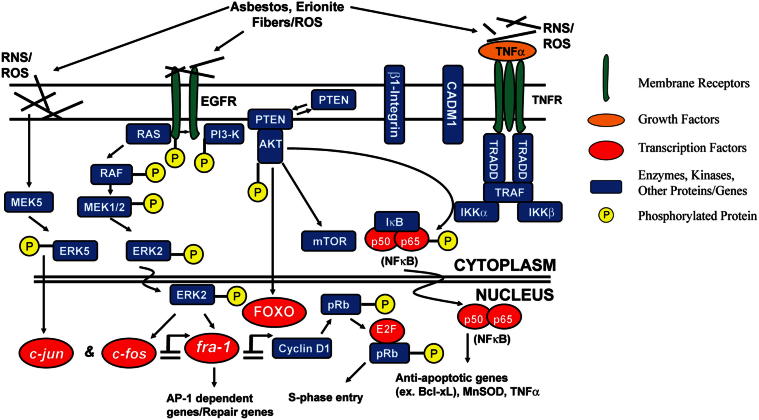
Figure 3.
A schematic diagram indicating the main players in transformation of mesothelial cells to MMs. Several receptors are activated directly by asbestos or oxidants, leading to phosphorylation of RTKs, mitogen-activated protein kinases, and stimulation of growth-promoting or antiapoptotic (survival) pathways that also may be initiated by cytokines such as TNF-α produced by macrophages or mesothelial cells.40,49 Cell-signaling cascades, such as ERKs, may govern plasticity of mesothelial cells and may impinge on early-response proto-oncogenes, such as fra-1, to modulate c-Jun recruitment to form AP-1, NF-κB, FOXO, and other transcription factors; these encode genes promoting cell proliferation, inflammation, and genetic instability. In subsets of MMs or mesothelial cells exposed to pathogenic asbestos fibers, genetic changes over time may include transient mutations by ROS that are subsequently repaired and mutations in genetic susceptibility or cell cycle genes. It is unclear whether these mutations are directly relevant to the pathogenesis of MMs. Epigenetic changes during carcinogenesis may be critical to silencing of tumor suppressor genes.
Modified from Heintz et al.22
Genetic and Epigenetic Events in Mesotheliomas
Although 70% to 80% of MMs are associated with occupational exposures to asbestos, <5% of asbestos workers develop MMs.53 These observations suggest there are significant interindividual barriers to tumorigenesis and genetic and other factors (eg, DNA repair) that affect susceptibility to and MM induction by asbestos fibers. For example, germline mutations in the tumor suppressor gene BAP1 are associated with familial MMs.54 Studies examining oxidative DNA damage on a number of cell types in vitro indicate that asbestos fibers, particularly high iron-containing types, are capable of inducing mutagenic lesions consistent with exposure to ROS,55 as well as DNA repair by the base excision DNA repair enzyme apurinic endonuclease,56,57 and other DNA repair enzymes,58 but as yet no signature of oxidative DNA damage has emerged from studies of human MMs. A unique signature associated with asbestos fiber exposures and carcinogenesis may exist, but this can only be demonstrated by determining genomic sequences in a large number of MMs. Other studies have documented epigenetic events (eg, DNA methylation profiles)10,11 and miRNA signatures59,60 that may be helpful in understanding the prognosis of MMs.
Chronic Inflammation and Proliferation in the Pathogenesis of MMs
Inhalation studies have found that asbestos induces an acute inflammatory response at sites of deposition of fibers that is typified by elaboration of inflammatory cytokines, recruitment of macrophages and neutrophils, and airway epithelial cell proliferation (reviewed in Mossman et al4). These inflammatory changes are followed by mesothelial cell proliferation after inhalation of crocidolite asbestos by rats.
On the basis of these observations, it is biologically plausible that an endless sequence of inflammatory episodes during the development of MM predisposes individuals, especially those exposed occupationally to oxidant-generating asbestos fibers, to malignant tumors. For example, the increased pathogenicity of long asbestos fibers may depend on their ability to be retained for longer periods in the pleura, producing repeated injury, tissue repair, and local inflammation.61,62 Inflammation by mesotheliomagenic fibers may reverse normal transpleural pressure, resulting in a net flow of fluid and fibers directly into the pleural space from the underlying lung parenchyma.63 Mesothelial function is likely altered either directly or indirectly by chemokines or cytokines released from epithelial cells of the lung, alveolar, or pleural macrophages. We recently reported in primary isolates and a telomerase-immortalized human mesothelial cell line that crocidolite asbestos caused increased gene expression and release of inflammatory mediators, including IL-13, basic fibroblast growth factor, vascular endothelial growth factor (VEGF), and granulocyte colony-stimulating factor.64,65 In vivo experiments confirm that increased levels of many of these chemokines and growth factors precede MM development in an intraperitoneal mouse model of MM.66
Novel studies show that inhaled chrysotile fibers and crocidolite asbestos in vitro activate the NLRP3 (NALP3) inflammasome, a cytoplasmic protein complex that is required for secretion of the cytokine IL-1β, in human macrophages and monocytes.67 The inflammasome is activated by a redox-dependent mechanism through oxidation of thioredoxin-interacting protein 1, which, in macrophages, results from oxidants produced by a NADPH oxidase during unsuccessful phagocytosis of long fibers. In response to inhalation of asbestos, recruitment of inflammatory cells and production of cytokines are reduced significantly in NLRP3 knockout mice.67 Surprisingly, human mesothelial cells also express components of the NLRP3 inflammasome and produce NLRP3 inflammasome–dependent cytokines and high-mobility group protein 1 in response to crocidolite or erionite fibers.49 Because critical cytokines are blocked with an IL-1 receptor antagonist, both in vitro and in a xenograft model of MM development,65 pathogenic fibers induce an autocrine pathway in human mesothelial cells that initiates and sustains inflammatory responses (Figure 4). This model also acknowledges the contributions of macrophages in initial inflammation and the tumor microenvironment [ie, tumor-associated macrophages (TAMs)]. In agreement with our hypothesis that asbestos and oxidants perpetuate a chronic inflammatory environment for MMs, exposure to crocidolite asbestos leads to ROS-dependent, transcriptional suppression of FUS1/TUSC2, a novel tumor suppressor gene, in MMs.68
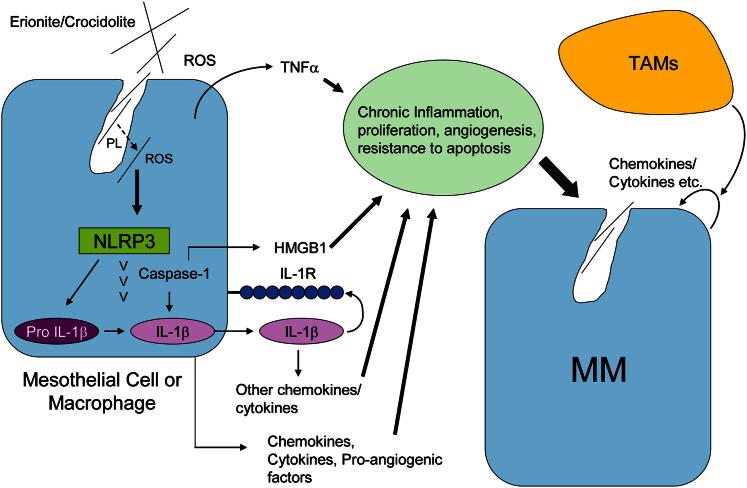
Figure 4.
The NLRP3 (NAPL3) inflammasome is a key player in initiation of inflammation and release of chemokines and cytokines in human mesothelial cells and macrophages in response to long, pathogenic fibers. ROS appear to play a role in both activation of NADPH during phagocytosis and lysosomal degradation, which then releases asbestos fibers into the cytoplasm, where they interact with NLRP3 and induce caspase-1 activity. As a consequence, mature IL-1β, high-mobility group protein 1, and IL-1β–related cytokines are released into the tumor milieu, creating episodic bouts of cell injury, inflammation, and compensatory proliferation. Levels of these key inflammatory factors are reduced in mesothelial cells transfected with small-interfering NLRP3 and enhanced in the presence of TNF-α released by mesothelial cells, TAMs, and macrophages in the tumor environment.49,67
Several studies have found that inflammatory profiles can be used as prognostic and therapeutic indicators of MM. For example, IL-6 is a multifunctional cytokine that regulates immune response and inflammation, and its overproduction has been shown to underlie a number of malignant tumors, including MMs. Others have investigated blood neutrophil-to-lymphocyte ratio and other inflammation-based prognostic factors in MM patients.69,70 These studies found that the neutrophil-to-lymphocyte ratio is an independent predictor of survival for patients with MM undergoing systemic therapy.69 Moreover, these indices correlate with sustained neoangiogenesis and increased proliferation.70
TAMs recently have been associated with MMs and exist in two major phenotypes: the M1 (antitumor) macrophage, which may modulate tumor cell death, and the M2 (protumor) phenotype, which may be critical to the development of cell proliferation and survival, angiogenesis, and tumor invasion. In a hypoxic tumor microenvironment, TAMs can undergo further modification of function and/or modulation or suppression of immunostimulatory cytokines. For example, TAMs assuming an immunosuppressive phenotype may also suppress the infiltration of neutrophils inducing proinflammatory events and ROS.71 An intriguing preventive and therapeutic possibility is the conversion of M2 to M1 TAMs in MMs and other solid tumors.72
Management of MMs
Conventional Therapies
Poor performance status, nonepithelioid histology, male sex, anemia, thrombocytosis, and leucocytosis are the major indicators of poor prognosis in mesothelioma.73 The rarity of disease, the limited number of patients in individual trials, and the difficulty in objectively assessing response are challenges in studying effective therapies. Although the role of surgery is not fully established, current guidelines recommend surgery for patients with clinical stages I through III MM if they are deemed medically operable based on cardiopulmonary evaluation and tolerance. Because surgical procedures themselves rarely result in a complete resection, they are usually performed as part of a multimodal approach, consisting of chemotherapy, surgery, and sometimes radiotherapy. Chemotherapy is the default treatment for patients with stage IV disease, sarcomatoid histology, and medically inoperable stage I through III disease and is used in multimodal therapy for patients with operable disease.74
Surgery
MMs have a specific propensity for adhesion and growth on mesothelial cells that can be related to expression of the cell adhesion molecule 1.75 Adhesion and migratory factors, including mesothelin76 and CD44,77 frequently promote localized intracavitary growth rather than distal metastasis of MMs. For these reasons, surgery and cytoreductive procedures in pleural MM are used, including extrapleural pneumonectomy (EPP) and pleurectomy/decortication (P/D), which spare the lung. The role of surgery in MM and the choice of surgical procedure has been a subject of much controversy.78 Because of the lack of randomized trials, clinical data to guide the selection of surgical procedure are largely derived from observational studies performed in tertiary referral centers that treat selected groups of patients. These series are biased with heterogeneous and selected patient populations, lack of control groups of nonsurgically treated patients, variable surgical techniques, and choice of variable adjuvant therapies.78
EPP [ie, en bloc resection of ipsilateral lung, pleura (parietal and visceral), pericardium, and hemidiaphragm] represents the most aggressive surgical option. The potential benefits of EPP include complete resection of all gross tumors and ability to deliver high-dose adjuvant hemithoracic radiation therapy. EPP is associated with high morbidity and mortality. In retrospective studies, the median overall survival of patients with resectable tumors that undergo EPP-based multimodality therapy ranged from 14 to 19 months.79–81 The Mesothelioma and Radical Surgery trial, the only reported randomized trial that compared EPP with a nonsurgical approach, randomized patients who completed platinum-based induction chemotherapy to EPP (n = 24) followed by postoperative hemithoracic radiation therapy or no EPP (n = 26).82 Only 16 patients assigned to EPP completed surgery, and 8 received hemithoracic radiation therapy. Patients in the EPP group had inferior median overall survival of 14.4 versus 19.5 months for the non-EPP group. In a systematic review of 2320 patients who underwent EPP in 34 studies, a similar overall survival was found, ranging from 9.4 to 27.5 months with 30-day mortality from 0% to 11.8%.80
P/D or lung-sparing surgery consists of complete removal of the involved pleura and all macroscopic tumors and is termed extended or radical P/D when the diaphragm or pericardium is resected.83 P/D has been evaluated in an effort to provide macroscopic clearance of disease with lower morbidity and mortality. The efficacy of P/D is limited by the inability to provide effective postoperative radiation treatment due to the risk of lung toxicity. There are no randomized trials comparing the outcomes of P/D with either a nonsurgical approach or EPP. P/D was found to be associated with only a marginal survival benefit compared with EPP [hazard ratio (HR) for survival with EPP = 1.4; P < 0.001].
Pleurodesis is a less invasive surgical procedure aimed primarily at palliation of dyspnea and pain arising from rapidly accumulating pleural effusions. It consists of pleural fluid drainage by tube thoracostomy or video thoracoscopy followed by instillation of an irritant (often sterile talc) to obliterate the pleural space.
Local adjuvant therapies are used to potentially overcome high rates of local recurrence after surgery by exposing tumor tissue to very high concentrations of active agents, while sparing the ipsilateral and contralateral lung parenchyma and adjacent critical organs. These therapies include intrapleural immunotherapy,84 chemotherapy with or without hyperthermia,85 and photodynamic therapy.86 Photodynamic therapy uses a nontoxic photosensitizing drug that when activated by the appropriate wavelength of visible light produces ROS that can trigger a number of tumoricidal cascades. These local therapies are usually administered as adjuvant treatments after surgical debulking. In summary, the benefit to any form of surgical cytoreduction in addition to systemic treatment remains unproven because of the lack of controlled studies. EPP may be an option for very highly selected patients with epithelial histology, operable early-stage disease, no nodal metastases, good performance status, and no comorbidities,74,79,87 whereas P/D may be considered for patients with operable advanced disease, mixed (biphasic) histology, poor performance status, or comorbidities.74,88 In patients with metastatic disease or sarcomatoid histology, surgery is not recommended, but experimental studies suggest that the latter tumor type is particularly sensitive to oxidative stress and is inhibited by selenite,89 a potential new approach for therapy.
Radiation Therapy
Although MM is sensitive to radiation, the pattern of spread surrounding the lung, proximity to heart, spinal cord, and other organs and its large surface area rather than localized bulk limit the delivery of therapeutic doses of radiation without serious toxic effects.90 Hence, the use of radiation therapy is limited to a single modality for palliation of symptoms90 and as part of a multimodality approach to improve local control after pneumonectomy.79 Improvements in radiation therapy planning and delivery, for example, intensity-modulated radiation therapy (IMRT), allow better dose distribution to regions at risk of recurrence and reduced radiation to surrounding organs.91 In the largest study to date, patients received IMRT (median dose, 45 Gy) with curative intent after EPP.92 Although excellent local control was achieved (13% locoregional failure), median overall survival was limited to only 14.2 months by distant metastases.
Chemotherapy
Because the benefits of single-agent first-line or second-line chemotherapy are limited, the current standard of care for first-line chemotherapy is cisplatin and pemetrexed (a folate inhibitor), the only first-line therapy approved by the US Food and Drug Administration for patients ineligible for surgery. Two randomized clinical trials established the survival benefit with cisplatin-based doublet chemotherapy over single-agent cisplatin.93,94 Compared with cisplatin alone, cisplatin plus pemexetred has been associated with improved response rates (41.3% versus 16.7%; P < 0.0001), longer time to progression (median, 5.7 versus 3.9 months; P = 0.001), and overall survival (median, 12.1 versus 9.3 months; HR = 0.77, P = 0.020). Cisplatin combined with 3 mg/m2 of raltitrexed, a quinazoline folate analog that is a pure and specific thymidylate synthase inhibitor,93 also resulted in improved response rates (23.6% versus 13.6%; P = 0.056) and survival (11.4 versus 8.8 months; HR = 0.76; 95% CI, 0.58 to 1.00; P = 0.48) compared with cisplatin alone. Carboplatin may be an alternative for cisplatin based on results of two phase II studies and an International Expanded Access Program, which showed similar activity between the two platinum analogs.95,96
Novel Therapies
As discussed, recent advances in DNA sequencing technology have provided a comprehensive view of MM genomes, transcriptomes, and epigenetic components. Despite an improved understanding of the mesothelioma genome, the translation of genomic data to identification of novel therapeutic targets has proven challenging.97 Mitotic checkpoints, histone deacetylases (HDAC),98 EGFR, and factors promoting angiogenesis are among the targets being evaluated for therapeutic inhibition in MM.
Epigenetic Modulations
A family of histone acetyltransferases and HDACs regulate tumor suppressor genes through chromatin condensation and decondensation. Their inhibition alters gene expression and the function of a wide range of proteins and cellular pathways regulating cell proliferation, differentiation, and cell death. No responses were observed in a phase II trial of belinostat, an inhibitor of class I and II HDACs, in recurrent MM.99 On the basis of the in vitro proapoptotic effect of valproic acid and its synergy with doxorubicin, a phase II study tested the combination of valproic acid and doxorubicin in patients with MM after prior platinum based chemotherapy (n = 45). Seven partial responses (16%) and two treatment-related deaths were observed, with a median survival of 6.7 months and a progression-free survival of 2.5 months.100 In a phase I trial, vorinostat, an orally administered HDAC inhibitor, had some clinical benefits among 13 patients with MM.101 A phase III randomized, double-blind, placebo-controlled trial of vorinostat, in patients with advanced MM previously treated with systemic chemotherapy, failed to demonstrate improvement in overall survival (primary end point) (median, 31 weeks for the vorinostat group versus 27 weeks for placebo).102 A phase I/II trial evaluating frontline vorinostat with pemetrexed/cisplatin in patients with MM is ongoing (NCT01353482).
Signaling Pathway Inhibition
As shown in Figure 3, EGFR is dimerized by asbestos fibers and up-regulated, mutated, and/or tyrosine-phosphorylated in some MMs, resulting in downstream activation of the mitogen-activated protein kinases, ERK1/2 and 5, and/or the AKT pathway. Although EGFR is overexpressed in 44% to 97% of MM specimens, no consistent association has been demonstrated between EGFR expression and outcome in MM.103 Moreover, in phase II trials, gefitinib or erlotinib, both orally administered, ATP-competitive, small-molecule EGFR tyrosine kinase inhibitors, demonstrated no significant clinical activity in front-line treatment of patients with unresectable MM.104,105 The apparent lack of clinical activity of EGFR inhibition despite EGFR expression and activation in mesothelioma is the subject of ongoing investigation.106 Absence of mutations in the EGFR kinase domain in patients with mesothelioma; concurrent activation of multiple RTKs, including EGFR and MET; PTEN loss; and the resultant activation of AKT may be possible mechanisms of resistance to EGFR inhibition. Recent multiagent studies suggest the efficacy of combinations of RTK inhibitors and inhibition of the RTK chaperone heat shock protein 90.107
Role of Antiangiogenesis
The role of antiangiogenesis as a prognostic marker for therapeutic targeting remains controversial. Although some studies convincingly demonstrate increased angiogenesis in MM as a factor predicting poor prognosis, others do not (reviewed in Ceresoli and Zucali95). Single-agent targeting with bevacizumab (a monoclonal antibody against VEGF), thalidomide, and VEGF receptor tyrosine kinase inhibitors have failed to alter the course of MM.108–110
As illustrated in Figure 3, some MMs may harbor mutations in tumor suppressors and/or oncogenes, which impair several DNA damage checkpoints by inhibiting the activity of multiple kinases involved in G2 arrest. In a phase I dose-escalation study, CBP501 (a G2 checkpoint abrogator) in combination with cisplatin produced clinical activity in three of eight patients with MM.111 A phase II part of a phase I/II trial is evaluating cisplatin and pemetrexed combined with CBP501 in MM patients and has completed accrual (NCT00700336).
Immunotherapies
The overexpression of unique proteins and the development of MMs in a tumor environment of chronic inflammation have prompted immunotherapeutic strategies for MM, including dendritic cell (DC) and WT1 analog peptide vaccines and antibodies targeting mesothelin. WT1 is a transcription factor, which is commonly overexpressed in mesothelioma but has limited expression in normal adult tissues.112,113 DCs are potent antigen-presenting cells found in peripheral tissues that induce activation and proliferation of CD8+ cytotoxic T lymphocytes and helper CD4+ lymphocytes. In a pilot trial, administration of vaccine comprising four WT1 analog peptides, following stimulation of injection sites with granulocyte-macrophage colony-stimulating factor in nine MM patients with WT1-expressing tumors, resulted in induction of immune responses in most patients.112 In a phase I study, autologous tumor lysate pulsed DCs were well tolerated and induced immune responses to tumor cells in MM patients who received them after a course of standard chemotherapy.114 Ongoing clinical trials are evaluating both WT1 vaccine and DC-based immunotherapy in MM (NCT01265433, NCT01241682).
Mesothelin
Mesothelin is an immunogenic glycoprotein that is highly overexpressed in pancreatic, ovarian, non–small cell lung cancers, and MMs and occurs at lower levels in normal mesothelial cells.115 Thus, it is an attractive candidate for tumor-specific immunotherapy. SS1 (dsFv) PE38 (SS1P) is a chimeric recombinant immunotoxin comprising antimesothelin disulfide-stabilized murine-antibody Fv fused to PE38, a 38-kDa portion of Pseudomonas exotoxin A. In preclinical studies, SS1P was cytotoxic to mesothelin-expressing cell lines and caused regression of mesothelin-expressing tumor xenografts in nude mice.116 In phase I studies, clinical activity was noted in a group of heavily pretreated patients with mesothelin-expressing cancers.117 Preclinical observations of synergistic antitumor activity of SS1P in combination with chemotherapies led to a trial of SS1P with six cycles of pemetrexed and cisplatin in front-line therapy for patients with advanced MM.118,119 Among the 14 evaluable patients treated at all dose levels, the overall response rate was 50%. Despite clinical activity, development of neutralizing antibodies to SS1P within 3 weeks of initiation precluded its use beyond two cycles.117 However, recent observations suggest that host immune depletion with pentostatin plus cyclophosphamide (a nonmyeloablative regimen, including durable host T-cell functional defects) safely prevents anti-immunotoxin antibody formation.120 An ongoing pilot study is evaluating the safety and immunogenicity of a conditioning regimen of pentostatin and cyclophosphamide in combination with SS1P in MM patients who have progressive disease after prior treatments, such as a platinum-containing chemotherapy regimen (NCT01362790).
Amatuximab (MORAb-009) is a fully humanized, high-affinity monoclonal chimeric IgG1/k antibody that targets mesothelin. It was generated by fusing the genes encoding the antimesothelin Fv (SS1 scFv) in frame with human IgG1 and κ constant regions.118 In preclinical studies, amatuximab elicited antibody-dependent cellular cytotoxicity against mesothelin-expressing tumor cell lines. In addition, combination of amatuximab with chemotherapy led to a greater reduction in the growth of mesothelin-expressing tumors in nude mice than either amatuximab or chemotherapy alone and was well tolerated in a phase I study with low incidence of immunogenicity.121 To date, disease stabilization has been observed in several heavily pretreated patients, and an open-label, multicenter, phase II clinical trial of combination of amatuximab with pemetrexed and cisplatin for treatment of malignant pleural mesothelioma with progression-free survival as the primary end point has recently completed accrual (NCT00738582).
Summary
This review illustrates how observations on key factors in the pathogenesis of MMs leads to design of therapies based on these experimental and preclinical studies. In concert, data suggest that MMs are a complex, pleiomorphic group of tumors with their phenotypes governed by a plethora of cytokines and growth factors produced in an autocrine fashion or by components of their microenvironment. Novel therapeutic approaches have been based on exploiting mechanisms important in the pathogenesis of MMs and might include promising combined approaches using immunotherapy, sequential blocking of antiapoptotic pathways,122,123 and targeting cell-cycle promoting and susceptibility genes.124,125
Acknowledgments
We thank Jennifer Díaz and Maximilian MacPherson (University of Vermont College of Medicine, Burlington, VT) for exceptional assistance in the preparation of the manuscript and illustrations.
Footnotes
Supported by grants from the Mesothelioma Applied Research Foundation (B.T.M., N.H.H., A.S.), National Institute of Environmental Health Sciences grants T32 ES007122 (B.T.M.) and R01 ES021110 (A.S.), National Cancer Institute grant P01 CA11407 (project 2, B.T.M.), and in part by the Intramural Research Program of the NIH National Cancer Institute, Center for Cancer Research (A.T. and R.H.)
References
- 1.Berman D.W., Crump K.S. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit Rev Toxicol. 2008;38(Suppl 1):49–73. doi: 10.1080/10408440802273156. [DOI] [PubMed] [Google Scholar]
- 2.Kielkowski D., Nelson G., Rees D. Risk of mesothelioma from exposure to crocidolite asbestos: a 1995 update of a South African mortality study. Occup Environ Med. 2000;57:563–567. doi: 10.1136/oem.57.8.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mossman B.T., Craighead J.E., MacPherson B.V. Asbestos-induced epithelial changes in organ cultures of hamster trachea: inhibition by retinyl methyl ether. Science. 1980;207:311–313. doi: 10.1126/science.7350661. [DOI] [PubMed] [Google Scholar]
- 4.Mossman B.T., Lippmann M., Hesterberg T.W., Kelsey K.T., Barchowsky A., Bonner J.C. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J Toxicol Environ Health B Crit Rev. 2011;14:76–121. doi: 10.1080/10937404.2011.556047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin A.A., Coplu L., Selcuk Z.T., Eryilmaz M., Emri S., Akhan O., Baris Y.I. Malignant pleural mesothelioma caused by environmental exposure to asbestos or erionite in rural Turkey: cT findings in 84 patients. AJR Am J Roentgenol. 1993;161:533–537. doi: 10.2214/ajr.161.3.8394641. [DOI] [PubMed] [Google Scholar]
- 6.Antao V.C., Larson T.C., Horton D.K. Libby vermiculite exposure and risk of developing asbestos-related lung and pleural diseases. Curr Opin Pulm Med. 2012;18:161–167. doi: 10.1097/MCP.0b013e32834e897d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jasani B., Gibbs A. Mesothelioma not associated with asbestos exposure. Arch Pathol Lab Med. 2012;136:262–267. doi: 10.5858/arpa.2011-0039-RA. [DOI] [PubMed] [Google Scholar]
- 8.Stahel R.A., Weder W., Felip E. Malignant pleural mesothelioma: eSMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):73–75. doi: 10.1093/annonc/mdp134. [DOI] [PubMed] [Google Scholar]
- 9.Taskin S., Gumus Y., Kiremitci S., Kahraman K., Sertcelik A., Ortac F. Malignant peritoneal mesothelioma presented as peritoneal adenocarcinoma or primary ovarian cancer: case series and review of the clinical and immunohistochemical features. Int J Clin Exp Pathol. 2012;5:472–478. [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen B.C., Houseman E.A., Godleski J.J., Marsit C.J., Longacker J.L., Roelofs C.R., Karagas M.R., Wrensch M.R., Yeh R.F., Nelson H.H., Wiemels J.L., Zheng S., Wiencke J.K., Bueno R., Sugarbaker D.J., Kelsey K.T. Epigenetic profiles distinguish pleural mesothelioma from normal pleura and predict lung asbestos burden and clinical outcome. Cancer Res. 2009;69:227–234. doi: 10.1158/0008-5472.CAN-08-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto Y., Shinjo K., Kondo Y., Shen L., Toyota M., Suzuki H., Gao W., An B., Fujii M., Murakami H., Osada H., Taniguchi T., Usami N., Kondo M., Hasegawa Y., Shimokata K., Matsuo K., Hida T., Fujimoto N., Kishimoto T., Issa J.P., Sekido Y. Epigenetic profiles distinguish malignant pleural mesothelioma from lung adenocarcinoma. Cancer Res. 2009;69:9073–9082. doi: 10.1158/0008-5472.CAN-09-1595. [DOI] [PubMed] [Google Scholar]
- 12.Gee G.V., Koestler D.C., Christensen B.C., Sugarbaker D.J., Ugolini D., Ivaldi G.P., Resnick M.B., Houseman E.A., Kelsey K.T., Marsit C.J. Downregulated microRNAs in the differential diagnosis of malignant pleural mesothelioma. Int J Cancer. 2010;127:2859–2869. doi: 10.1002/ijc.25285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherpereel A., Lee Y.C. Biomarkers for mesothelioma. Curr Opin Pulm Med. 2007;13:339–443. doi: 10.1097/MCP.0b013e32812144bb. [DOI] [PubMed] [Google Scholar]
- 14.Kanamori-Katayama M., Kaiho A., Ishizu Y., Okamura-Oho Y., Hino O., Abe M., Kishimoto T., Sekihara H., Nakamura Y., Suzuki H., Forrest A.R., Hayashizaki Y. LRRN4 and UPK3B are markers of primary mesothelial cells. PLoS One. 2011;6:e25391. doi: 10.1371/journal.pone.0025391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutsaers S.E., Wilkosz S. Structure and function of mesothelial cells. Cancer Treat Res. 2007;134:1–19. doi: 10.1007/978-0-387-48993-3_1. [DOI] [PubMed] [Google Scholar]
- 16.Herrick S.E., Mutsaers S.E. The potential of mesothelial cells in tissue engineering and regenerative medicine applications. Int J Artif Organs. 2007;30:527–540. doi: 10.1177/039139880703000611. [DOI] [PubMed] [Google Scholar]
- 17.Que J., Wilm B., Hasegawa H., Wang F., Bader D., Hogan B.L. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci U S A. 2008;105:16626–16630. doi: 10.1073/pnas.0808649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asahina K., Zhou B., Pu W.T., Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–995. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortes-Dericks L., Carboni G.L., Schmid R.A., Karoubi G. Putative cancer stem cells in malignant pleural mesothelioma show resistance to cisplatin and pemetrexed. Int J Oncol. 2010;37:437–444. doi: 10.3892/ijo_00000692. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson K., Murphy F.A., Duffin R., Poland C.A. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni Z., Liu Y., Keshava N., Zhou G., Whong W., Ong T. Analysis of K-ras and p53 mutations in mesotheliomas from humans and rats exposed to asbestos. Mutat Res. 2000;468:87–92. doi: 10.1016/s1383-5718(00)00043-7. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama Y., Suwa H., Okamoto K., Fukumoto M., Hiai H., Toyokuni S. Low incidence of point mutations in H-. K- and N-ras oncogenes and p53 tumor suppressor gene in renal cell carcinoma and peritoneal mesothelioma of Wistar rats induced by ferric nitrilotriacetate. Jpn J Cancer Res. 1995;86:1150–1158. doi: 10.1111/j.1349-7006.1995.tb03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papp T., Schipper H., Pemsel H., Bastrop R., Muller K.M., Wiethege T., Weiss D.G., Dopp E., Schiffmann D., Rahman Q. Mutational analysis of N-ras, p53, p16INK4a, p14ARF and CDK4 genes in primary human malignant mesotheliomas. Int J Oncol. 2001;18:425–433. doi: 10.3892/ijo.18.2.425. [DOI] [PubMed] [Google Scholar]
- 24.Heintz N.H., Janssen-Heininger Y.M., Mossman B.T. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol. 2010;42:133–139. doi: 10.1165/rcmb.2009-0206TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altomare D.A., You H., Xiao G.H., Ramos-Nino M.E., Skele K.L., De Rienzo A., Jhanwar S.C., Mossman B.T., Kane A.B., Testa J.R. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene. 2005;24:6080–6089. doi: 10.1038/sj.onc.1208744. [DOI] [PubMed] [Google Scholar]
- 26.Opitz I., Soltermann A., Abaecherli M., Hinterberger M., Probst-Hensch N., Stahel R., Moch H., Weder W. PTEN expression is a strong predictor of survival in mesothelioma patients. Eur J Cardiothorac Surg. 2008;33:502–506. doi: 10.1016/j.ejcts.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 27.Wang H., Gillis A., Zhao C., Lee E., Wu J., Zhang F., Ye F., Zhang D.Y. Crocidolite asbestos-induced signal pathway dysregulation in mesothelial cells. Mutat Res. 2011;723:171–176. doi: 10.1016/j.mrgentox.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Shukla A., Hillegass J.M., MacPherson M.B., Beuschel S.L., Vacek P.M., Butnor K.J., Pass H.I., Carbone M., Testa J.R., Heintz N.H., Mossman B.T. ERK2 is essential for the growth of human epithelioid malignant mesotheliomas. Int J Cancer. 2011;129:1075–1086. doi: 10.1002/ijc.25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos-Nino M.E., Vianale G., Sabo-Attwood T., Mutti L., Porta C., Heintz N., Mossman B.T. Human mesothelioma cells exhibit tumor cell-specific differences in phosphatidylinositol 3-kinase/AKT activity that predict the efficacy of Onconase. Mol Cancer Ther. 2005;4:835–842. doi: 10.1158/1535-7163.MCT-04-0243. [DOI] [PubMed] [Google Scholar]
- 30.Sekido Y. Genomic abnormalities and signal transduction dysregulation in malignant mesothelioma cells. Cancer Sci. 2010;101:1–6. doi: 10.1111/j.1349-7006.2009.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bianchi A.B., Mitsunaga S.I., Cheng J.Q., Klein W.M., Jhanwar S.C., Seizinger B., Kley N., Klein-Szanto A.J., Testa J.R. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci U S A. 1995;92:10854–10858. doi: 10.1073/pnas.92.24.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curto M., McClatchey A.I. Nf2/Merlin: a coordinator of receptor signalling and intercellular contact. Br J Cancer. 2008;98:256–262. doi: 10.1038/sj.bjc.6604002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao G.H., Gallagher R., Shetler J., Skele K., Altomare D.A., Pestell R.G., Jhanwar S., Testa J.R. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol. 2005;25:2384–2394. doi: 10.1128/MCB.25.6.2384-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altomare D.A., Menges C.W., Xu J., Pei J., Zhang L., Tadevosyan A., Neumann-Domer E., Liu Z., Carbone M., Chudoba I., Klein-Szanto A.J., Testa J.R. Losses of both products of the Cdkn2a/Arf locus contribute to asbestos-induced mesothelioma development and cooperate to accelerate tumorigenesis. PLoS One. 2011;6:e18828. doi: 10.1371/journal.pone.0018828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altomare D.A., Vaslet C.A., Skele K.L., De Rienzo A., Devarajan K., Jhanwar S.C., McClatchey A.I., Kane A.B., Testa J.R. A mouse model recapitulating molecular features of human mesothelioma. Cancer Res. 2005;65:8090–8095. doi: 10.1158/0008-5472.CAN-05-2312. [DOI] [PubMed] [Google Scholar]
- 36.Gordon G.J., Rockwell G.N., Jensen R.V., Rheinwald J.G., Glickman J.N., Aronson J.P., Pottorf B.J., Nitz M.D., Richards W.G., Sugarbaker D.J., Bueno R. Identification of novel candidate oncogenes and tumor suppressors in malignant pleural mesothelioma using large-scale transcriptional profiling. Am J Pathol. 2005;166:1827–1840. doi: 10.1016/S0002-9440(10)62492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bahnassy A.A., Zekri A.R., Abou-Bakr A.A., El-Deftar M.M., El-Bastawisy A., Sakr M.A., El-Sherif G.M., Gaafar R.M. Aberrant expression of cell cycle regulatory genes predicts overall and disease free survival in malignant pleural mesothelioma patients. Exp Mol Pathol. 2012;93:154–161. doi: 10.1016/j.yexmp.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Janssen Y.M., Heintz N.H., Mossman B.T. Induction of c-fos and c-jun proto-oncogene expression by asbestos is ameliorated by N-acetyl-L-cysteine in mesothelial cells. Cancer Res. 1995;55:2085–2089. [PubMed] [Google Scholar]
- 39.Shukla A., Jung M., Stern M., Fukagawa N.K., Taatjes D.J., Sawyer D., Van Houten B., Mossman B.T. Asbestos induces mitochondrial DNA damage and dysfunction linked to the development of apoptosis. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1018–L1025. doi: 10.1152/ajplung.00038.2003. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg J.L., Zanella C.L., Janssen Y.M., Timblin C.R., Jimenez L.A., Vacek P., Taatjes D.J., Mossman B.T. Novel cell imaging techniques show induction of apoptosis and proliferation in mesothelial cells by asbestos. Am J Respir Cell Mol Biol. 1997;17:265–271. doi: 10.1165/ajrcmb.17.3.2991. [DOI] [PubMed] [Google Scholar]
- 41.Zanella C.L., Posada J., Tritton T.R., Mossman B.T. Asbestos causes stimulation of the extracellular signal-regulated kinase 1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res. 1996;56:5334–5338. [PubMed] [Google Scholar]
- 42.Pache J.C., Janssen Y.M., Walsh E.S., Quinlan T.R., Zanella C.L., Low R.B., Taatjes D.J., Mossman B.T. Increased epidermal growth factor-receptor protein in a human mesothelial cell line in response to long asbestos fibers. Am J Pathol. 1998;152:333–340. [PMC free article] [PubMed] [Google Scholar]
- 43.Berken A., Abel J., Unfried K. Β1-integrin mediates asbestos-induced phosphorylation of AKT and ERK1/2 in a rat pleural mesothelial cell line. Oncogene. 2003;22:8524–8528. doi: 10.1038/sj.onc.1207195. [DOI] [PubMed] [Google Scholar]
- 44.Wilson S.M., Barbone D., Yang T.M., Jablons D.M., Bueno R., Sugarbaker D.J., Nishimura S.L., Gordon G.J., Broaddus V.C. mTOR mediates survival signals in malignant mesothelioma grown as tumor fragment spheroids. Am J Respir Cell Mol Biol. 2008;39:576–583. doi: 10.1165/rcmb.2007-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos-Nino M.E., Timblin C.R., Mossman B.T. Mesothelial cell transformation requires increased AP-1 binding activity and ERK-dependent Fra-1 expression. Cancer Res. 2002;62:6065–6069. [PubMed] [Google Scholar]
- 46.Taniguchi T., Karnan S., Fukui T., Yokoyama T., Tagawa H., Yokoi K., Ueda Y., Mitsudomi T., Horio Y., Hida T., Yatabe Y., Seto M., Sekido Y. Genomic profiling of malignant pleural mesothelioma with array-based comparative genomic hybridization shows frequent non-random chromosomal alteration regions including JUN amplification on 1p32. Cancer Sci. 2007;98:438–446. doi: 10.1111/j.1349-7006.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan Z., Taatjes D.J., Mossman B.T., Heintz N.H. The duration of nuclear extracellular signal-regulated kinase 1 and 2 signaling during cell cycle reentry distinguishes proliferation from apoptosis in response to asbestos. Cancer Res. 2004;64:6530–6536. doi: 10.1158/0008-5472.CAN-04-0946. [DOI] [PubMed] [Google Scholar]
- 48.Shukla A., Hillegass J.M., MacPherson M.B., Beuschel S.L., Vacek P.M., Pass H.I., Carbone M., Testa J.R., Mossman B.T. Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Mol Cancer. 2010;9:314. doi: 10.1186/1476-4598-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shukla A., Miller J.M., Hillegass J.M., MacPherson M.B., Beuschel S.L., Pass H.I., Mossman B.T. Role of the NLRP3 inflammasome in the development and drug resistance of malignant mesothelioma. Cancer Res. 2012;72:5461. [abstract 5461] [Google Scholar]
- 50.Newick K., Cunniff B., Preston K., Held P., Arbiser J., Pass H., Mossman B., Shukla A., Heintz N. Peroxiredoxin 3 is a redox-dependent target of thiostrepton in malignant mesothelioma cells. PLoS One. 2012;7:e39404. doi: 10.1371/journal.pone.0039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tabata C., Terada T., Tabata R., Yamada S., Eguchi R., Fujimori Y., Nakano T. Serum thioredoxin-1 as a diagnostic marker for malignant peritoneal mesothelioma. J Clin Gastroenterol. 2013;47:e7–e11. doi: 10.1097/MCG.0b013e31824e901b. [DOI] [PubMed] [Google Scholar]
- 52.Stapelberg M., Gellert N., Swettenham E., Tomasetti M., Witting P.K., Procopio A., Neuzil J. Alpha-tocopheryl succinate inhibits malignant mesothelioma by disrupting the fibroblast growth factor autocrine loop: mechanism and the role of oxidative stress. J Biol Chem. 2005;280:25369–25376. doi: 10.1074/jbc.M414498200. [DOI] [PubMed] [Google Scholar]
- 53.Below J.E., Cox N.J., Fukagawa N.K., Hirvonen A., Testa J.R. Factors that impact susceptibility to fiber-induced health effects. J Toxicol Environ Health B Crit Rev. 2011;14:246–266. doi: 10.1080/10937404.2011.556052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Testa J.R., Cheung M., Pei J., Below J.E., Tan Y., Sementino E., Cox N.J., Dogan A.U., Pass H.I., Trusa S., Hesdorffer M., Nasu M., Powers A., Rivera Z., Comertpay S., Tanji M., Gaudino G., Yang H., Carbone M. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang S.X., Jaurand M.C., Kamp D.W., Whysner J., Hei T.K. Role of mutagenicity in asbestos fiber-induced carcinogenicity and other diseases. J Toxicol Environ Health B Crit Rev. 2011;14:179–245. doi: 10.1080/10937404.2011.556051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fung H., Kow Y.W., Van Houten B., Taatjes D.J., Hatahet Z., Janssen Y.M., Vacek P., Faux S.P., Mossman B.T. Asbestos increases mammalian AP-endonuclease gene expression, protein levels, and enzyme activity in mesothelial cells. Cancer Res. 1998;58:189–194. [PubMed] [Google Scholar]
- 57.Liu W., Ernst J.D., Broaddus V.C. Phagocytosis of crocidolite asbestos induces oxidative stress. DNA damage, and apoptosis in mesothelial cells. Am J Respir Cell Mol Biol. 2000;23:371–378. doi: 10.1165/ajrcmb.23.3.4094. [DOI] [PubMed] [Google Scholar]
- 58.Pietruska J.R., Johnston T., Zhitkovich A., Kane A.B. XRCC1 deficiency sensitizes human lung epithelial cells to genotoxicity by crocidolite asbestos and Libby amphibole. Environ Health Perspect. 2010;118:1707–1713. doi: 10.1289/ehp.1002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pass H.I., Goparaju C., Ivanov S., Donington J., Carbone M., Hoshen M., Cohen D., Chajut A., Rosenwald S., Dan H., Benjamin S., Aharonov R. hsa-miR-29c∗ is linked to the prognosis of malignant pleural mesothelioma. Cancer Res. 2010;70:1916–1924. doi: 10.1158/0008-5472.CAN-09-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghawanmeh T., Thunberg U., Castro J., Murray F., Laytragoon-Lewin N. miR-34a expression, cell cycle arrest and cell death of malignant mesothelioma cells upon treatment with radiation, docetaxel or combination treatment. Oncology. 2011;81:330–335. doi: 10.1159/000334237. [DOI] [PubMed] [Google Scholar]
- 61.Moalli P.A., MacDonald J.L., Goodglick L.A., Kane A.B. Acute injury and regeneration of the mesothelium in response to asbestos fibers. Am J Pathol. 1987;128:426–445. [PMC free article] [PubMed] [Google Scholar]
- 62.Donaldson K., Brown G.M., Brown D.M., Bolton R.E., Davis J.M. Inflammation generating potential of long and short fibre amosite asbestos samples. Br J Ind Med. 1989;46:271–276. doi: 10.1136/oem.46.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219–225. doi: 10.1183/09031936.97.10010219. [DOI] [PubMed] [Google Scholar]
- 64.Shukla A., MacPherson M.B., Hillegass J., Ramos-Nino M.E., Alexeeva V., Vacek P.M., Bond J.P., Pass H.I., Steele C., Mossman B.T. Alterations in gene expression in human mesothelial cells correlate with mineral pathogenicity. Am J Respir Cell Mol Biol. 2009;41:114–123. doi: 10.1165/rcmb.2008-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hillegass J.M., Shukla A., MacPherson M.B., Bond J.P., Steele C., Mossman B.T. Utilization of gene profiling and proteomics to determine mineral pathogenicity in a human mesothelial cell line (LP9/TERT-1) J Toxicol Environ Health A. 2010;73:423–436. doi: 10.1080/15287390903486568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hillegass J.M., Shukla A., Lathrop S.A., MacPherson M.B., Beuschel S.L., Butnor K.J., Testa J.R., Pass H.I., Carbone M., Steele C., Mossman B.T. Inflammation precedes the development of human malignant mesotheliomas in a SCID mouse xenograft model. Ann N Y Acad Sci. 2010;1203:7–14. doi: 10.1111/j.1749-6632.2010.05554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dostert C., Petrilli V., Van Bruggen R., Steele C., Mossman B.T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ivanova A.V., Ivanov S.V., Prudkin L., Nonaka D., Liu Z., Tsao A., Wistuba I., Roth J., Pass H.I. Mechanisms of FUS1/TUSC2 deficiency in mesothelioma and its tumorigenic transcriptional effects. Mol Cancer. 2009;8:91. doi: 10.1186/1476-4598-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kao S.C., Pavlakis N., Harvie R., Vardy J.L., Boyer M.J., van Zandwijk N., Clarke S.J. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16:5805–5813. doi: 10.1158/1078-0432.CCR-10-2245. [DOI] [PubMed] [Google Scholar]
- 70.Pinato D.J., Mauri F.A., Ramakrishnan R., Wahab L., Lloyd T., Sharma R. Inflammation-based prognostic indices in malignant pleural mesothelioma. J Thorac Oncol. 2012;7:587–594. doi: 10.1097/JTO.0b013e31823f45c1. [DOI] [PubMed] [Google Scholar]
- 71.Solinas G., Germano G., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 72.Ruffell B., Affara N.I., Coussens L.M. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edwards J.G., Abrams K.R., Leverment J.N., Spyt T.J., Waller D.A., O’Byrne K.J. Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax. 2000;55:731–735. doi: 10.1136/thorax.55.9.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ettinger D.S., Akerley W., Borghaei H., Chang A., Cheney R.T., Chirieac L.R., D’Amico T.A., Demmy T.L., Ganti A.K., Govindan R., Grannis F.W., Horn L., Jahan T.M., Jahanzeb M., Kessinger A., Komaki R., Kong F.M., Kris M.G., Krug L.M., Lennes I.T., Loo B.W., Martins R., O’Malley J., Osarogiagbon R.U., Otterson G.A., Patel J.D., Schenck M.P., Pisters K.M., Reckamp K., Riely G.J., Rohren E., Swanson S.J., Wood D.E., Yang S.C. Malignant pleural mesothelioma. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;10:26–41. doi: 10.6004/jnccn.2012.0006. [DOI] [PubMed] [Google Scholar]
- 75.Ito A., Hagiyama M., Mimura T., Matsumoto M., Wakayama T., Iseki S., Yokozaki H., Okada M. Expression of cell adhesion molecule 1 in malignant pleural mesothelioma as a cause of efficient adhesion and growth on mesothelium. Lab Invest. 2008;88:504–514. doi: 10.1038/labinvest.2008.15. [DOI] [PubMed] [Google Scholar]
- 76.Gubbels J.A., Belisle J., Onda M., Rancourt C., Migneault M., Ho M., Bera T.K., Connor J., Sathyanarayana B.K., Lee B., Pastan I., Patankar M.S. Mesothelin-MUC16 binding is a high affinity: N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramos-Nino M.E., Blumen S.R., Pass H., Mossman B.T. Fra-1 governs cell migration via modulation of CD44 expression in human mesotheliomas. Mol Cancer. 2007;6:81. doi: 10.1186/1476-4598-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Perrot M., Feld R., Cho B.C., Bezjak A., Anraku M., Burkes R., Roberts H., Tsao M.S., Leighl N., Keshavjee S., Johnston M.R. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol. 2009;27:1413–1418. doi: 10.1200/JCO.2008.17.5604. [DOI] [PubMed] [Google Scholar]
- 79.Sugarbaker D.J., Flores R.M., Jaklitsch M.T., Richards W.G., Strauss G.M., Corson J.M., DeCamp M.M., Jr., Swanson S.J., Bueno R., Lukanich J.M., Baldini E.H., Mentzer S.J. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117:54–63. doi: 10.1016/s0022-5223(99)70469-1. [DOI] [PubMed] [Google Scholar]
- 80.Cao C.Q., Yan T.D., Bannon P.G., McCaughan B.C. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Oncol. 2010;5:1692–1703. doi: 10.1097/JTO.0b013e3181ed0489. [DOI] [PubMed] [Google Scholar]
- 81.Kaufman A.J., Flores R.M. Surgical treatment of malignant pleural mesothelioma. Curr Treat Options Oncol. 2011;12:201–216. doi: 10.1007/s11864-011-0154-4. [DOI] [PubMed] [Google Scholar]
- 82.Weder W., Stahel R.A., Bernhard J., Bodis S., Vogt P., Ballabeni P., Lardinois D., Betticher D., Schmid R., Stupp R., Ris H.B., Jermann M., Mingrone W., Roth A.D., Spiliopoulos A. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol. 2007;18:1196–1202. doi: 10.1093/annonc/mdm093. [DOI] [PubMed] [Google Scholar]
- 83.Rice D., Rusch V., Pass H., Asamura H., Nakano T., Edwards J., Giroux D.J., Hasegawa S., Kernstine K.H., Waller D., Rami-Porta R. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol. 2011;6:1304–1312. doi: 10.1097/JTO.0b013e3182208e3f. [DOI] [PubMed] [Google Scholar]
- 84.Castagneto B., Zai S., Mutti L., Lazzaro A., Ridolfi R., Piccolini E., Ardizzoni A., Fumagalli L., Valsuani G., Botta M. Palliative and therapeutic activity of IL-2 immunotherapy in unresectable malignant pleural mesothelioma with pleural effusion: results of a phase II study on 31 consecutive patients. Lung Cancer. 2001;31:303–310. doi: 10.1016/s0169-5002(00)00192-6. [DOI] [PubMed] [Google Scholar]
- 85.Tilleman T.R., Richards W.G., Zellos L., Johnson B.E., Jaklitsch M.T., Mueller J., Yeap B.Y., Mujoomdar A.A., Ducko C.T., Bueno R., Sugarbaker D.J. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg. 2009;138:405–411. doi: 10.1016/j.jtcvs.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 86.Pass H.I., Temeck B.K., Kranda K., Thomas G., Russo A., Smith P., Friauf W., Steinberg S.M. Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann Surg Oncol. 1997;4:628–633. doi: 10.1007/BF02303746. [DOI] [PubMed] [Google Scholar]
- 87.Treasure T., Lang-Lazdunski L., Waller D., Bliss J.M., Tan C., Entwisle J., Snee M., O’Brien M., Thomas G., Senan S., O’Byrne K., Kilburn L.S., Spicer J., Landau D., Edwards J., Coombes G., Darlison L., Peto J. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763–772. doi: 10.1016/S1470-2045(11)70149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flores R.M., Pass H.I., Seshan V.E., Dycoco J., Zakowski M., Carbone M., Bains M.S., Rusch V.W. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–626. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 89.Nilsonne G., Sun X., Nystrom C., Rundlof A.K., Potamitou Fernandes A., Bjornstedt M., Dobra K. Selenite induces apoptosis in sarcomatoid malignant mesothelioma cells through oxidative stress. Free Radic Biol Med. 2006;41:874–885. doi: 10.1016/j.freeradbiomed.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 90.de Graaf-Strukowska L., van der Zee J., van Putten W., Senan S. Factors influencing the outcome of radiotherapy in malignant mesothelioma of the pleura: a single-institution experience with 189 patients. Int J Radiat Oncol Biol Phys. 1999;43:511–516. doi: 10.1016/s0360-3016(98)00409-x. [DOI] [PubMed] [Google Scholar]
- 91.Chi A., Liao Z., Nguyen N.P., Howe C., Gomez D., Jang S.Y., Komaki R. Intensity-modulated radiotherapy after extrapleural pneumonectomy in the combined-modality treatment of malignant pleural mesothelioma. J Thorac Oncol. 2011;6:1132–1141. doi: 10.1097/JTO.0b013e3182199819. [DOI] [PubMed] [Google Scholar]
- 92.Rice D.C., Stevens C.W., Correa A.M., Vaporciyan A.A., Tsao A., Forster K.M., Walsh G.L., Swisher S.G., Hofstetter W.L., Mehran R.J., Roth J.A., Liao Z., Smythe W.R. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2007;84:1685–1692. doi: 10.1016/j.athoracsur.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 93.van Meerbeeck J.P., Gaafar R., Manegold C., Van Klaveren R.J., Van Marck E.A., Vincent M., Legrand C., Bottomley A., Debruyne C., Giaccone G. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;23:6881–6889. doi: 10.1200/JCO.20005.14.589. [DOI] [PubMed] [Google Scholar]
- 94.Vogelzang N.J., Rusthoven J.J., Symanowski J., Denham C., Kaukel E., Ruffie P., Gatzemeier U., Boyer M., Emri S., Manegold C., Niyikiza C., Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 95.Ceresoli G.L., Zucali P.A. Anti-angiogenic therapies for malignant pleural mesothelioma. Expert Opin Investig Drugs. 2012;21:833–844. doi: 10.1517/13543784.2012.681641. [DOI] [PubMed] [Google Scholar]
- 96.Santoro A., O’Brien M.E., Stahel R.A., Nackaerts K., Baas P., Karthaus M., Eberhardt W., Paz-Ares L., Sundstrom S., Liu Y., Ripoche V., Blatter J., Visseren-Grul C.M., Manegold C. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaive patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol. 2008;3:756–763. doi: 10.1097/JTO.0b013e31817c73d6. [DOI] [PubMed] [Google Scholar]
- 97.Zauderer M.G., Krug L.M. Novel therapies in phase II and III trials for malignant pleural mesothelioma. J Natl Compr Canc Netw. 2012;10:42–47. doi: 10.6004/jnccn.2012.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paik P.K., Krug L.M. Histone deacetylase inhibitors in malignant pleural mesothelioma: preclinical rationale and clinical trials. J Thorac Oncol. 2010;5:275–279. doi: 10.1097/JTO.0b013e3181c5e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramalingam S.S., Belani C.P., Ruel C., Frankel P., Gitlitz B., Koczywas M., Espinoza-Delgado I., Gandara D. Phase II study of belinostat (PXD101), a histone deacetylase inhibitor, for second line therapy of advanced malignant pleural mesothelioma. J Thorac Oncol. 2009;4:97–101. doi: 10.1097/JTO.0b013e318191520c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scherpereel A., Berghmans T., Lafitte J.J., Colinet B., Richez M., Bonduelle Y., Meert A.P., Dhalluin X., Leclercq N., Paesmans M., Willems L., Sculier J.P. Valproate-doxorubicin: promising therapy for progressing mesothelioma: a phase II study. Eur Respir J. 2011;37:129–135. doi: 10.1183/09031936.00037310. [DOI] [PubMed] [Google Scholar]
- 101.Kelly W.K., O’Connor O.A., Krug L.M., Chiao J.H., Heaney M., Curley T., MacGregore-Cortelli B., Tong W., Secrist J.P., Schwartz L., Richardson S., Chu E., Olgac S., Marks P.A., Scher H., Richon V.M. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krug L.M., Kindler H., Calvert H., Manegold C., Tsao A.S., Fennell D., Lubiniecki G.M., Sun X., Smith M., Baas P. VANTAGE 014: vorinostat (V) in patients with advanced malignant pleural mesothelioma (MPM) who have failed prior pemetrexed and either cisplatin or carboplatin therapy: a phase III, randomized, double-blind, placebo-controlled trial. Eur J Cancer. 2011;47:2–3. [abstract] [Google Scholar]
- 103.Destro A., Ceresoli G.L., Falleni M., Zucali P.A., Morenghi E., Bianchi P., Pellegrini C., Cordani N., Vaira V., Alloisio M., Rizzi A., Bosari S., Roncalli M. EGFR overexpression in malignant pleural mesothelioma: an immunohistochemical and molecular study with clinico-pathological correlations. Lung Cancer. 2006;51:207–215. doi: 10.1016/j.lungcan.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 104.Govindan R., Kratzke R.A., Herndon J.E., 2nd, Niehans G.A., Vollmer R., Watson D., Green M.R., Kindler H.L. Gefitinib in patients with malignant mesothelioma: a phase II study by the Cancer and Leukemia Group B. Clin Cancer Res. 2005;11:2300–2304. doi: 10.1158/1078-0432.CCR-04-1940. [DOI] [PubMed] [Google Scholar]
- 105.Garland L.L., Rankin C., Gandara D.R., Rivkin S.E., Scott K.M., Nagle R.B., Klein-Szanto A.J., Testa J.R., Altomare D.A., Borden E.C. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol. 2007;25:2406–2413. doi: 10.1200/JCO.2006.09.7634. [DOI] [PubMed] [Google Scholar]
- 106.Agarwal V., Lind M.J., Cawkwell L. Targeted epidermal growth factor receptor therapy in malignant pleural mesothelioma: where do we stand? Cancer Treat Rev. 2011;37:533–542. doi: 10.1016/j.ctrv.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 107.Ou W.B., Hubert C., Corson J.M., Bueno R., Flynn D.L., Sugarbaker D.J., Fletcher J.A. Targeted inhibition of multiple receptor tyrosine kinases in mesothelioma. Neoplasia. 2011;13:12–22. doi: 10.1593/neo.101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laurie S.A., Gupta A., Chu Q., Lee C.W., Morzycki W., Feld R., Foo A.H., Seely J., Goffin J.R., Laberge F., Murray N., Rao S., Nicholas G., Laskin J., Reiman T., Sauciuc D., Seymour L. Brief report: a phase II study of sunitinib in malignant pleural mesothelioma. the NCIC Clinical Trials Group. J Thorac Oncol. 2011;6:1950–1954. doi: 10.1097/JTO.0b013e3182333df5. [DOI] [PubMed] [Google Scholar]
- 109.Baas P., Boogerd W., Dalesio O., Haringhuizen A., Custers F., van Zandwijk N. Thalidomide in patients with malignant pleural mesothelioma. Lung Cancer. 2005;48:291–296. doi: 10.1016/j.lungcan.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 110.Kindler H.L., Karrison T.G., Gandara D.R., Lu C., Krug L.M., Stevenson J.P., Janne P.A., Quinn D.I., Koczywas M.N., Brahmer J.R., Albain K.S., Taber D.A., Armato S.G., III, Vogelzang N.J., Chen H.X., Stadler W.M., Vokes E.E. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol. 2012;30:2509–2515. doi: 10.1200/JCO.2011.41.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shapiro G.I., Tibes R., Gordon M.S., Wong B.Y., Eder J.P., Borad M.J., Mendelson D.S., Vogelzang N.J., Bastos B.R., Weiss G.J., Fernandez C., Sutherland W., Sato H., Pierceall W.E., Weaver D., Slough S., Wasserman E., Kufe D.W., Von Hoff D., Kawabe T., Sharma S. Phase I studies of CBP501, a G2 checkpoint abrogator, as monotherapy and in combination with cisplatin in patients with advanced solid tumors. Clin Cancer Res. 2011;17:3431–3442. doi: 10.1158/1078-0432.CCR-10-2345. [DOI] [PubMed] [Google Scholar]
- 112.Krug L.M., Dao T., Brown A.B., Maslak P., Travis W., Bekele S., Korontsvit T., Zakhaleva V., Wolchok J., Yuan J., Li H., Tyson L., Scheinberg D.A. WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1467–1479. doi: 10.1007/s00262-010-0871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Keilholz U., Menssen H.D., Gaiger A., Menke A., Oji Y., Oka Y., Scheibenbogen C., Stauss H., Thiel E., Sugiyama H. Wilms’ tumour gene 1 (WT1) in human neoplasia. Leukemia. 2005;19:1318–1323. doi: 10.1038/sj.leu.2403817. [DOI] [PubMed] [Google Scholar]
- 114.Hegmans J.P., Veltman J.D., Lambers M.E., de Vries I.J., Figdor C.G., Hendriks R.W., Hoogsteden H.C., Lambrecht B.N., Aerts J.G. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med. 2010;181:1383–1390. doi: 10.1164/rccm.200909-1465OC. [DOI] [PubMed] [Google Scholar]
- 115.Hassan R., Bera T., Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Y., Xiang L., Hassan R., Paik C.H., Carrasquillo J.A., Jang B.S., Le N., Ho M., Pastan I. Synergistic antitumor activity of taxol and immunotoxin SS1P in tumor-bearing mice. Clin Cancer Res. 2006;12:4695–4701. doi: 10.1158/1078-0432.CCR-06-0346. [DOI] [PubMed] [Google Scholar]
- 117.Hassan R., Bullock S., Premkumar A., Kreitman R.J., Kindler H., Willingham M.C., Pastan I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 118.Hassan R., Broaddus V.C., Wilson S., Liewehr D.J., Zhang J. Anti-mesothelin immunotoxin SS1P in combination with gemcitabine results in increased activity against mesothelin-expressing tumor xenografts. Clin Cancer Res. 2007;13:7166–7171. doi: 10.1158/1078-0432.CCR-07-1592. [DOI] [PubMed] [Google Scholar]
- 119.Hassan R., Sharon E., Schuler B., Mallory Y., Zhang J., Ling A. Antitumor activity of SS1P with pemetrexed and cisplatin for front-line treatment of pleural mesothelioma and utility of serum mesothelin as a marker of tumor response. J Clin Oncol. 2011;29 [abstract] [Google Scholar]
- 120.Mossoba M.E., Onda M., Taylor J., Massey P.R., Treadwell S., Sharon E., Hassan R., Pastan I., Fowler D.H. Pentostatin plus cyclophosphamide safely and effectively prevents immunotoxin immunogenicity in murine hosts. Clin Cancer Res. 2011;17:3697–3705. doi: 10.1158/1078-0432.CCR-11-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hassan R., Cohen S.J., Phillips M., Pastan I., Sharon E., Kelly R.J., Schweizer C., Weil S., Laheru D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16:6132–6138. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Du X., Xiang L., Mackall C., Pastan I. Killing of resistant cancer cells with low Bak by a combination of an antimesothelin immunotoxin and a TRAIL receptor 2 agonist antibody. Clin Cancer Res. 2011;17:5926–5934. doi: 10.1158/1078-0432.CCR-11-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee M.J., Ye A.S., Gardino A.K., Heijink A.M., Sorger P.K., MacBeath G., Yaffe M.B. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149:780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mizuno T., Murakami H., Fujii M., Ishiguro F., Tanaka I., Kondo Y., Akatsuka S., Toyokuni S., Yokoi K., Osada H., Sekido Y. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012;31:5117–5122. doi: 10.1038/onc.2012.5. [DOI] [PubMed] [Google Scholar]
- 125.Ladanyi M., Zauderer M.G., Krug L.M., Ito T., McMillan R., Bott M., Giancotti F. New strategies in pleural mesothelioma: bAP1 and NF2 as novel targets for therapeutic development and risk assessment. Clin Cancer Res. 2012;18:4485–4490. doi: 10.1158/1078-0432.CCR-11-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]