Abstract
Purpose
The prognostic significance of germline BRCA1 and BRCA2 mutations in Jewish patients with pancreatic adenocarcinoma (PAC) is unknown. Our objective was to define the prevalence of BRCA1 and BRCA2 in an unselected group of Jewish patients and to compare the clinical characteristics and overall survival (OS) of patients with resected BRCA mutation-associated PAC to PAC patients without mutations.
Patients and Methods
Jewish patients with PAC resected between January 1986 and January 2004 were identified. DNA was extracted from the archived material, anonymized, and genotyped for founder mutations in BRCA1 (185delAG, 5382insC) and BRCA2 (6174delT). Standard two-sided statistical tests were utilized.
Results
Of the 187 Jewish patients who underwent resection for PAC, tissue was available for 145 patients. Eight subjects (5.5%) had a BRCA founder mutation (two with BRCA1 [1.3%], six with BRCA2 [4.1%]). The BRCA2 founder mutation was identified in 4.1% of patients with pancreatic adenocarcinoma compared with only 1.1% of cancer-free Washington, DC,–area controls (4.1% v 1.1%; P = .007; odds ratio, 3.85; 95% CI, 2.1 to 10.8). Patients with and without BRCA1 or BRCA2 mutations did not differ in age (mean, 66 v 73 years; P = .6) or other clinicopathologic features. OS was not significantly different (median, 6 v 16 months; P = .35). A previous cancer was reported by 24% (35 of 145) of patients with the most common sites being breast cancer (9 of 35; 74%) and prostate cancer (8 of 35; 23%).
Conclusion
Founder mutations for BRCA1 and BRCA2 were identified in 5.5% of Ashkenazi patients operated on for PAC. BRCA2 mutations were more prevalent than documented by population studies. Consistent with previous reports, BRCA2 mutations are associated with an increased risk of PAC.
INTRODUCTION
Over the past decade it has become increasingly clear that the study of germline variation is of great relevance to the field of oncology. The genes underlying a number of highly penetrant susceptibility syndromes have been defined. Less well studied is whether cancers that arise as a result of a germline predisposition are clinically distinct from sporadic cancers in clinical characteristics, clinical behavior, or response to therapy. Mutations in the tumor suppressor genes BRCA1 and BRCA2 are inherited in an autosomal dominant fashion with incomplete penetrance.1 The two BRCA proteins are involved in transcriptional regulation of gene expression and recognition and repair of DNA damage, particularly double-strand breaks. Although mutations are relatively rare in the general population, they are more common in certain ethnic groups, such as Ashkenazi Jews. Testing of 5,318 Jewish men and women from the Washington, DC, area documented a founder mutation prevalence of 1.1% for BRCA1 and 1.1% for BRCA2.2 Breast cancer is the most well studied cancer associated with BRCA1 and BRCA2 founder mutations.3-6 Several studies have also demonstrated that BRCA mutations increase the risk of pancreatic adenocarcinoma.7-10
Survival continues to be poor for the approximately 37,170 patients who will be diagnosed with pancreatic adenocarcinoma in the United States in 2007.11 Patients’ fortunate enough to undergo resection experience 5-year survival rates between 12% and 17%.12-14 The majority of pancreatic adenocarcinoma cases are thought to be sporadic and associated with various environmental and lifestyle risk factors,15 occupational exposures,16 and medical conditions.17-19 In 1989, the existence of familial pancreatic cancer was initially suggested by a systematic study of a large cohort of families with pancreatic carcinoma.20 Currently it is estimated that as many as 10% of patients with pancreatic cancer may have an inherited form of the disease.21 Inherited mutations currently known to be associated with pancreatic adenocarcinoma include p1622,23(multiple mole melanoma), hMSH2 and hMLH1 associated with HNPCC,24,25 as well as in breast-ovarian cancer families with BRCA1 and BRCA2 mutations.26-29
Most studies evaluating the prevalence of genetic mutations have used familial data or population based series of probands, which could lead to a substantial ascertainment bias. To address this we have used an unselected group of Jewish patients with pancreatic cancer to estimate the contribution of BRCA mutations to the development of pancreatic cancer. The objectives of the present study were to determine the prevalence of BRCA1 and BRCA2 mutations in unselected Jewish patients who had their pancreatic adenocarcinoma resected and to compare the clinical characteristics and overall survival of patients with BRCA-associated pancreatic cancer treated at a single institution to that of patients without founder mutations.
PATIENTS AND METHODS
Through the review of hospital pathology records between January 1986 and January 2004 all patients with pancreatic adenocarcinoma were identified. Patients who self declared at the time of registration to be Jewish were selected as potential subjects for this study. In unpublished validation studies by the clinical genetics service at our institution, more than 90% of individuals who report Jewish religious preference are of Ashkenazi (Central/Eastern European) ethnicity. Hospital records were abstracted to collect clinical information including age at diagnosis, sex, weight loss, pain, jaundice, site of tumor, cytology results, date of operation, postoperative chemotherapy and/or radiation therapy, and status at last follow-up. Pathologic factors included TNM stage, margin, and differentiation. After all clinicopathologic data was collected, each patient was assigned a unique study number. Overall survival was calculated from date of operation to the date of last known status at which point patients were either deceased or censored.
For each potential subject, paraffin-embedded, formalin-fixed material was retrieved. Of the patients who had archived tissue, nontumor tissue (eg, noninvolved lymph node) was used as the source of DNA for testing. Ten-μm curls were shaved from the archival pathology material and placed in a vial labeled with a unique study number. All links between personal identifiers and the unique study number were then irretrievably destroyed, thus anonymizing the samples. The study design was approved by the Memorial Sloan-Kettering institutional review board, including all data collection and anonymization procedures.
DNA was extracted from the formalin-fixed paraffin-embedded material using standard protocols. Samples were then analyzed for the presence of the three common Ashkenazi Jewish BRCA founder mutations (185delAG and 5382insC in BRCA1 and 6174delT in BRCA2) as described previously.30 Polymerase chain reaction products containing each mutation were generated with the primers 5′-TCTGCTCTTCGCGTTGAAGAA-3′ and 5′-CACTCTTGTGCTGACTTACCA-3′ for BRCA1 185delAG (90-bp product), 5′-CAGCATGATTTTGAAGTCAG-3′ and 5′-AGGGAGCTTTACCTTTCTGTC-3′ for BRCA1 5382insC (99-bp product), and 5′GGGAAGCTTCATAAGTCAGTC-3′ and 5′-TTTGTAATGAAGCATCTGATACC-3′ for BRCA2 6174delT (97-bp product). Radiolabeled products were produced using a forward primer end-labeled with [g-33P] adenosine triphosphate and then visualized by denaturing polyacrylamide gel electrophoresis followed by autoradiography as previously described in detail. All mutations were confirmed by independent polymerase chain reaction amplification from the corresponding DNA sample.
Once all samples were genotyped, linkage between genotype and clinical data was established through the unique study number and characteristics and outcomes of subjects with and without mutations were compared. Statistical analysis was performed using the χ2 and t test, as appropriate. Overall survival was estimated using the Kaplan-Meier method, and comparison between groups was performed with the log-rank test. Lifetime risks of BRCA2-associated PAC were estimated according to methods previously described for BRCA mutation carriers with ovarian or breast cancer.31,32 A control group of 5,318 Ashkenazi Jews was described in a previously published series of volunteers in the Washington, DC, area.33 This group was used to calculate a population BRCA2 mutation prevalence and, in conjunction with the current series, the relative risk for PAC associated with BRCA2 6174delT. The lifetime risk of PAC was obtained from the Surveillance, Epidemiology, and End Results database provided by the National Cancer Institute. The lifetime risk is expressed in terms of the population lifetime risk of PAC (to age 95), the mutation prevalence and the relative risk of PAC in mutation carriers as follows:
LRC = LRP × RR/(PPMUTRR + 1 − PPMUT), where LRC is the lifetime risk of PAC in the BRCA2 heterozygotes, LRP is the lifetime risk of PAC in the general population, RR is the relative risk of PAC in BRCA2 heterozygotes, PPMUT is the prevalence of BRCA2 mutations in controls.
RESULTS
Of the 187 Jewish patients who underwent pancreatic resection for pancreatic adenocarcinoma, 145 patients had tissue available for evaluation. Eight patients (5.5%) were found to have BRCA mutations. Two patients (1.3%) carried a BRCA1 mutation (BRCA1 185delAG and BRCA1 5382insC) and six patients (4.1%) carried a BRCA2 mutation (6174delT). The BRCA2 6174delT founder mutation was identified in 4.1% of patients with pancreatic adenocarcinoma in this cohort compared to only 1.1% of cancer-free Washington, DC–area controls (4.1% v 1.1%; P = .007; odds ratio, 3.85; 95% CI, 2.1 to 10.8). Patients with BRCA1 and BRCA2 founder mutations did not differ in age at presentation from Jewish patients without a founder mutation (mean, 66 v 73 years; P = .6) or other clinicopathologic factors. Clinical characteristics are listed in Table 1.
Table 1.
Clinicopathologic Factors of Patients With Pancreatic Adenocarcinoma According to BRCA Mutation Status
| Characteristic | Total (N = 145) |
BRCA (n = 8) |
P | ||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Median age, years | 71 | 66 | |||||
| Range | 42-84 | 60-79 | NS | ||||
| Female sex | 60 | 41 | 4 | 50 | NS | ||
| Pain | 45 | 31 | 4 | 50 | NS | ||
| Jaundice | 46 | 32 | 1 | 13 | NS | ||
| Surgery | NS | ||||||
| Pancreaticoduodenectomy | 126 | 87 | 6 | 75 | |||
| Distal pancreatectomy | 16 | 11 | 1 | 13 | |||
| Total pancreatectomy | 3 | 2 | 1 | 13 | |||
| Margin positive | 39 | 27 | 4 | 50 | NS | ||
| Median size, cm | 3.3 | 3.5 | NS | ||||
| Range | 1.8-2 | 3.1-7 | |||||
| Perineural invasion | 107 | 74 | 5 | 62 | NS | ||
| Perivascular invasion | 55 | 38 | 3 | 38 | NS | ||
| Tumor location | NS | ||||||
| Head | 127 | 87 | 6 | 75 | |||
| Body | 7 | 5 | 2 | 25 | |||
| Tail | 11 | 8 | |||||
| Differentiation | NS | ||||||
| Well | 16 | 11 | 0 | 0 | |||
| Moderate | 77 | 53 | 4 | 50 | |||
| Poor | 44 | 30 | 2 | 25 | |||
| Unknown | 8 | 6 | 2 | 25 | |||
| Stage of resected patients | NS | ||||||
| IA | 3 | 2 | 0 | 0 | |||
| IB | 23 | 16 | 0 | 0 | |||
| IIA | 27 | 19 | 3 | 38 | |||
| IIB | 89 | 61 | 5 | 62 | |||
| IV | 3 | 2 | 0 | 0 | |||
| Adjuvant chemotherapy | 58 | 40 | 2 | 25 | NS | ||
| Adjuvant radiation | 40 | 28 | 2 | 25 | NS | ||
| Vital status as last follow up | NS | ||||||
| NED | 6 | 4 | 0 | 0 | |||
| AWD | 4 | 3 | 1 | 13 | |||
| DOD | 125 | 86 | 7 | 87 | |||
| DOC | 12 | 7 | 0 | 0 | |||
Abbreviations: NS, not significant; NED, no evidence of disease; AWD, alive with disease; DOD, dead as a result of disease; DOC, dead as a result of another cause.
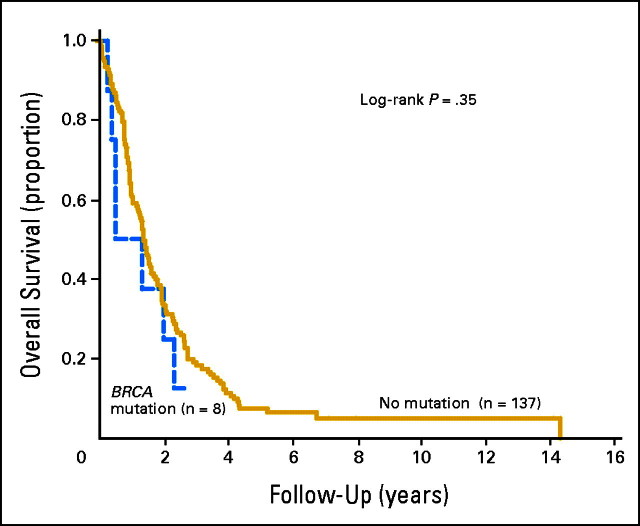
At last follow-up (median, 1.38 years; mean, 1.91), six patients had no evidence of disease, four were alive with disease, 12 dead of another cause, and 123 dead of disease. Mean and median overall survivals were 2.2 years and 1.3 years, respectively. Two- and 3-year overall survival rates were 30% and 14%, respectively. Overall survival was not significantly shorter for patients with BRCA founder mutations relative to the entire cohort of Jewish patients (median, 6 v 16 months; P = .35), and there was no significant difference in 2-year survival (32% v 25%; P = NS; Fig 1).
Fig 1.
Overall survival of patients stratified by BRCA mutation.
In this cohort of patients 24% (35 of 145) had a second primary cancer, which preceded the diagnosis of pancreatic adenocarcinoma. Of these second primaries 74% (26 of 35) were cancers previously reported to be associated with BRCA1 and BRCA2 mutations.33 The two most prevalent previous cancers were breast cancer (15%; nine of 61 women) and prostate cancer (10%; eight of 84 men). Of the eight patients with PAC associated with a documented BRCA mutation, only two had a previous second primary tumor (Table 2). Both patients had a prior breast cancer. Conversely, two of nine patients with breast cancer preceding their pancreatic cancer diagnosis had a founder mutation (22%; two of nine).
Table 2.
Characteristics of Pancreatic Adenocarcinomas Associated With BRCA Mutations
| No. | Mutation | Sex | Age (years) | Location | Stage | Status | Length of Survival (years) | Second Primary | Family History |
|---|---|---|---|---|---|---|---|---|---|
| 1 | BRCA1 185delAG | M | 78.7 | Head | IIB | DOD | 1.96 | Breast | No |
| 2 | BRCA1 5382insC | M | 64.7 | Body | IIB | DOD | 2.29 | No | No |
| 3 | BRCA2 6174delT | F | 74.4 | Head | IIB | DOD | 0.46 | Breast | Breast, endometrial |
| 4 | BRCA2 6174delT | F | 59.5 | Body | IIB | DOD | 0.48 | No | No |
| 5 | BRCA2 6174delT | F | 67.9 | Head | IIA | AWD | 2.59 | No | Prostate, gastric |
| 6 | BRCA2 6174delT | F | 6403 | Head | IIA | DOD | 1.32 | No | No |
| 7 | BRCA2 6174delT | M | 75.2 | Head | IIA | DOD | 0.21 | No | Unknown |
| 8 | BRCA2 6174delT | M | 62.4 | Head | IIB | DOD | 0.33 | No | Breast, ovarian |
Abbreviations: M, male; DOD, dead as a result of disease; F, female; AWD, alive with disease.
A family history was documented in 105 patients, of whom eight (7.6%; eight of 105) had a first-degree relative with pancreatic adenocarcinoma. None of these patients had a BRCA founder mutation. A positive family history in a first-degree relative for a BRC-associated cancer including breast, ovarian, and prostate was reported in 36 patients (34%; 36 of 105). The most prevalent cancers in a first-degree relative were breast (16%; 17 of 105), ovarian (7.6%; eight of 105), and prostate cancer (7.6%; eight of 105). Only 5.6% (two of 36) of patients with a documented family history of breast, ovarian, or prostate cancer had a founder mutation.
For individuals with a BRCA2 mutation, the estimated lifetime risk of PAC was 4.9%, corresponding to a relative risk of 3.85 (95% CI, 2.1 to 10.8; Table 3).
Table 3.
Mutations, RRs, and LRP in Patients and Controls
| Mutation | PAC (n = 145) |
Controls (n = 5,318) |
LRP | PPMUT | RR | LRC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | RR | 95% CI | |||||||||
| BRCA26174delT | 6 | 4.1 | 59 | 1.1 | 3.85 | 2.1 to 10.8 | 0.0131 | 0.011 | 3.85 | 0.049 | ||||
Abbreviations: RR, relative risk of pancreatic adenocarcinoma; PAC, pancreatic adenocarcinoma; LRP, lifetime risk of pancreatic adenocarcinoma in the general population; PPMUT, prevalence of mutations in controls; LRC, lifetime risk of pancreatic adenocarcinoma in the BRCA2 heterozygotes.
DISCUSSION
A number of studies have suggested that patients whose cancers arise as the result of an inherited predisposition may have different clinical characteristics and different prognosis from patients without an inherited predisposition with cancers of the same type of primary site. Specific design flaws compromise many of these studies. It is difficult to completely genotype unselected groups of patients with a specific type of cancer as not all subjects in a retrospective cohort are survivors at the time of genotyping, resulting in a survivor bias or Neyman bias. In prospective studies, not all patients will choose to be genotyped, introducing a selection bias. For these reasons, it is difficult to determine the impact of germline mutations on clinical outcomes. The most effective means of accurately determining the impact of germline mutations on clinical outcomes is to genotype all individuals of a subject population in whom the clinical outcomes are known. To avoid the theoretical minimal risk of harm due to inadvertent disclosure of germline status, the genotype must not be linkable to personal identifying information. This is the largest series of unselected patients with pancreatic cancer who were tested for both BRCA1 and BRCA2 mutations.
The Breast Cancer Linkage Consortium study of BRCA2 families estimated the cumulative risk of all cancers to be 32% for men by the age of 70 years, compared to 90% for women.34 BRCA1 has been reported to be associated with increases in the risk of breast,35 prostate,36 gastric, hepatobiliary, renal, testis cancers, and leukemia,37 although the strength and reproducibility of these associations has varied. BRCA2 mutations increase the risk of prostate cancer, pancreatic cancer, melanoma, gall bladder, and stomach cancer,34 again with variable reproducibility. Pancreatic cancer has been a consistent association. The Breast Cancer Linkage Consortium determined the relative risk of pancreatic cancer to be 2.26 for BRCA1 mutation carriers and 3.55 for BRCA2 mutation carriers.38 Based on the relative risk calculated in this study (3.85; 95% CI, 2.1 to 10.8), the estimated lifetime risk of pancreatic cancer for individuals with a BRCA2 mutation is 4.9%.
Approximately 1.1% of the Jewish population carries a BRCA1 founder mutation and 1.1% carries a BRCA2 founder mutation.2,39 An estimated 1.7% to 10% of patients of Jewish origin with pancreatic cancer carry a BRCA mutation (Table 4).7-10,40,41 By genotyping all subjects from the 145 patients in the study population, we identified BRCA1 mutations in 1.3% and BRCA2 mutations in 4.1% of our cohort. This study demonstrates that the 6174delT BRCA2 mutation is significantly more prevalent in Jewish patients with pancreatic adenocarcinoma compared with the general Jewish population while BRCA1 mutations are not. The sample size, however, is too small to exclude an increased risk associated with BRCA1 mutations.
Table 4.
Other Studies Evaluating BRCA Mutations in Patients With Pancreatic Adenocarcinoma
| Study | Population | BRCA1 | BRCA2 | % |
|---|---|---|---|---|
| Hahn et al7 | Familial* (n = 26 families, n = 64 individuals) | No | 4075delGT | 19 (n = 3 families) |
| 6672insT | ||||
| 6819delTG | ||||
| R2034C | ||||
| G3076E | ||||
| 10323delCins11 | ||||
| Goggins et al10 | Sporadic (n = 245) | No | (n = 4) 6174delT 6158insT | 9.8 |
| Ozcelik et al9 | Ashkenazi Jews (n = 39) | No | 4 (6174delT) | 10 |
| Murphy et al8 | Familial† (n = 29 families) | No | (n = 5) 6174delT | 17 |
| Point mutation M192T | ||||
| Two splice mutations | ||||
| This study | Jewish patients (n = 145) | (n = 2) | (n = 6) 6174delT | 5.5 |
Familial was defined as two first-degree relatives with pancreatic adenocarcinoma but do not fulfill the criteria for other familial cancer syndromes. No Ashkenazi Jewish heritage.
Families with three or more cases of pancreatic cancer with at least two cases in first-degree relatives. Six families of Ashkenazi Jewish descent.
BRCA1 and BRCA2 are involved in DNA repair, especially in double-strand breaks. BRCA protein function has been attributed to cellular regulation of DNA damage repair and gene transcription events.42 Specifically, BRCA1 is thought to serve as a scaffold in large protein ensembles that function in DNA double-strand break repair, DNA mismatch repair, gene transcription, transcription-coupled DNA damage repair, and chromatin remodeling. Similar functions have been attributed to BRCA2.36,42
Based on the preclinical data, patients with pancreatic cancer and BRCA mutations may be more responsive to certain types of chemotherapy, such as mitomycin or cisplatin. However, no clinical trials have conclusively demonstrated differential sensitivity. Results of the Charité Onkologie 01 trial demonstrate an improvement in median disease-free survival from 6.9 to 13.4 months for patients who receive adjuvant gemcitabine postresection of their pancreatic adenocarcinoma.43 Gemcitabine appears to be the only agent that demonstrates an improvement in disease-free survival postresection of pancreatic adenocarcinoma in phase III testing. In two phase III trials by Colucci et al44 and Heinemann et al45 gemcitabine alone was compared with gemcitabine plus cisplatin based on preclinical data demonstrating gemcitabine’s ability to increase cisplatin induced DNA cross-links and effective inhibition of DNA repair. Both studies demonstrated a statistically significant prolonged median progression-free survival, but neither trial demonstrated an improvement in overall survival. Although phase II data in the metastatic setting has not demonstrated a survival advantage to gemcitabine in combination with multiple other chemotherapeutic agents, patients with BRCA mutations may benefit from gemcitabine and platinum combinations to a greater degree than those without mutations, given the role of the BRCA proteins in double-strand DNA damage repair.
Preliminary data in animal models and cell lines has demonstrated that polyadenosine diphosphate ribose polymerase (PARP) inhibitors may be specifically active in tumors with BRCA mutations. PARP enzymes are involved in the repair of single strand DNA breaks, while BRCA1 and BRCA2 are important for homologous recombination. Farmer et al46 demonstrated that BRCA1 and BRCA 2–deficient cells were extremely sensitive to PARP inhibitors relative to heterozygous mutants or wild-type cells. Early clinical studies have demonstrated activity for PARP inhibitors as single agents in BRCA mutation-associated ovarian cancer.47 McCabe et al48 demonstrated that BRCA2 deficient pancreatic cancer cell lines are extremely sensitive to PARP inhibitors. Jacob et al49 demonstrated synergy between gemcitabine and PARP inhibitors when treating nude mice injected with BRCA2–deficient pancreatic cancer cells. This new class of drugs may be a therapeutic option, either alone or in combination, for patients with BRCA1 and BRCA2 deficient pancreatic cancer. Clinical trials are in development.
The strengths of this study include the analysis of Jewish patients consecutively acquired from a single institution, limiting ascertainment bias. All three mutations are clearly deleterious, and Jewish patients without one of these three mutations are unlikely to have undetected BRCA mutations based on breast and ovarian cancer data.50,51 With 5.5% of Jewish patients having a BRCA mutation there is a unique opportunity to investigate an alternative chemotherapy regimen, which may improve the survival of a population with a dismal prognosis. Even absent a direct therapeutic implication, the identification of a BRCA mutation in an individual with pancreatic cancer may have significant implications for other family members. As many mutation carriers in this series did not have a family history of typical BRCA-associated cancers, clinicians may wish to consider the diagnosis of pancreatic cancer in an Ashkenazi individual an independent reason to consider BRCA founder mutation testing.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Cristina R. Ferrone
Financial support: Murray F. Brennan
Provision of study materials or patients: Peter J. Allen, William Jarnagin, Murray F. Brennan
Collection and assembly of data: Laura H. Tang
Data analysis and interpretation: Cristina R. Ferrone, Douglas A. Levine, Kenneth Offit, Mark E. Robson
Manuscript writing: Cristina R. Ferrone, Douglas A. Levine, Murray F. Brennan, Mark E. Robson
Final approval of manuscript: Mark E. Robson
Footnotes
published online ahead of print at www.jco.org on December 8, 2008.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Thull DL, Vogel VG: Recognition and management of hereditary breast cancer syndromes. Oncologist 9:13-24, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Hartge P, Struewing JP, Wacholder S, et al: The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet 64:963-970, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robson M, Dabney MK, Rosenthal G, et al: Prevalence of recurring BRCA mutations among Ashkenazi Jewish women with breast cancer. Genet Test 1:47-51, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Robson M, Rajan P, Rosen PP, et al: BRCA-associated breast cancer: Absence of a characteristic immunophenotype. Cancer Res 58:1839-1842, 1998 [PubMed] [Google Scholar]
- 5.Robson ME, Offit K: New BRCA2 mutation in an Ashkenazi Jewish family with breast and ovarian cancer. Lancet 350:117-118, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Robson M, Gilewski T, Haas B, et al: BRCA-associated breast cancer in young women. J Clin Oncol 16:1642-1649, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Hahn SA, Greenhalf B, Ellis I, et al: BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst 95:214-221, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Murphy KM, Brune KA, Griffin C, et al: Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: Deleterious BRCA2 mutations in 17%. Cancer Res 62:3789-3793, 2002 [PubMed] [Google Scholar]
- 9.Ozcelik H, Schmocker B, Di Nicola N, et al: Germline BRCA2 6174delT mutations in Ashkenazi Jewish pancreatic cancer patients. Nat Genet 16:17-18, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Goggins M, Schutte M, Lu J, et al: Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res 56:5360-5364, 1996 [PubMed] [Google Scholar]
- 11.American Cancer Society: Facts and Figures 2007. Atlanta, GA, American Cancer Society, 2007
- 12.Winter JM, Cameron JL, Campbell KA, et al: 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg 10:1199-1210, 2006; discussion, 10:1210-1211, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Ferrone CR, Kattan MW, Tomlinson JS, et al: Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol 23:7529-7535, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrone CR, Brennan MF, Gonen M, et al: Pancreatic adenocarcinoma: The actual 5-year survivors. J Gastrointest Surg 12:701-706, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Gold EB, Goldin SB: Epidemiology of and risk factors for pancreatic cancer. Surg Oncol Clin N Am 7:67-91, 1998 [PubMed] [Google Scholar]
- 16.Kauppinen T, Partanen T, Degerth R, et al: Pancreatic cancer and occupational exposures. Epidemiology 6:498-502, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Mack TM, Yu MC, Hanisch R, et al: Pancreas cancer and smoking, beverage consumption, and past medical history. J Natl Cancer Inst 76:49-60, 1986 [PubMed] [Google Scholar]
- 18.Bansal P, Sonnenberg A: Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology 109:247-251, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Everhart J, Wright D: Diabetes mellitus as a risk factor for pancreatic cancer: A meta-analysis. JAMA 273:1605-1609, 1995 [PubMed] [Google Scholar]
- 20.Lynch HT, Lanspa SJ, Fitzgibbons RJ Jr, et al: Familial pancreatic cancer (part 1): Genetic pathology review. Nebr Med J 74:109-112, 1989 [PubMed] [Google Scholar]
- 21.Silverman DT, Schiffman M, Everhart J, et al: Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer 80:1830-1837, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergman W, Watson P, de Jong J, et al: Systemic cancer and the FAMMM syndrome. Br J Cancer 61:932-936, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein AM, Fraser MC, Struewing JP, et al: Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med 333:970-974, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Bronner CE, Baker SM, Morrison PT, et al: Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 368:258-261, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Fishel R, Lescoe MK, Rao MR, et al: The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 75:1027-1038, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Tulinius H, Olafsdottir GH, Sigvaldason H, et al: Neoplastic diseases in families of breast cancer patients. J Med Genet 31:618-621, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonin P, Serova O, Simard J, et al: The gene for hereditary breast-ovarian cancer, BRCA1, maps distal to EDH17B2 in chromosome region 17q12-q21. Hum Mol Genet 3:1679-1682, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Tonin P, Serova O, Lenoir G, et al: BRCA1 mutations in Ashkenazi Jewish women. Am J Hum Genet 57:189, 1995 [PMC free article] [PubMed] [Google Scholar]
- 29.Simard J, Tonin P, Durocher F, et al: Common origins of BRCA1 mutations in Canadian breast and ovarian cancer families. Nat Genet 8:392-398, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Levine DA, Argenta PA, Yee CJ, et al: Fallopian tube and primary peritoneal carcinomas associated with BRCA mutations. J Clin Oncol 21:4222-4227, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Satagopan JM, Offit K, Foulkes W, et al: The lifetime risks of breast cancer in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev 10:467-473, 2001 [PubMed] [Google Scholar]
- 32.Satagopan JM, Boyd J, Kauff ND, et al: Ovarian cancer risk in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Clin Cancer Res 8:3776-3781, 2002 [PubMed] [Google Scholar]
- 33.Struewing JP, Hartge P, Wacholder S, et al: The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 336:1401-1408, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Cancer risks in BRCA2 mutation carriers: The Breast Cancer Linkage Consortium. J Natl Cancer Inst 91:1310-1316, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Brose MS, Rebbeck TR, Calzone KA, et al: Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst 94:1365-1372, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Thompson D, Easton DF: Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst 94:1358-1365, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Offit K: BRCA mutation frequency and penetrance: New data, old debate. J Natl Cancer Inst 98:1675-1677, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Lynch HT, Brand RE, Deters CA, et al: Hereditary pancreatic cancer. Pancreatology 1:466-471, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Struewing JP, Abeliovich D, Peretz T, et al: The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet 11:198-200, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Real FX, Malats N, Lesca G, et al: Family history of cancer and germline BRCA2 mutations in sporadic exocrine pancreatic cancer. Gut 50:653-657, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lal G, Liu G, Schmocker B, et al: Inherited predisposition to pancreatic adenocarcinoma: Role of family history and germ-line p16, BRCA1, and BRCA2 mutations. Cancer Res 60:409-416, 2000 [PubMed] [Google Scholar]
- 42.Venkitaraman AR: Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108:171-182, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Oettle H, Post S, Neuhaus P, et al: Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 297:267-277, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Colucci G, Giuliani F, Gebbia V, et al: Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: A prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer 94:902-910, 2002 [PubMed] [Google Scholar]
- 45.Heinemann V, Quietzsch D, Gieseler F, et al: Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 24:3946-3952, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Farmer H, McCabe N, Lord CJ, et al: Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434:917-921, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Fong PC, Boss DS, Carden CP, et al. AZD2281 (KU-0059436), a PARP (poly ADP-ribose polymerase) inhibitor with single agent anticancer activity in patients with BRCA deficient ovarian cancer: Results from a phase I study. J Clin Oncol 26;295s, 2008. (suppl; abstr 5510) [Google Scholar]
- 48.McCabe N, Lord CJ, Tutt AN, et al: BRCA2-deficient CAPAN-1 cells are extremely sensitive to the inhibition of Poly (ADP-Ribose) polymerase: An issue of potency. Cancer Biol Ther 4:934-936, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Jacob DA, Bahra M, Langrehr JM, et al: Combination therapy of poly (ADP-ribose) polymerase inhibitor 3-aminobenzamide and gemcitabine shows strong antitumor activity in pancreatic cancer cells. J Gastroenterol Hepatol 22:738-748, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Boyd J, Sonoda Y, Federici MG, et al: Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA 283:2260-2265, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Shattuck-Eidens D, Oliphant A, McClure M, et al: BRCA1 sequence analysis in women at high risk for susceptibility mutations: Risk factor analysis and implications for genetic testing. JAMA 278:1242-1250, 1997 [PubMed] [Google Scholar]