Abstract
Exogenous kainate receptor agonists have been shown to modulate inhibitory synaptic transmission in the hippocampus, but the pathways involved in physiological activation of the receptors remain largely unknown. Accumulating evidence indicates that astrocytes can release glutamate in a Ca2+-dependent manner and signal to neighboring neurons. We tested the hypothesis that astrocyte-derived glutamate activates kainate receptors on hippocampal interneurons. We report here that elevation of intracellular Ca2+ in astrocytes, induced by uncaging Ca2+, o-nitrophenyl-EGTA, increased action potential-driven spontaneous inhibitory postsynaptic currents in nearby interneurons in rat hippocampal slices. This effect was blocked by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate glutamate receptor antagonists, but not by selective AMPA receptor or N-methyl-d-aspartate receptor antagonists. This pharmacological profile indicates that kainate receptors were activated during Ca2+ elevation in astrocytes. Kainate receptors containing the GluR5 subunit seemed to mediate the observed effect because a selective GluR5-containing kainate receptor antagonist blocked the changes in sIPSCs induced by Ca2+ uncaging, and bath application of a selective GluR5-containing receptor agonist robustly potentiated sIPSCs. When tetrodotoxin was included to block action potentials, Ca2+ uncaging induced a small decrease in the frequency of miniature inhibitory postsynaptic currents, which was not affected by AMPA/kainate receptor antagonists. Our data suggest that an astrocyte-derived, nonsynaptic source of glutamate represents a signaling pathway that can activate neuronal kainate receptors. By modulating the activity of interneurons, astrocytes may play a critical role in circuit function of hippocampus.
Astrocytes have traditionally been considered to function as supporting cells in the central nervous system. During the past decade, it has become increasingly clear that they actively participate in intercellular communication in the brain (1-3). They are endowed with a variety of ion channels and receptors (4-6), and they display a form of excitability by changing the intracellular Ca2+ concentration ([Ca2+]i) (1-3, 7-9). Both application of neurotransmitters (1, 5, 10, 11) and electrical stimulation of neuronal pathways (12, 13) increase [Ca2+]i in astrocytes. Thus, astrocytes sense and respond to neuronal activity by increasing [Ca2+]i. The functional significance of this calcium signaling is still not well understood, but growing evidence suggests that it may trigger the release of glutamate and other neuroactive substances from astrocytes and thereby play a pivotal role in providing feedback to adjacent neurons and blood vessels. Such feedback can result in changes in neuronal excitability (8, 14-17), modulation of synaptic strength (18-20), an increase in neuronal [Ca2+]i (8, 11), and a decrease in vascular tone (21).
The study of astrocyte-neuron interactions is hampered by the lack of specific stimuli that activate only astrocytes (1). Indeed, most neurotransmitter receptors expressed on neurons are also expressed by astrocytes (5). Photolysis of caged compounds (uncaging) has been used to deliver targeted stimulation in many cell types. We used UV laser pulses to uncage caged Ca2+, o-nitrophenyl-EGTA (NP-EGTA), and to increase [Ca2+]i selectively in astrocytes. NP-EGTA releases Ca2+ upon uncaging by lowering its affinity with Ca2+ (22). Through the use of Ca2+ uncaging in astrocytes and whole-cell patch clamp recording in neighboring neurons, we examined how astrocytic calcium signaling influences γ-aminobutyric acid-releasing inhibitory interneurons in hippocampal slices. It has been shown in recent years that exogenous kainate receptor agonists modulate inhibitory synaptic transmission in the hippocampus (23-27). However, it is not well understood how glutamate, the excitatory transmitter, activates the receptors on inhibitory interneurons. Here, we test the hypothesis that calcium-dependent glutamate release from astrocytes (10, 11, 18-20) activates kainate receptors on interneurons. We provide evidence that Ca2+ elevation in astrocytes increases the frequency of action potential-driven spontaneous inhibitory postsynaptic currents (sIPSCs) by activating kainate receptors on neighboring interneurons.
Materials and Methods
Preparation of Hippocampal Slices. Hippocampal slices were prepared from 11- to 15-day-old Sprague-Dawley rats, similar to that described previously (18). Briefly, rats were decapitated, and the whole brains were rapidly removed and immersed in an ice-cold solution containing (in mM): 240 sucrose, 2.5 KCl, 0.5 CaCl2, 10 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, and 10 glucose and saturated with 95% O2/5% CO2. Transverse or coronal hippocampal slices (300 μm) were cut by using a Vibratome (Vibratome Company, St. Louis) and transferred to oxygenated artificial cerebrospinal fluid that contained (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, and 10 glucose.
Calcium Imaging and Photolysis of Caged Compound. After recovering for 30 min at room temperature, slices were incubated at 30-32°C for 60-80 min in artificial cerebrospinal fluid containing acetoxymethyl (AM) ester of fluo-4 (5 μM) and 0.02-0.04% pluronic F-127, in the presence or absence of NP-EGTA AM (10 μM). In some experiments, fluo-4 AM (5 μM) was replaced with fura-2 AM (10 μM) to coload with NP-EGTA AM. After loading, slices were washed in artificial cerebrospinal fluid for 20 min and used for recordings for 2 h thereafter.
Most imaging was performed with an Olympus Fluoview confocal microscope. The 488-nm line of the argon laser was used for excitation, and emission was low pass filtered at 510 nm. In experiments where fura-2 AM was used, calcium imaging was carried out with a custom-built two-photon laser scanning microscope and a Chamelon laser (Coherent, Santa Clara, CA) tuned to 780 nm for excitation. A rectangular window containing one or a few astrocytes was scanned repetitively with a time resolution of 0.3-0.5 s per frame. Fluorescence intensities were analyzed by using Fluoview software. Changes in [Ca2+]i are given as ΔF/F0, where F0 is the baseline fluorescence level and ΔF is determined by the relative change from the base level. A photolysis system (Prairie Tech, Madison, WI) was used to uncage NP-EGTA and release calcium. A UV laser beam (337 nm) from a nitrogen-pulsed laser (VSL-337ND; Laser Science, Cambridge, MA) was focused through the 40× water-immersion objective to an optical spot of 15 μm in diameter. A red spot from a weak 635-nm diode laser was coupled with the UV beam to guide the positioning of the uncaging. To trigger calcium elevation in astrocytes, a train of 12 UV laser pulses was delivered at 10-s intervals to the targeted astrocytes in the stratum radiatum.
Electrophysiological Recording. Whole-cell recordings were made from interneurons in the stratum radiatum of CA1. Interneurons, pyramidal neurons, and astrocytes were identified by their morphology and location and by their electrophysiological properties, as those described previously in our laboratory (18). The pipette solution used for recording sIPSCs contained (in mM): 140 CsCl, 2 MgCl2, 10 Hepes, 2 EGTA, 5 lidocaine N-ethyl chloride (QX314), 5 sodium phosphocreatine, 4 MgATP, and 0.3 GTP (pH 7.2 with CsOH, osmolarity was ≈295 mM). QX314 was omitted when recording miniature IPSCs (mIPSCs). The pipettes had a resistance of 3-5 MΩ. For intracellular loading of NP-EGTA in astrocytes and pyramidal neurons through whole-cell recording, the pipette solution contained (in mM): 10 KCl, 130 KCH3SO3, 2 MgCl2, 20 Hepes, 5 sodium phosphocreatine, 4 MgATP, 0.3 GTP, 0.1 fluo-4 potassium salt, 1 (astrocytes) or 1.2 (neurons) CaCl2, and 2 NP-EGTA. For perforated whole-cell recording, the pipette solution contained (in mM): 10 KCl, 130 KCH3SO3, 2 MgCl2, 20 Hepes, and 400 μg/ml amphotericin B. Membrane currents were filtered at 1-2 kHz, digitized at 5-10 kHz by using an Axopatch 700A amplifier, clampex8 software, and DigiData 1332A interface (Axon Instruments, Foster City, CA). Series resistance (<20 MΩ) was carefully monitored throughout the experiments and was not allowed to vary by more than 15%. All experiments were performed at room temperature (21-23°C).
Data Analysis. All data are presented as the mean ± SEM. For multiple group comparisons, statistical differences were calculated by one-way ANOVA followed by Dunnett's post hoc test. For comparison of means from the same group of cells, Student's paired t test was used. Results were considered to be significant at P < 0.05. sIPSCs and mIPSCs were analyzed with minianalysis (Synaptosoft). The cumulative probability (28) of amplitude and interevent intervals was compared using the Kolmogorov-Smirnov test. Two cumulative sets of data were considered to be significantly different at P < 0.01.
Chemicals. 2-Amino-3-(5-tert-butyl-3-hydroxy-4-isoxazolyl)propionic acid (ATPA), 4-(4-aminophenyl)-1,2-dihydro-1-methyl-2-propylcarbamoyl-6,7-methylenedioxyphthalazine (SYM 2206), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX), and 3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP) were purchased from Tocris Cookson (St. Louis). AM esters and potassium salts of NP-EGTA and fluo-4, and pluronic F-127 were from Molecular Probes. LY293558 was a generous gift from Eli Lilly. All other chemicals were from Sigma.
Results
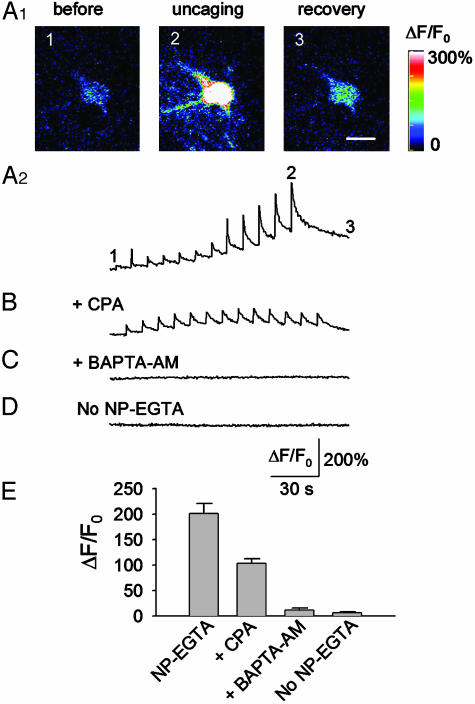
Modulation of sIPSCs by Ca2+ Uncaging in Astrocytes. Slices were preloaded with the calcium fluorescence indicator fluo-4 and caged Ca2+, NP-EGTA. Whole-cell voltage-clamp recordings (-60 mV) were made from interneurons in stratum radiatum in the CA1 region. Most (>95%) of the spontaneous postsynaptic currents were blocked by 5 μM bicuculline, whereas the remaining currents were blocked by 5 μM CNQX (data not shown). Thus, the spontaneous postsynaptic currents are predominately γ-aminobutyric acid receptor-mediated sIPSCs. Spontaneous excitatory postsynaptic currents (EPSCs) decay much faster than sIPSCs, a feature that allows us to separate sIPSCs from sEPSCs by using minianalysis software. After a stable baseline recording of sIPSCs was achieved, an astrocyte near the recorded interneurons was targeted and stimulated with a train of 12 UV pulses (0.1 Hz, 2 min). Ca2+ uncaging produced a stepwise increase in [Ca2+]i in the astrocyte (Fig. 1A2), as reflected by change of ΔF/F0 (Fig. 1 A2 and E). Calcium increases were limited to the astrocytes that received direct UV stimulation, and we did not observe any calcium wave propagation at the cell body level. However, it is not clear whether the elevated calcium levels can propagate through the fine processes of neighboring astrocytes. Loading slices with fluo-4 AM does not allow high-resolution calcium imaging of fine astrocytic processes.
Fig. 1.
Ca2+ uncaging in astrocytes. (A1) An astrocyte was stimulated with a train of 12 UV laser pulses (0.1 Hz) to uncage NP-EGTA. Images were taken before, during, and after the UV pulses, as indicated. Scale bar in recovery, 10 μm. (A2) Time course of changes in calcium fluorescence, ΔF/F0, of the experiment shown in A1. (B) The Ca2+ uncaging-induced change in ΔF/F0 was significantly attenuated by CPA (10 μM), which depletes internal Ca2+ stores. (C) Preloading with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate AM (BAPTA-AM; 10 μM), a calcium chelator, prevented the calcium change induced by Ca2+ uncaging. (D) UV pulses had no effect on ΔF/F0 when slices were loaded with fluo-4 alone (No NP-EGTA). (E) Averaged peak values of ΔF/F0 under the various conditions; n = 8-11 for each group.
Ca2+-induced Ca2+ release from internal stores seemed to amplify the calcium responses induced by Ca2+ uncaging, because a significant attenuation of the peak fluorescence level occurred in slices pretreated with cyclopiazonic acid (CPA, 10 μM for 10 min), which depletes the internal Ca2+ stores (Fig. 1 B and E). Furthermore, Ca2+ uncaging after CPA treatment produced only individual Ca2+ transients but no significant buildup (Fig. 1B). Interestingly, in the presence of CPA, the amplitudes of the first three to five Ca2+ transients were often larger than those observed under control conditions. CPA inhibits the endoplasmic reticular Ca2+-ATPase and prevents Ca2+ sequestration into endoplasmic reticulum (29). The inhibition of Ca2+ uptake by CPA may therefore underlie the observed amplification of the Ca2+ transients.
Slices incubated with 10 μM BAPTA-AM (20-30 min at 30-32°C) after loading with NP-EGTA and fluo-4 displayed no increase in [Ca2+]i in response to uncaging NP-EGTA (Fig. 1 C and E). The astrocytic calcium responses were not due to photo damage or nonspecific effects produced by the UV beam because UV flashings failed to increase [Ca2+]i in slices that were loaded with fluo-4 alone (Fig. 1 D and E).
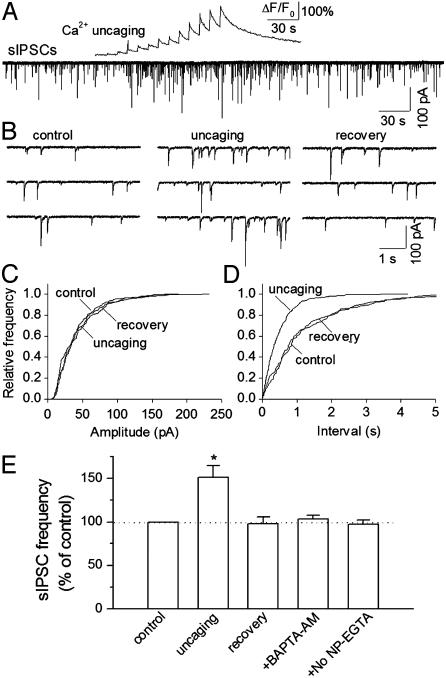
Ca2+ uncaging in nearby astrocytes was accompanied by a reversible increase in the frequency of sIPSCs in the interneurons (Fig. 2). The cell bodies of the stimulated astrocytes were within 30 μm of proximal dendrites, which were within 100 μm from the soma of recorded interneurons. Cumulative frequency plots showed that Ca2+ uncaging had no significant effect on the amplitude distribution of sIPSCs (Fig. 2C), whereas it produced a significant shift of the interevent interval toward the left in 7 of 11 interneurons examined (Fig. 2D, P < 0.01, Kolmogorov-Smirnov test). The time course of increase in the frequency of sIPSCs in interneurons followed that of calcium elevation in astrocytes (Fig. 2A). The anatomical proximity of the stimulated astrocytes with the dendrites of the recorded interneurons is required for modulation of sIPSCs. In astrocytes that were 60-100 μm away from the dendrites of recorded interneurons, Ca2+ uncaging elevated calcium levels in astrocytes (peak ΔF/F0 = 208 ± 19%, n = 8) but did not significantly alter the frequency of sIPSCs (100 ± 6% of baseline frequency, n = 8, P > 0.5, ANOVA). Therefore, only astrocytes within 30 μm of the proximal dendrites of recorded interneurons were chosen for the following experiments.
Fig. 2.
Effects of Ca2+ uncaging on sIPSCs in interneurons. (A) Ca2+ uncaging produced a change of ΔF/F0 in an astrocyte (Upper) and a reversible increase in the frequency of sIPSCs in nearby interneuron (Lower). (B) Traces are sIPSCs taken from A but shown in an expanded time scale. (C and D) Cumulative frequency plots show that Ca2+ uncaging has no significant effect on the amplitude distribution (P > 0.1, Kolmogorov-Smirnov test, C) but shifts the interevent interval toward the left (P < 0.001, Kolmogorov-Smirnov test, D). (E) Normalized frequency of sIPSCs under different conditions. No NP-EGTA indicates that slices were loaded with fluo-4 alone. *, P < 0.05, uncaging versus other groups by ANOVA with Dunnett's test; n = 8-11 for each group.
Pretreatment with BAPTA-AM prevented the uncaging-induced increase in sIPSCs in interneurons (Fig. 2E). In slices loaded with fluo-4 alone, UV flashing of astrocytes had no significant effect on the sIPSCs (Fig. 2E). Taken together, these results suggest that calcium elevation in astrocytes is required for the potentiation of sIPSCs in interneurons.
It was apparent that astrocytes were preferentially loaded with fluo-4, whereas the fluorescent dye was virtually absent from neurons. This loading of fluo-4 and other calcium fluorescent dyes selectively into astrocytes in brain slices has been reported extensively (8, 9, 18, 30), but its mechanism is not yet clear. It is possible that astrocytes may also be preferentially loaded with NP-EGTA under these conditions. Two independent approaches were used to test this possibility. First, fura-2 AM was used to replace fluo-4 AM in some experiments where NP-EGTA AM was coloaded.
Fura-2 AM was chosen because it can still be loaded into neurons in slices prepared from 11- to 15-day-old rats. The neuronal loading of fura-2 in hippocampal slices decreased with the animal age; pyramidal neurons and interneurons were only sparsely loaded with fura-2 in 11- to 15-day-old rats used in the present study. Two-photon excitation of fura-2 at 780 nm was used to image the calcium fluorescence. Fura-2 shows a decrease in fluorescence on binding Ca2+ rather than an increase as with fluo-4. Uncaging at astrocytes clearly increased calcium levels (peak ΔF/F0 = -68 ± 10%, n = 6, P < 0.005, t test), whereas no significant changes of calcium levels were found by uncaging at either pyramidal neurons (peak ΔF/F0 = -6 ± 4%, n = 7, P > 0.1, t test) or interneurons (peak ΔF/F0 = -1 ± 8%, n = 7, P > 0.5, t test). Second, using perforated whole-cell recordings (31) from the NP-EGTA-loaded slices, we found that exposing pyramidal neurons (voltage-clamped at -60 mV) in the CA1 region to UV flashing failed to elicit any outward Ca2+-activated K currents (n = 7), whereas depolarizing voltage steps (50 ms to +10 mV) did induce such currents in the same neurons. When NP-EGTA (2 mM with 1.2 mM Ca2+) was loaded into pyramidal neurons through conventional whole-cell recordings, Ca2+-activated K currents were readily detected in virtually every pyramidal neuron upon uncaging (n = 7; 120 ± 19 pA). Because the Ca2+-activated K channels have a high affinity for Ca2+ (32), recording channel activity should provide a sensitive assay for detecting changes in [Ca2+]i in cells. Our inability to detect Ca2+-activated K currents suggests that loading of NP-EGTA in neurons, at least in pyramidal neurons, is insignificant.
Activation of GluR5-Containing Kainate Receptors Underlies the Modulation of sIPSCs. Next, we investigated the mechanisms underlying the increase in the frequency of sIPSCs. Calcium-dependent release of glutamate from astrocytes has been shown to mediate several types of neuronal responses (10, 11, 14, 16, 19, 20). Because kainate receptor agonists have been shown to drastically enhance sIPSCs in hippocampal pyramidal and interneurons (23-25), we tested whether Ca2+ uncaging-induced potentiation of sIPSCs was mediated by activation of kainate and/or other ionotropic glutamate receptors.
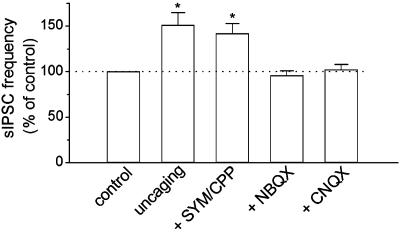
The effect of Ca2+ uncaging in astrocytes on sIPSCs was tested in the presence of different ionotropic glutamate receptor antagonists. In the presence of the selective α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist SYM 2206 (50 μM) (33, 34) together with the N-methyl-d-aspartate (NMDA) receptor antagonist CPP (5 μM), Ca2+ uncaging in astrocytes (peak ΔF/F0 = 189 ± 23%, n = 9) produced a similar increase in the frequency of sIPSCs in interneurons (Fig. 3), which was not significantly different from that observed in control slices, in the absence of the blockers (P > 0.3, ANOVA). Both NBQX (50 μM) and CNQX (50 μM), two broad-spectrum AMPA/kainate glutamate receptor antagonists, completely blocked the increase in the frequency of sIPSCs (Fig. 3). However, neither of these compounds affected astrocytic calcium elevation during Ca2+ uncaging (NBQX: peak ΔF/F0 = 201 ± 17%, n = 8; CNQX: peak ΔF/F0 = 194 ± 20%, n = 9).
Fig. 3.
Effects of ionotropic glutamate receptor antagonists on the uncaging-induced increase in the frequency of sIPSCs. Pooled data of normalized frequency of sIPSCs during Ca2+ uncaging. The data for uncaging was taken from that shown in Fig. 2E for comparison. NBQX and CNQX (50 μM) each prevented the increased frequency of sIPSCs in response to Ca2+ uncaging, whereas the selective AMPA antagonist SYM 2206 and the selective NMDA receptor antagonist CPP did not. *, P < 0.05 compared with control, NBQX, or CNQX groups by ANOVA with Dunnett's test, n = 8-9 for each group.
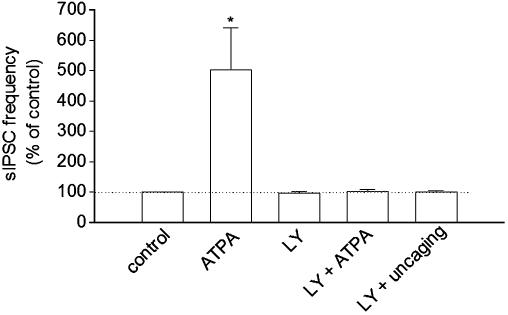
The pharmacological profile indicates that activation of kainate receptors is responsible for modulating sIPSCs in interneurons. GluR5-containing kainate receptors are expressed primarily in interneurons (35). We examined the effect of the selective GluR5-containing kainate receptor agonist ATPA (36) and antagonist LY293558 (25) on sIPSCs in interneurons. In the presence of SYM 2206 (50 μM) and CPP (5 μM) to block AMPA and NMDA receptors, bath application of ATPA (1 μM) produced a reversible increase in the frequency of sIPSCs in interneurons. LY293558 (30 μM) had no significant effect on baseline frequency of sIPSCs, but it did prevent the ATPA-induced increase in the frequency of sIPSCs (Fig. 4). In the presence of LY293558 (30 μM), Ca2+ uncaging in astrocytes (peak ΔF/F0 = 213 ± 18%, n = 8) did not significantly alter sIPSCs in interneurons (Fig. 4).
Fig. 4.
Effects of selective GluR5-containing kainate receptor agonist ATPA and antagonist LY293558 (LY) on sIPSCs. ATPA (1 μM) increased the frequency of sIPSCs. LY (30 μM) had no effect on the frequency of sIPSCs but prevented ATPA and Ca2+ uncaging-induced increases. *, P < 0.001, ATPA versus other groups by ANOVA with Dunnett's test, n = 8 or 9 for each group.
Modulation of mIPSCs by Astrocytic Calcium Elevation. Kainate receptor agonists increase the frequency of sIPSCs in hippocampal interneurons (24), but their effect on mIPSCs remains controversial. Kainate receptor agonists have been either reported to increase (26, 37) or have no effect on the frequency of mIPSCs (24). We tested the effect of the Ca2+ uncaging on mIPSCs recorded in the presence of 0.5 μM tetrodotoxin to block action potentials. The frequency of mIPSCs was 13 ± 4% of that of sIPSCs (n = 7, P < 0.001, t test), indicating that most sIPSCs were driven by action potentials. In the presence of tetrodotoxin, most of the spontaneous postsynaptic currents were bicuculline-sensitive mIPSCs. The frequency of mIPSCs ranged from 0.1 to 2 Hz. The effects of uncaging NP-EGTA on mIPSCs in astrocytes were tested in 14 interneurons with relatively high baseline frequencies (>0.5 Hz). Ca2+ uncaging in astrocytes (peak ΔF/F0 = 212 ± 22%, n = 7) produced an unexpected decrease in the frequency of mIPSCs (81 ± 4% of baseline frequency, n = 7, P < 0.05) in nearby interneurons (recovered to 96 ± 5% of baseline level, P > 0.3, t test). There was no significant change in the amplitude distribution of mIPSCs, as determined by the Kolmogorov-Smirnov test.
In the presence of CNQX (50 μM) and CPP (5 μM), Ca2+ uncaging (peak ΔF/F0 = 197 ± 18%, n = 7) still produced a significant decrease in the frequency of mIPSCs (78 ± 3% of baseline frequency, n = 7, P < 0.05, t test), which is not significantly different from that obtained in the absence of CNQX and CPP (P > 0.5, ANOVA). The mechanism by which the mIPSCs are depressed remains to be determined. However, these results do not support any involvement of ionotropic glutamate receptors, including kainate receptors, in the uncaging-induced modulation of mIPSCs.
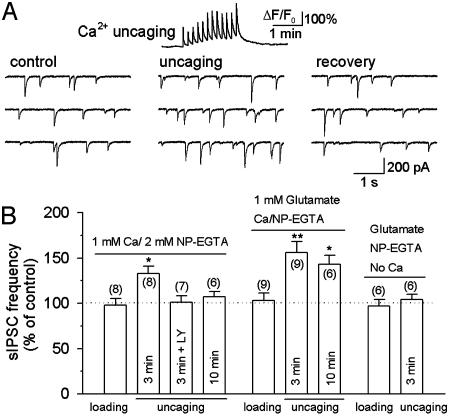
Whole-Cell Loading of NP-EGTA in Astrocytes. Although the UV laser beams were focused onto astrocytes, and astrocytes might preferentially take up NP-EGTA, the possibility that neuronal components are stimulated by uncaging cannot be completely excluded. Therefore, we used whole-cell recording to load NP-EGTA (2 mM, preloaded with 1 mM Ca2+) directly into astrocytes. Dual whole-cell recordings were made, first from an interneuron and then from an astrocyte, to examine the effect of Ca2+ uncaging on sIPSCs. After a stable baseline recording of sIPSCs was made from an interneuron, whole-cell recording was made from a nearby astrocyte. After breaking into whole-cell mode, we waited for 3 min to allow NP-EGTA to diffuse into the astrocyte while sIPSCs were continuously monitored. There was no significant change in the frequency of sIPSCs during the 3-min loading of NP-EGTA (Fig. 5B). The astrocyte was then stimulated with a train of UV pulses to uncage NP-EGTA. As shown in Fig. 5A, uncaging NP-EGTA produced distinct Ca2+ transients (peak ΔF/F0 = 197 ± 19%, n = 8), which were accompanied by a reversible increase in the frequency of sIPSCs (Fig. 5). In the continuous presence of the GluR5-containing kainate receptor antagonist, LY293558 (30 μM), Ca2+ uncaging after loading of NP-EGTA for 3 min (peak ΔF/F0 = 210 ± 17%, n = 7) had no significant effect on sIPSCs in interneurons (Fig. 5B). Thus, the modulation of sIPSCs by Ca2+ uncaging was also mediated by activation of kainate receptors.
Fig. 5.
Modulation of sIPSCs by uncaging NP-EGTA loaded through whole-cell recording. (A Upper) A representative trace showing the time course of Ca2+ transients (ΔF/F0) induced by Ca2+ uncaging. Ca2+/NP-EGTA (1 mM/2 mM) was loaded into the astrocyte for 3 min before uncaging. (A Lower) Ca2+ uncaging produced a reversible increase in the frequency of sIPSCs in interneurons. (B) Pooled data show the effect of NP-EGTA loading, uncaging, and glutamate infusion on the frequency of sIPSCs in interneurons. Whole-cell loading of glutamate and/or NP-EGTA had no effect on sIPSCs. After loading 1 mM Ca2+/2 mM NP-EGTA for 3 min in astrocytes, Ca2+ uncaging produced a significant increase in the frequency of sIPSCs, which was blocked by the GluR5-containing kainate receptor antagonist LY293558 (LY, 30 μM). After loading Ca2+/NP-EGTA for 10 min, Ca2+ uncaging had no significant effect on sIPSCs (Left). The time-dependent rundown of the responses was mainly due to the washout of glutamate in whole-cell recording, because including 1 mM glutamate in the recording solution significantly retarded the rundown (Center). Uncaging NP-EGTA alone (in the absence of added Ca2+) had no effect on sIPSCs (Right). The number of cells tested in each group is shown in parentheses. *, P < 0.05; **, P < 0.01 compared with control or loading groups by ANOVA with Dunnett's test.
The Ca2+ uncaging-induced increase in the frequency of sIPSCs by whole-cell loading of NP-EGTA is significantly less than that observed in response to slice loading of NP-EGTA AM (P < 0.05, ANOVA). Time-dependent washout of intracellular components during whole-cell recordings may account for this difference. Indeed, we found that Ca2+ uncaging after loading NP-EGTA in astrocytes for 10 min increased [Ca2+]i (peak ΔF/F0 = 201 ± 25%, n = 6) but did not significantly alter the frequency of sIPSCs (Fig. 5B).
Whole-cell recording from the giant presynaptic terminal (the calyx of Held) caused washout of glutamate and time-dependent rundown of excitatory postsynaptic currents in postsynaptic neurons, whereas infusion of glutamate into the presynaptic terminal prevented the rundown (38). A similar mechanism may underlie the reduced effect on sIPSCs produced by Ca2+ uncaging. To test this possibility, we added 1 mM glutamate into the pipette solution for recording from astrocytes. Three minutes after loading NP-EGTA into astrocytes, Ca2+ uncaging (peak ΔF/F0 = 188 ± 16%, n = 9) produced a significant increase in the frequency of sIPSCs (Fig. 5B). This is not significantly different from that observed in response to slice loading of NP-EGTA AM (P > 0.5, ANOVA). In six of nine astrocytes in which long-term recordings (≥15 min) were made, we were able to test the effect of a second round of uncaging on sIPSCs 10 min after loading NP-EGTA. Ca2+ uncaging in astrocytes (peak ΔF/F0 = 198 ± 23%, n = 6) still significantly increased the frequency of sIPSCs (Fig. 5B), which is significantly different from that obtained after loading NP-EGTA for 10 min without adding glutamate (P < 0.001, ANOVA).
Photolysis of NP-EGTA releases free Ca2+ and by-products. To test whether Ca2+ is the only factor that is responsible for the modulation of sIPSCs, we added NP-EGTA without Ca2+. Glutamate (1 mM) was included in the recording solution to prevent rundown of the responses. After loading Ca2+-free NP-EGTA into astrocytes for 3 min, uncaging did not produce calcium elevation, nor did it affect the frequency of sIPSCs in the interneurons (Fig. 5B).
Discussion
We have identified a form of astrocyte-neuron interaction that is mediated by activation of kainate receptors in hippocampal interneurons. By uncaging NP-EGTA to release free calcium, we have shown that calcium elevation in astrocytes increases the frequency of sIPSCs in neighboring interneurons in a kainate-receptor-dependent manner. Our data suggest that calcium-dependent release of glutamate from astrocytes (10, 11, 18-20) activates neuronal kainate receptors and modulates inhibitory synaptic transmission in hippocampal interneurons.
Uncaging NP-EGTA in astrocytes, which were either patch pipette loaded or slice loaded, was shown to activate kainate receptors on interneurons. Whole-cell patch loading of NP-EGTA would certainly allow “pure” astrocyte stimulation, but the washout of intracellular components could compromise the downstream effect. Indeed, Ca2+ uncaging induced increases in the frequency of sIPSC rundown during whole-cell recording of astrocytes. The rundown was ameliorated by including glutamate in the recording solution; washout of glutamate from astrocytes may therefore underlie the rundown. Slice loading of NP-EGTA AM in astrocytes prevented this type of washout. Neuronal uptake of NP-EGTA in slice loading was insignificant because we were not able to detect changes of fura-2 fluorescence or Ca2+-activated K currents during the uncaging. Nevertheless, we used whole-cell recording to load NP-EGTA to completely eliminate possible neuronal stimulation in response to uncaging. These two approaches gave similar results, suggesting that calcium elevation in astrocytes, but not in neurons, is required for modulation of sIPSCs in interneurons.
The increase in sIPSCs in interneurons in response to astrocytic calcium elevation was blocked by the AMPA/kainate receptor antagonists, CNQX and NBQX, and by LY293558, a selective GluR5-containing kainate receptor antagonist (25), but not by a SYM 2206, a selective AMPA receptor antagonist (33, 34). These findings suggest that the effect is mediated by activation of GluR5-containing kainate receptors. Kainate receptor agonists have been shown to produce three effects on hippocampal interneurons: (i) a robust increase in the frequency of sIPSCs (24), (ii) direct depolarization or increased action potential firing (23, 25), and (iii) an increase (26, 37) or no change (24) in the frequency of mIPSCs. Ca2+ uncaging mimicked the first effect, but we did not observe a significant change in baseline holding current in the voltage-clamp mode, which would suggest a direct depolarization in interneurons, nor did we find kainate receptor-mediated effect on mIPSCs. Activation of somatodendritic (39) and axonal (24) kainate receptors has been suggested to underlie the increase in the frequency of sIPSCs. If glutamate released from astrocytes activates kainate receptors at distal sites of axons, as suggested by a recent study (24), an increase in sIPSCs could occur in the absence of any significant change in membrane potential recorded at the soma of interneurons. The lack of kainate receptor-mediated effects on mIPSCs in response to Ca2+ uncaging could be due to the ineffectiveness of kainate receptor activation on mIPSCs (24).
Exogenous kainate receptor agonists have often been used to study the function of kainate receptors (40-43). The source of endogenous glutamate that activates the kainate receptors on inhibitory interneurons remains unknown. High-frequency stimulation can trigger the spillover of glutamate to activate kainate receptors on inhibitory terminals (24, 26, 44), suggesting that the receptors are activated during intense excitatory synaptic activity. Here, we demonstrate that astrocytes can serve as a nonsynaptic source of glutamate that activates kainate receptors on neighboring interneurons. Because astrocytic calcium elevation or oscillation occurs either spontaneously (8, 9, 30) or in response to neuronal activity (12, 13, 18), activation of kainate receptors by astrocytes is likely to play a physiological role in regulation of inhibitory transmission in interneurons.
Our results further strengthen the idea that astrocytes may release neuroactive substances to signal to neurons (8, 10, 11, 14-16, 18-21, 45). Calcium-dependent glutamate release from astrocytes (10, 11, 18-20) is believed to account for most of the observed effects. Vesicular exocytosis has been proposed as the mechanism for glutamate release (10, 46, 47), although this issue remains controversial (48). Activation of NMDA receptors (20), metabotropic glutamate receptors (19), and AMPA/NMDA receptors (18) by glutamate released from astrocytes has been implicated in astrocyte-neuron signaling. However, the involvement of kainate receptors in this process has not, to our knowledge, been demonstrated. Because kainate receptors are ubiquitously expressed on many types of neurons, the regulatory mechanism revealed here may occur throughout the brain.
Electron microscopy studies indicate that astrocytes and neurons are in close contact. In the cerebellum, subcellular compartments (glial microdomains) within Bergmann glial cell processes (astrocytic cells of the cerebellum) have been identified as sites of contact between glia and neurons (13). In the hippocampus, thin sheets of astrocytic processes make close contact with dendrites, axons, and synapses (49). The intimate relationship between astrocytes and neurons allows released neuroactive substances to reach and activate target receptors. In the CA1 region of rat hippocampus, it has been estimated that a single astrocyte makes contact with 140,000 synapses (50). Thus, glutamate or other neuroactive substances released from astrocytes may influence a great number of synapses and receptors that are in contact with astrocytic processes.
Interneurons in the stratum radiatum provide a powerful feed-forward γ-aminobutyric acid (GABA)-ergic inhibition onto pyramidal cells (51). They are richly connected to form a dynamic network that participates in complicated brain functions (52, 53). For example, it has been shown that interneurons are crucial in generating rhythmic activity, such as theta oscillations, which reliably correlate with a variety of behaviors (52, 53). Thus, modulation of interneuron activities represents an important mechanism in shaping hippocampal function. Afferent synaptic input shapes the rate and pattern of action potential firing and the final output of interneurons. By altering the strength of inhibitory inputs of interneurons through activation of kainate receptors, astrocytes may play a critical role in the circuit function of the hippocampus.
Acknowledgments
This work was supported by a National Institutes of Health grant.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: [Ca2+]i, intracellular Ca2+ concentration; IPSCs, inhibitory postsynaptic currents; sIPSCs, spontaneous IPSCs; mIPSCs, miniature IPSCs; NP-EGTA, o-nitrophenyl-EGTA; AM, acetoxymethyl; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA, N-methyl-d-aspartate; ATPA, 2-amino-3-(5-tert-butyl-3-hydroxy-4-isoxazolyl)propionic acid; Sym 2206, 4-(4-aminophenyl)-1,2-dihydro-1-methyl-2-propylcarbamoyl-6,7-methylenedioxyphthalazine; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; NBQX, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt; CPP, 3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid; CPA, cyclopiazonic acid; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate.
See Commentary on page 2649.
References
- 1.Newman, E. A. (2003) Trends Neurosci. 26, 536-542. [DOI] [PubMed] [Google Scholar]
- 2.Fields, R. D. & Stevens-Graham, B. (2002) Science 298, 556-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmignoto, G. (2000) Prog. Neurobiol. 62, 561-581. [DOI] [PubMed] [Google Scholar]
- 4.Sontheimer, H. (1994) Glia 11, 156-172. [DOI] [PubMed] [Google Scholar]
- 5.Porter, J. T. & McCarthy, K. D. (1997) Prog. Neurobiol. 51, 439-455. [DOI] [PubMed] [Google Scholar]
- 6.Gallo, V. & Ghiani, C. A. (2000) Trends Pharmacol. Sci. 21, 252-258. [DOI] [PubMed] [Google Scholar]
- 7.Cornell-Bell, A. H., Finkbeiner, S. M., Cooper, M. S. & Smith, S. J. (1990) Science 247, 470-473. [DOI] [PubMed] [Google Scholar]
- 8.Parri, H. R., Gould, T. M. & Crunelli, V. (2001) Nat. Neurosci. 4, 803-812. [DOI] [PubMed] [Google Scholar]
- 9.Aguado, F., Espinosa-Parrilla, J. F., Carmona, M. A. & Soriano, E. (2002) J. Neurosci. 22, 9430-9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasti, L., Zonta, M., Pozzan, T., Vicini, S. & Carmignoto, G. (2001) J. Neurosci. 21, 477-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bezzi, P., Carmignoto, G., Pasti, L., Vesce, S., Rossi, D., Rizzini, B. L., Pozzan, T. & Volterra, A. (1998) Nature 391, 281-285. [DOI] [PubMed] [Google Scholar]
- 12.Porter, J. T. & McCarthy, K. D. (1996) J. Neurosci. 16, 5073-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosche, J., Matyash, V., Moller, T., Verkhratsky, A., Reichenbach, A. & Kettenmann, H. (1999) Nat. Neurosci. 2, 139-143. [DOI] [PubMed] [Google Scholar]
- 14.Hassinger, T. D., Atkinson, P. B., Strecker, G. J., Whalen, L. R., Dudek, F. E., Kossel, A. H. & Kater, S. B. (1995) J. Neurobiol. 28, 159-170. [DOI] [PubMed] [Google Scholar]
- 15.Newman, E. A. (2003) J. Neurosci. 23, 1659-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parpura, V. & Haydon, P. G. (2000) Proc. Natl. Acad. Sci. USA 97, 8629-8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman, E. A. & Zahs, K. R. (1998) J. Neurosci. 18, 4022-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang, J., Jiang, L., Goldman, S. A. & Nedergaard, M. (1998) Nat. Neurosci. 1, 683-692. [DOI] [PubMed] [Google Scholar]
- 19.Araque, A., Parpura, V., Sanzgiri, R. P. & Haydon, P. G. (1998) Eur. J. Neurosci. 10, 2129-2142. [DOI] [PubMed] [Google Scholar]
- 20.Araque, A., Sanzgiri, R. P., Parpura, V. & Haydon, P. G. (1998) J. Neurosci. 18, 6822-6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zonta, M., Angulo, M. C., Gobbo, S., Rosengarten, B., Hossmann, K. A., Pozzan, T. & Carmignoto, G. (2003) Nat. Neurosci. 6, 43-50. [DOI] [PubMed] [Google Scholar]
- 22.Ellis-Davies, G. C. & Kaplan, J. H. (1994) Proc. Natl. Acad. Sci. USA 91, 187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frerking, M., Malenka, R. C. & Nicoll, R. A. (1998) Nat. Neurosci. 1, 479-486. [DOI] [PubMed] [Google Scholar]
- 24.Semyanov, A. & Kullmann, D. M. (2001) Nat. Neurosci. 4, 718-723. [DOI] [PubMed] [Google Scholar]
- 25.Cossart, R., Esclapez, M., Hirsch, J. C., Bernard, C. & Ben-Ari, Y. (1998) Nat. Neurosci. 1, 470-478. [DOI] [PubMed] [Google Scholar]
- 26.Cossart, R., Tyzio, R., Dinocourt, C., Esclapez, M., Hirsch, J. C., Ben-Ari, Y. & Bernard, C. (2001) Neuron 29, 497-508. [DOI] [PubMed] [Google Scholar]
- 27.Jiang, L., Xu, J., Nedergaard, M. & Kang, J. (2001) Neuron 30, 503-513. [DOI] [PubMed] [Google Scholar]
- 28.Van der Kloot, W. (1991) Prog. Neurobiol. 36, 93-130. [DOI] [PubMed] [Google Scholar]
- 29.Missiaen, L., De Smedt, H., Droogmans, G. & Casteels, R. (1992) Eur. J. Pharmacol. 227, 391-394. [DOI] [PubMed] [Google Scholar]
- 30.Nett, W. J., Oloff, S. H. & McCarthy, K. D. (2002) J. Neurophysiol. 87, 528-537. [DOI] [PubMed] [Google Scholar]
- 31.Rae, J., Cooper, K., Gates, P. & Watsky, M. (1991) J. Neurosci. Methods 37, 15-26. [DOI] [PubMed] [Google Scholar]
- 32.Sah, P. & Clements, J. D. (1999) J. Neurosci. 19, 3657-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Moreno, A., Lopez-Garcia, J. C. & Lerma, J. (2000) Proc. Natl. Acad. Sci. USA 97, 1293-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, P., Wilding, T. J., Kim, S. J., Calejesan, A. A., Huettner, J. E. & Zhuo, M. (1999) Nature 397, 161-164. [DOI] [PubMed] [Google Scholar]
- 35.Bureau, I., Bischoff, S., Heinemann, S. F. & Mulle, C. (1999) J. Neurosci. 19, 653-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke, V. R., Ballyk, B. A., Hoo, K. H., Mandelzys, A., Pellizzari, A., Bath, C. P., Thomas, J., Sharpe, E. F., Davies, C. H., Ornstein, P. L., et al. (1997) Nature 389, 599-603. [DOI] [PubMed] [Google Scholar]
- 37.Mulle, C., Sailer, A., Swanson, G. T., Brana, C., O'Gorman, S., Bettler, B. & Heinemann, S. F. (2000) Neuron 28, 475-484. [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa, T., Sahara, Y. & Takahashi, T. (2002) Neuron 34, 613-621. [DOI] [PubMed] [Google Scholar]
- 39.Frerking, M., Petersen, C. C. & Nicoll, R. A. (1999) Proc. Natl. Acad. Sci. USA 96, 12917-12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lerma, J., Morales, M., Vicente, M. A. & Herreras, O. (1997) Trends Neurosci. 20, 9-12. [DOI] [PubMed] [Google Scholar]
- 41.Kullmann, D. M. (2001) Neuron 32, 561-564. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Ari, Y. & Cossart, R. (2000) Trends Neurosci. 23, 580-587. [DOI] [PubMed] [Google Scholar]
- 43.Nicoll, R. A., Mellor, J., Frerking, M. & Schmitz, D. (2000) Nature 406, 957. [DOI] [PubMed] [Google Scholar]
- 44.Min, M. Y., Melyan, Z. & Kullmann, D. M. (1999) Proc. Natl. Acad. Sci. USA 96, 9932-9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nedergaard, M. (1994) Science 263, 1768-1771. [DOI] [PubMed] [Google Scholar]
- 46.Araque, A., Li, N., Doyle, R. T. & Haydon, P. G. (2000) J. Neurosci. 20, 666-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calegari, F., Coco, S., Taverna, E., Bassetti, M., Verderio, C., Corradi, N., Matteoli, M. & Rosa, P. (1999) J. Biol. Chem. 274, 22539-22547. [DOI] [PubMed] [Google Scholar]
- 48.Nedergaard, M., Takano, T. & Hansen, A. J. (2002) Nat. Rev. Neurosci. 3, 748-755. [DOI] [PubMed] [Google Scholar]
- 49.Ventura, R. & Harris, K. M. (1999) J. Neurosci. 19, 6897-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bushong, E. A., Martone, M. E., Jones, Y. Z. & Ellisman, M. H. (2002) J. Neurosci. 22, 183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alger, B. E. & Nicoll, R. A. (1982) J. Physiol. (London) 328, 105-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McBain, C. J. & Fisahn, A. (2001) Nat. Rev. Neurosci. 2, 11-23. [DOI] [PubMed] [Google Scholar]
- 53.Buzsaki, G. (2001) Neurochem. Res. 26, 899-905. [DOI] [PubMed] [Google Scholar]