Abstract
The mammalian hippocampus processes sensory information into memory. The neurobiological basis of this representation, as well as the type of information that is encoded, is central to understanding how memories are formed. Normally, there is an infinite amount of information that could be encoded for any given stimulus. Thus, the question arises as to how the hippocampus selects and encodes features of a given stimulus. Here, we show that neurons in the hippocampus of the monkey appear to categorize types of visual stimuli presented in a delayed-match-to-sample memory task. By extracting unique combinations of features, these category cells are able to encode aspects of behaviorally important images instead of encoding all visual details. The subject is then able to rapidly select an appropriate response to that stimulus when distracting stimuli are presented simultaneously, thereby facilitating performance. Moreover, across animals, this specific type of encoding differed considerably. Just as in humans, different monkeys attended to and selected different aspects of the same stimulus image, most likely reflecting different histories, strategies, and expectations residing within individual hippocampal networks.
It has been known for some time that damage to the hippocampus and related structures produces memory deficits in humans (1-5) and animals (6-9). Degeneration of the hippocampus, such as in Alzheimer's disease, renders individuals unable to function in a normal environment due to memory impairments (10, 11). One of the intriguing questions regarding hippocampal function is how information is actually processed by cells within that structure to encode, retain, and retrieve memories (12-15). In this paper, we show that hippocampal neurons in monkeys exhibit activity consistent with a role in encoding task-relevant information. Instead of encoding a mere verbatim representation of individual sensory elements (16-20), we demonstrate that hippocampal neurons may have the capacity to respond to categories of stimulus features across different task-related images, in a manner that facilitates performance under conditions where potential information content is excessive. Whereas such responses could also represent high-level feature detection by these cells, the fact remains that the number of images to which these neurons responded ranged across a wide spectrum, and several cells in different monkeys responded in the same manner to the same collection of images. Thus, it is indeterminate as to whether hippocampal neurons encode events by means of a compilation of stimulus elements that occur in different images or through a scheme in which those elements all represent some aspect of the same conceptual theme; i.e., a category, irrespective of the physical similarity between elements.
The ability to categorize information is a highly efficient process because it reduces the number of items that must be retained for later recall, and to some extent defines how humans resolve memory challenges (21-23). An important characteristic of such perceptual categorization is the equivalent encoding of all stimuli within a category, but a sharp boundary between categories (24-26). Thus, category encoding not only suggests facilitated performance when selecting from different stimuli but also implies that performance may be impaired if stimuli that share features of the category must be discriminated. Our findings indicate that both processes occur in the hippocampus of monkeys performing a visual delayed-match-to-sample (DMS) task, depending on the number of images presented and the likelihood that stimuli from different categories may overlap. Interestingly, these results show that the classification schemes determined to be present in individual hippocampal neurons may also be specific to individual subjects, because in some cases the same stimuli were classified differently by different monkeys. Hence, individual classification strategies appear to determine the information that is represented by hippocampal neurons and therefore the accuracy and/or relevance of that representation for different task demands (14, 27, 28).
Materials and Methods
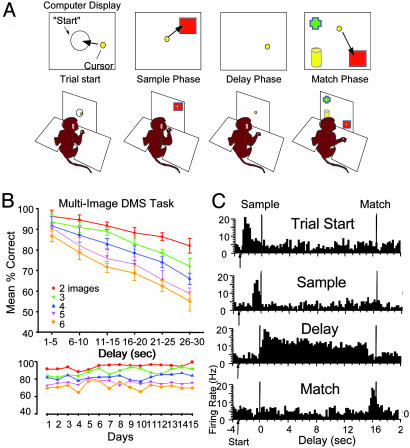
Behavioral Training. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee, in accordance with U.S. Department of Agriculture, American Association of Laboratory Animal Care, and National Institutes of Health guidelines. Four adult male rhesus monkeys (Macaca mulatta) were trained to sit in a primate chair and perform a multiobject visual DMS task by moving a cursor on a computer display projected in front of the animal (Fig. 1A). The cursor was controlled by the monkey's hand movements. Each trial consisted of four successive phases: a Trial-Start (target), a Sample (image presentation), a Delay (blank screen), and a Match (multiimage display) phase. Trials were initiated by the monkey moving the computer cursor into a start target centered on the screen, which then produced the Sample image consisting of randomly selected Internet clip art (see Fig. 2). Placement of the cursor into the Sample image (200-300 msec) then blanked the screen for a variable Delay interval of 1-30 sec interposed between the Sample and Match phases. After the delay interval timed out, the Match phase was presented, consisting of two to six images, only one of which was the Sample image; the other (one to five) images were nonmatch or distractor images. Selection of the match image delivered 0.5 ml of fruit juice to the monkey through a sipper tube. All images (Sample, Match, and distractors) were presented randomly in one of nine positions on the display screen; in the Match phase, the match image was never placed in the same position as in the Sample phase. None of the clip-art images presented on a trial were repeated within a session, all were trial-unique. Clip-art images were used because their distinctive features (e.g., faces, people, photographs, cartoons, animals, toys, colors, etc.) and abundance prevented transference of outcomes across trials due to past associations. Approximately 500-600 unique images per session were chosen randomly from a collection of >5,000 different clip-art images. Trials occurred every 3-10 sec and totaled 150-200 trials per session.
Fig. 1.
(A) Illustration of visual DMS task: (i) Trial-start display (circle) for initiation of trial; (ii) presentation of Sample image; (iii) Interposed 1-30 sec Delay, screen blanked; and (iv) Match phase presentation of Sample image (square) plus 1-5 nonmatch distractor images (e.g., cross and cylinder). (B Upper) DMS performance curves (mean ± SEM, percent correct responses) plotted as a function of duration of the Delay phase and number of images (Sample plus distractors) in the Match phase (n = 4 monkeys). (Lower) Mean correct responses per day sorted by number of images per trial in the Match phase over 15 consecutive DMS sessions for one monkey. (C) Examples of task-relevant firing of four different hippocampal cell types recorded during performance of the DMS task. TBHs of single-neuron activity were summed over 150 trials for the four hippocampal cell types (Trial Start, Sample, Delay, and Match cells) to show specific firing patterns within the DMS task. Vertical time marks in TBH reflect the mean latency from start of trial (arrow) to Sample- and Match-phase responses and demarcate Sample, Delay, and Match phases.
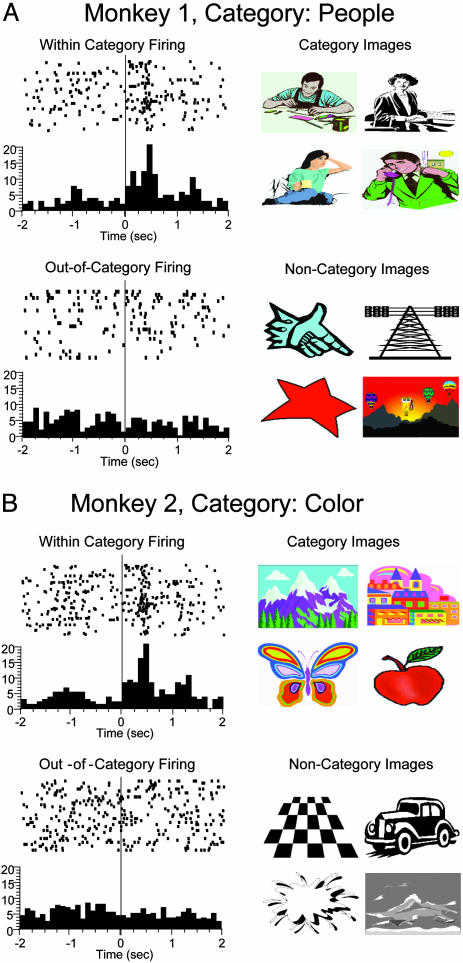
Fig. 2.
Examples of category cells recorded from the hippocampus in two different monkeys. Identification of category cells was determined by sorting trials with images that produced significantly increased (z >3.09) firing rates. Rastergrams (Left) show individual cell discharges to Sample image. Each dot indicates when the neuron fired, synchronized to Sample image presentation (time = 0 sec); each row represents one trial. Histograms (beneath) show normalized firing rates across trials with different images sorted independently, according to the presence or absence of significant cell firing peaks. (Right) Examples of Sample images associated with trial sort procedure. (A) Monkey 1: category cell that increased firing (within-category firing) to images of people (category images), but not (out-of-category firing) to other objects, shapes, or colors (noncategory images). (B) Monkey 2: category cell that showed increased discharge to a wide assortment of color images (category images), but not to black/white images (noncategory images). Note that trials shown in rasters (i.e., rows) were sorted according to the above procedures and were not necessarily successive during the 150-trial session.
Surgery. All procedures were performed under sterile conditions. Animals were sedated with ketamine (10 mg/kg), were intubated, and were maintained throughout surgery with isoflurane (1-3% to effect) and oxygen. Craniotomy sites were selected to overlie the stereotaxic coordinates of the hippocampus as certified from an atlas (29) and normalized by individual MRIs for each monkey. An access cylinder (Crist Instruments) was placed on the skull above the craniotomy sites to allow daily placement of microelectrodes into the brain. The cylinder was then fixed to the skull with screws and dental cement. Each animal received antibiotics (cefazolin, 25 mg/kg) for 7 days after surgery, and acetaminophen (10 mg/kg) and/or buprenorphine (0.7 mg/kg) as needed for pain. Animals were allowed to recover from the procedure for 5-15 days before resuming training.
Electrode Positioning and Recording. At the beginning of daily experiments, the recording cylinder was opened, cleaned, and disinfected. Recording electrodes (etched 125-μm tungsten wires, 5-μm tip diameter) were introduced and were slowly advanced into the brain (1-2 mm/min) to the appropriate depth for hippocampal recording (30-35 mm ventral to top of brain), while neural activity was monitored to determine final positioning. Once the electrode was positioned in a hippocampal cell layer (CA1 or CA3), as determined by cell discharge characteristics and MRI-assisted depth coordinates, cells were isolated and recording commenced over a 90- to 100-min behavioral session. After completion of the recording session, the electrodes were removed, and the cylinder was disinfected and sealed.
Single-neuron waveforms were discriminated and analyzed with a multineuron acquisition processor and neuroexplorer software (Plexon). Perievent histograms, trial-based histograms (TBHs), and rastergrams of hippocampal neuron activity were constructed for each of the four phases of the task. Standard scores [z = (peak firing rate - baseline firing rate)/SD of baseline firing rate] were calculated for each event. Cell types were identified by the presence (or absence) of significant (z >3.09, P < 0.001) firing peaks in the perivent histograms derived from specific phases of the task. Individual Sample, Match, and distractor images were also examined to determine the common features of the images that significantly increased firing rate of neurons during the session (see Table 2 and Figs. 5-7, which are published as supporting information on the PNAS web site).
Results
Results from the four monkeys trained in the multiobject visual DMS task are shown in Fig. 1B. Performance on the task varied consistently with differences in: (i) the duration of the delay between Sample and Match choices, and (ii) the number of distractor images appearing in the Match phase. Fig. 1B shows that performance was severely affected by these two variables [F(28, 872) = 4.82, P < 0.001] specifically, duration of delay [F(5, 872) = 11.31, P < 0.001] and number of distractor images [F(4, 872) = 7.18, P < 0.001], but each variable influenced performance independent of the other [delay × image interaction: F(1, 872) = 1.98, P = 0.16].
Single-neuron activity was recorded from one to three presumed hippocampal pyramidal cells per session in two different monkeys performing the DMS task. Two other monkeys were tested behaviorally on the same task, but hippocampal recording was not conducted. Fig. 1C shows the representative TBHs of four different functional hippocampal cell types identified during performance of the DMS task (Table 1). Each type of neuron showed significantly increased firing during a different phase of the task (Trial-Start, Sample, Delay, and/or Match), which agrees with prior findings in rodents performing spatial delayed-nonmatch-to-sample tasks (12, 19, 30). The specific correlates of these four cell types were quite distinct. Trial-Start cells showed increased firing [mean baseline: 3.2 ± 0.4 Hz; mean peak: 18.3 ± 1.1 Hz; F(1, 1,531) = 13.9, P < 0.001] only when the trial-start stimulus was presented (Fig. 1). Increased firing occurred in Sample [baseline: 2.2 ± 0.3 Hz; peak: 17.6 ± 0.9 Hz; F(1, 1,531) = 18.4, P < 0.001], and Match phase cells [baseline: 3.5 ± 0.6 Hz; peak: 16.2 ± 0.7 Hz; F(1, 1,531) = 15.6, P < 0.001] to images that occurred in their respective phases of the task, but not when the image appeared in other phases (Fig. 1C). Delay cells showed increased discharge only during the variable delay interval (31) when the screen was blanked, irrespective of the stimulus image on that trial [baseline: 2.9 ± 0.2 Hz; peak: 19.2 ± 0.7 Hz; F(1, 1,531) = 22.1, P < 0.001]. These four types of hippocampal neurons comprised the most frequently encountered cells (59% and 54% of total cells) in each monkey (Table 1).
Table 1. No. of identified hippocampal cells.
| DMS task correlate | Monkey 1 | Monkey 2 |
|---|---|---|
| Trial start | 15 | 11 |
| Sample phase | 24 | 27 |
| Match phase | 17 | 19 |
| Delay interval | 16 | 11 |
| Category (image) | 23 | 26 |
| Unclassified | 28 | 31 |
| Total cells | n = 123 | n = 125 |
Devices for eye and hand tracking confirmed that firing of Sample- and Match-phase neurons in the above classification was synchronized to image presentation and not to completion of cursor movement in the target image (Fig. 1C). In addition, firing of these neurons occurred in the appropriate phase and was independent of image location on the screen. The vertical lines in the TBHs in Fig. 1C signify occurrence of task-relevant responses during the trial. The functional relevance of the above demarcation of Sample- and Match-cell-firing tendencies was analyzed by sorting and grouping trials by binned firing rates at 0.5-Hz resolution, then by determining the percentage of correct trials within each 0.5-Hz bin. A correlation was then calculated between percentage of correct trials within a bin and the median rate of that bin over a firing rate range of 2-20 Hz (baseline to maximum). A strong positive correlation [r2 = 0.639, F(1, 1,531) = 16.71, P < 0.001] indicated that correct behavioral performance was in fact consistently associated with high firing rates in these two cell types during the trial.
Hippocampal Neurons Encode Categories of Image Stimuli. The above four cell types provided a coherent segregation of the phases of the DMS task within hippocampal ensembles (12, 19). However, because the stimulus images to which animals responded were different on each trial, none of the above four neuron types could by themselves, or even together, accomplish the objective of providing the match-to-sample information required to perform the task correctly on a particular trial. To obtain the level of performance shown in Fig. 1B, the encoding and retrieval of some unique feature of the Sample image was required on each trial.
A major insight into how the animals achieved this requirement was provided by the discovery of a fifth type of hippocampal neuron that responded selectively to certain types of images presented on various trials within a session. These cells did not fire above background levels in the Delay phase or at any time when a trial was not presented. The majority of these cells showed increased firing relative to background in the Sample [mean baseline: 3.3 ± 0.2 Hz; mean peak: 21.4 ± 0.8 Hz, F(1, 1,531) = 25.5, P < 0.001] and Match [mean baseline: 3.3 ± 0.2 Hz; mean peak: 18.8 ± 0.9 Hz F(1, 1,531) = 20.6, P < 0.001] phases on a select number of trials during the 150-trial session (mean = 22.6 ± 1.1 trials per session, range = 12-34 trials, n = 49 cells). More importantly, it was discovered that the images which elicited increased firing of these cells on different trials, even though clearly not the same with respect to overall appearance, had common visual features that could be classified within broad but distinct groups or categories; i.e., people, colors, objects, etc. The fact that the session consisted of exposure to large numbers of clip-art images (500-600) allowed a sufficient sampling of trials to determine the particular image categories for individual cells. Independent sorts of these images revealed the individual categorization schemes for each cell. Examples of these category cells recorded in each monkey are shown in Fig. 2 (see also Table 2 and Figs. 5-7). For the cell shown in Monkey 1, the category was people vs. other objects (Fig. 2A), and for the cell shown in Monkey 2, a distinction between color vs. black/white images (Fig. 2B) was the appropriate category that increased firing. The rate of category cell firing did not differ with respect to the Sample or Match phases of the task under normal testing conditions [F(1, 1,531) = 2.2, P < 0.14]; however, firing of these cells was significantly greater if the trial was correct compared to when it was an error [mean correct = 16.9 ± 0.5 Hz; mean error 6.3 ± 0.6 Hz; F(1, 1,531) = 14.99, P < 0.001].
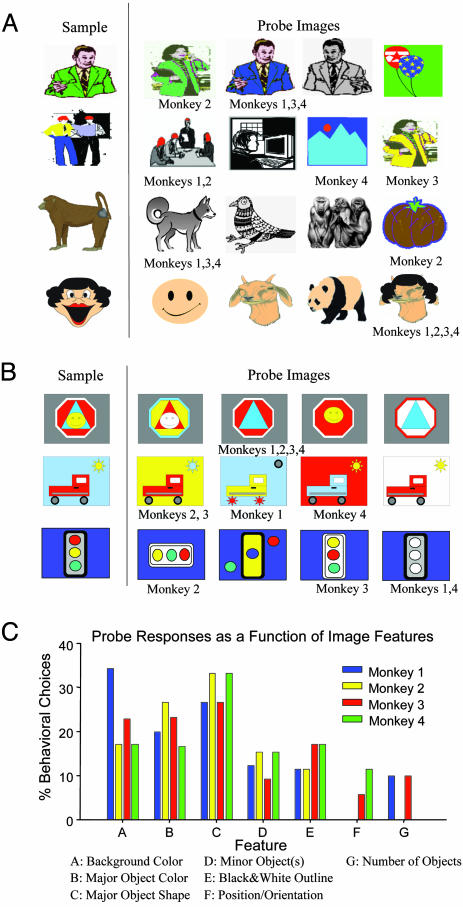
Manipulation of Image Features Reveals Different Categorization Strategies. Because the firing of category cells appeared to reflect particular elements of the Sample image, a direct manipulation of image features was used to determine: (i) whether particular features that fit selected categories were sufficient to significantly alter firing in these cells, and/or (ii) if the features responded to were behaviorally relevant. In addition, we determined whether a given Sample image was classified by the same or different features in each of four monkeys. To accomplish this goal, images which had previously elicited category cell firing in normal test sessions were used to construct a subset of probe trials consisting of morphed versions of the Sample image that were then presented in the Match phase. On the probe trials, none of the four probe images was an exact replica of the Sample image; however, each morphed image did contain at least one distinct feature of the Sample image. The monkey was therefore forced to select one of the four probe images as a match to the Sample image on probe trials, and any choice in the Match phase was rewarded. Fig. 3A shows examples of the Sample and probe images as presented on representative trials (each row). The image selected by each monkey is indicated below the four possible probe images for that trial. Probe trials occurred infrequently, once per 10-20 normal trials within a session, and a given probe was only presented once per session.
Fig. 3.
Probe trials reveal different encoding of image features across all four monkeys. (A) Four different probe trials in which Match phase images were morphed, or changed from a Sample image that previously fired a category cell. None of the four morphed probe images were exact replicas of Sample images; however, monkeys were rewarded for responses to any Match-phase image on probe trials. The respective selections by each of the four monkeys are indicated below each morphed probe image. (B) Three probe trials consisting of images that were not clip art, but were constructed with elements (features) that could be mixed to provide different combinations of the Sample-image elements. As in A, image selections by each monkey are indicated below the respective probe images on each trial (row). (C) Choice profiles determined for each monkey from the features in each chosen probe image. Profiles were constructed from response choices of 100 different types of probe images as in A and B. Each bar represents the percentage of trials in which a probe image with the indicated feature (A-G, below) was selected in the Match phase. This selection was normalized by the total number of presentations of that type of probe to all monkeys. Feature classification was performed blind by three staff members shown probe images and asked to sort them according to the seven features listed as A-G above.
Two important outcomes were immediately apparent from these results: (i) all monkeys responded selectively to the morphed probe images in the Match phase; however, (ii) the probe image selected was not necessarily the same for all monkeys. The top row in Fig. 3A shows that one monkey chose the green coat feature of the Sample image of a man, even though it was illustrated on a woman in a different posture. The other three monkeys all chose an image with the feature of the man, but with a blue instead of green coat. In rows 2 and 3, monkeys also chose differently, but this time with respect to different categories (red dots vs. people, four-legged animal vs. brown color, etc.). Row 4 (Fig. 3A) shows a probe trial where all monkeys selected the image with a distinct feature (hair) that was in the Sample image. The probe trials indicate that just as category cell firing was specific to individual Sample image features, individual monkeys' behavioral selections among probe images likely reflected different ways of categorizing features of the same Sample image.
We next examined how similar the individual features of the Sample and probe images could be to each other, and still be classified as different in the task. Therefore, a second set of specifically constructed probe images was designed with features that could be systematically recombined to retain a high degree of similarity to the Sample image. These images, shown in Fig. 3B, were less familiar to the monkeys because they were not clip art. This type of probe trial was presented with the same low frequency during the session as clip-art probe trials. In the top row of Fig. 3B it is clear that all monkeys chose the stop sign-triangle probe (second probe image, row 1) as representative of the Sample image, whereas in rows 2 and 3, different monkeys again chose different probe images.
To quantify differences in category selection, individual image features for all probe trials (clip-art and reconstructed images) were sorted blind by three randomly selected observers into separate predetermined classifications consisting of the seven major attributes (features) shown in Fig. 3C. The choice profile of each monkey was determined by scoring trial selections on the basis of these seven putative image features. Whereas there was some consistency of selection (i.e., feature C), features chosen for a given Sample image differed significantly across monkeys [F(3, 395) = 5.72, P < 0.001], indicating that different monkeys seeing the same Sample images tended to categorize them differently. All four monkeys were exposed to the same 100 probe trials a second time after at least five normal sessions intervened, and on 98% of the trials they selected the same morphed images as in the original test session. The latter finding indicates that the monkeys did not choose the morphed probe images randomly even though they were forced to respond in the Match phase.
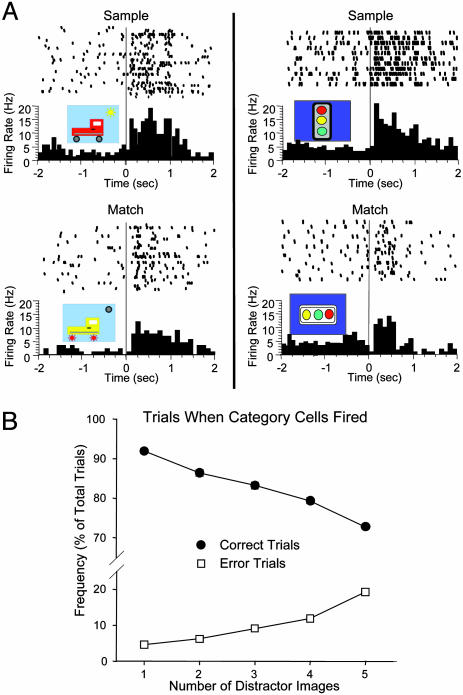
Category Cell Firing and Behavioral Errors. To assess the relevance to performance, some category cells (n = 13) were recorded on probe trials. Fig. 4A shows examples of category cell firing to different probe trials within the same session. The rastergrams and histograms indicate firing to the Sample image (Upper) and to the morphed Match image (Lower) across different trials within a session for two different category cells. Each line of the raster represents a different trial with unique probe images. One example of a Sample and morphed probe image is presented for each cell (first trial in rastergram). Once a category was tentatively identified to which a given cell showed increased firing, half of the probe trials within the session were selected for suspected features contained in that category to test and validate the cells' selectivity (Fig. 4A). Across all category cells tested in this manner, firing rates to morphed probe images in the Match phase were significantly elevated [mean baseline = 2.5 ± 0.6 Hz; mean morphed image peak = 12.4 ± 0.5 Hz; F(1, 34) = 16.22, P < 0.001], but was diminished relative to the original Sample image firing on the same trial [mean Sample image peak = 16.7 ± 0.8 Hz; vs. morphed image peak, F(1, 34) = 8.13, P < 0.01].
Fig. 4.
Reduced firing of two category cells to morphed probe images. (A) Perievent histograms and rastergrams from two different category cells recorded in the same monkey depict probe trials on which Sample images (Upper) evoked firing that was reduced in response to presentation of the morphed probe image in the Match phase (Lower) of the same trial. Each row (trial) in the rastergram represents firing in response to a different Sample (Upper) or morphed (Lower) probe image within the same session. (Insets) The images presented in the first trial (top row) of each rastergram are shown: In all circumstances, firing to the morphed probe image (Lower) was reduced, compared with the Sample image (Upper) for both cells. (B) Relationship between category cell firing and performance as a function of number of distractor images. Standard (nonprobe) trials on which category cells fired (n = 1,200 trials, 49 cells) were sorted according to the number of distractor images presented and whether the trials were correct or errors. Each point represents the frequency of correct (•) or error (□) trials plotted as a percentage of the total trials on which category cells showed significantly increased firing. Performance decreased as the number of distractor clip-art images increased on trials in which category cells fired.
Such reduced firing to probe images suggests a possible functional basis for elevated category cell firing on correct vs. error trials (see above). For example, in normal testing, if distractor images (nonmatch) happened to contain similar features to the Sample image (Fig. 4A), could such similarities influence category cell firing and behavioral performance? On average, a given category cell showed increased firing on 15.1 ± 0.7% of total trials in the session (see above). This percentage was comprised of 11.6 ± 0.9% correct trials, and 3.5 ± 0.3% error trials. It is interesting that on these error trials, (i) category cell firing was either reduced or absent during the Match phase, or (ii) the category cell fired inappropriately to a distractor image. Moreover, on trials in which a category cell fired and there were only two images presented in the Match phase, performance was correct 91.9 ± 0.5% of the time (Fig. 4B), but performance decreased significantly to 72.7 ± 0.6% [F(4, 193) = 5.32, P < 0.001] on trials in which five distractor images were presented (Fig. 4B, correct curve). Thus, it is possible that category cells which encoded Sample features were susceptible to a higher likelihood of firing to distractor images as the number of features (images) presented was increased (Fig. 1B). This result was substantiated by the fact that the number of error trials on which category cell firing occurred increased proportionately with trials that had an increased number of distractor images (Fig. 4B, errors). Finally, a separate analysis of the data in Fig. 3C revealed that 47.4 ± 3.7% of error trials consisted of choices of distractor images that shared at least one feature with the Sample image.
Discussion
Because Trial-Start, Sample, Delay, and Match hippocampal cell types were recorded abundantly in both monkeys (Table 1), it is likely that these four cell types provided a representation of the temporal structure of the DMS task within which the described category cells could operate. The DMS task appeared to be partitioned by hippocampal neurons into separate encoding domains (phases), each providing the necessary binding of contextual stimuli to: (i) delineate different task phases (Sample, Delay, and Match cells), and (ii) identify task-relevant image content when it reappeared after the delay interval (category cells). That such classification was critical to the task was indicated by: (i) the inverse relationship between performance accuracy and duration of the delay, and (ii) the increased difficulty imposed by larger numbers of distractor images (Figs. 1B and 4B). The existence of category cells, therefore, would appear to be an attempt to limit interference by other combinations or groupings of stimulus features (21, 32), as shown by the relationship shown in Fig. 4B, indicating that on average, 82.7% of trials were correct when category cells showed significantly increased firing.
To be effective, category cells must have a broad enough range to share common features with images that were presented on different trials, but a sharp boundary between categories to exclude images with similar but inappropriate physical characteristics (25, 26). Therefore, all stimuli within a category would be encoded the same by a given category neuron, but images outside of the category would not be encoded. Thus, from a behavioral standpoint, this type of encoding was only effective with small numbers of distractor images, because with larger numbers, the risk of selecting a different image from the same category increased (Fig. 4B). However, the alternative strategy of attempting to precisely encode all of the visual features of each Sample image during the session would be less effective than categorization because all visually specific Sample images appeared only once during the session. Unique to this study is the observation that hippocampal cells encoded images in terms of inherent features, or categories with dimensional segregation. Behavioral testing revealed that such categories were used by all four monkeys. It is possible that hippocampal category cell firing reflected the end result of associative neural networks individually trained to pattern complete and detect specific images when only a small number of features are present (27, 33, 34). In this context, it is difficult to distinguish between true category encoding and visual tuning to image similarities. On standard trials, the Match phase typically contained distractor images with quite different features; hence, category encoding could quickly eliminate distractor images (Fig. 1B). The fact that there were instances in which the same probe images were chosen by all four monkeys (Fig. 3A, row 4) illustrates the utility of the categorization scheme. However, when the Sample image features were morphed to mix features across different images on probe trials (e.g., Fig. 3 A and B), different animals exhibited significantly different feature selection profiles (Fig. 3C). Such disparity between monkeys suggests that the hippocampal networks established for this purpose (27) may have been quite different in each monkey. This divergence argues against visual tuning as the mechanism of category cell firing because the Sample image was the same for each monkey, but there were many different probe image selections, whereas visual tuning with respect to image similarity should have resulted in nearly the same choices by all monkeys.
Evidently monkeys, like humans (21-23, 32, 35), recall different things if asked to describe relevant features of a given stimulus image (Fig. 3). Clearly, such encoding interacts intimately with attentional processes (36), which through learning (14) may be largely responsible for developing category cell firing in the DMS task. Category-cell encoding provides an advantage by eliminating firing of other hippocampal neurons to distractor images that have few or no common features with the Sample image. Because category neurons in this task were driven by Sample- and Match-phase image presentations, they were not susceptible to the effects of the interposed delay. They therefore provided a strong basis for satisfying the match-to-sample contingency across different trials within the same session.
Category neurons have been identified in other brain regions (25, 37-39). In an elegant study, Freedman et al. (24) showed that deliberate morphing of visual stimuli from one distinct category to another differentially activated prefrontal cortical neurons, which encoded specific categories. In that report, neurons fired robustly when the stimulus image could be classified in one category, but less robustly when the image was morphed to contain fewer original features, which was similar to the effect shown in Fig. 4A. Likewise, Matsumoto et al. (37) demonstrated that prefrontal cortical neurons encoded not just the category, but the complete visual, motor, and reinforcement context (i.e., the trial type) associated with a given stimulus. Wirth et al. (14) recently identified hippocampal neurons in the monkey in which firing to specific scenes correlated with the acquisition of a trained response. The property of hippocampal neurons to selectively encode features of visual stimuli has also been reported (32, 33, 40-42).
The properties of hippocampal category cells reported here complement and extend the above reports. First, the fact that category cell firing correlated with performance (Fig. 4) demonstrates that stimulus feature categorization is an important learned, or acquired mechanism (14). Second, individual differences with respect to probe-trial responses (Fig. 3) indicate that the specific type of categorization by hippocampal neurons, although task-driven, may vary across individuals (21, 22, 33). Third, in agreement with Freedman et al. (24), diminished firing of category cells to morphed images (Fig. 4A) indicates that the firing rate of category cells may be an important metric of how well a given image fits the criteria of the category. Finally, the correlation between firing tendencies of category cells and errors on trials with increased numbers of distractor images (Fig. 4B), reveals a well known weakness of such categorization schemes (21, 22), i.e., that features of a given category can be present in more than one image from which to choose.
The demonstration that cells in prefrontal and temporal cortex, and now in hippocampus, retain the capacity to categorize stimuli implies that such encoding may in fact be the expression of synergistic synaptic processes across different brain regions (43, 44). Such simultaneous activation may be required for the accurate extraction and retention of information necessary for accurate performance in recognition type tasks (14, 27, 39). Firing of category neurons in hippocampus may herald the presence of items that are similar to past events and therefore, through prior experience, have a high probability of being significant to the individual (34). Indeed, the presence of such neurons may be critical for the ability to detect and/or encode critical features present in ambiguous stimuli. Finally, we have shown that what is categorized by these hippocampal neurons with respect to past events differs markedly among individual subjects. Even so, such individual encoding differences appear to be equally effective as long as the events they represent exhibit those encoded features on future occasions.
Supplementary Material
Acknowledgments
We thank Charles West, Stephanie Hodge, Ashley Morgan, Santos Ramirez, Christopher K. Craig, Lucy Fasano, Terry Bunn, and Michael Todd for their assistance on this project. This work was supported by National Institutes of Health Grants MH61397 (to R.E.H.), DA06634, and DA00119, Defense Advanced Research Projects Agency Contracts N66001-02-C-8058 (Space and Naval Warfare Systems Command) and DAAD19-02-1-0060 (Army Research Office; to S.A.D.) and NS048106 (to T.P.P.).
Abbreviations: DMS, delayed-match-to-sample; TBH, trial-based histogram.
References
- 1.Maguire, E. A. (2001) Rev. Neurol. (Paris) 157, 791-794. [PubMed] [Google Scholar]
- 2.Squire, L. R. & Zola, S. M. (1997) Philos. Trans. R. Soc. London B 352, 1663-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scoville, W. B. & Milner, B. (1957) J. Neurol. Neurosurg. Pysciatry 20, 11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, N. J., Ryan, J., Hunt, C., Romine, L., Wszalek, T. & Nash, C. (1999) Hippocampus 9, 83-98. [DOI] [PubMed] [Google Scholar]
- 5.Mishkin, M., Vargha-Khadem, F. & Gadian, D. G. (1998) Hippocampus 8, 212-216. [DOI] [PubMed] [Google Scholar]
- 6.Murray, E. A., Baxter, M. G. & Gaffan, D. (1998) Behav. Neurosci. 112, 1291-1303. [DOI] [PubMed] [Google Scholar]
- 7.Zola, S. M. & Squire, L. R. (2001) Hippocampus 11, 92-98. [DOI] [PubMed] [Google Scholar]
- 8.Hampson, R. E., Jarrard, L. E. & Deadwyler, S. A. (1999) J. Neurosci. 19, 1492-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarrard, L. E. (2001) Hippocampus 11, 43-49. [DOI] [PubMed] [Google Scholar]
- 10.Perry, R. J. & Hodges, J. R. (1996) Curr. Opin. Neurol. 9, 281-285. [DOI] [PubMed] [Google Scholar]
- 11.Markowitsch, H. J. & Pritzel, M. (1985) Prog. Neurobiol. 25, 189-287. [DOI] [PubMed] [Google Scholar]
- 12.Hampson, R. E., Simeral, J. D. & Deadwyler, S. A. (1999) Nature 402, 610-614. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro, M. L. & Eichenbaum, H. (1999) Hippocampus 9, 365-384. [DOI] [PubMed] [Google Scholar]
- 14.Wirth, S., Yanike, M., Frank, L. M., Smith, A. C., Brown, E. N. & Suzuki, W. A. (2003) Science 300, 1578-1581. [DOI] [PubMed] [Google Scholar]
- 15.Ono, T. & Nishijo, H. (1999) Hippocampus 9, 458-466. [DOI] [PubMed] [Google Scholar]
- 16.Murray, E. A. & Mishkin, M. (1998) J. Neurosci. 18, 6568-6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squire, L. R. & Zola, S. M. (1998) Hippocampus 8, 205-211. [DOI] [PubMed] [Google Scholar]
- 18.Rudy, J. W. & Sutherland, R. J. (1989) Behav. Brain Res. 34, 97-109. [DOI] [PubMed] [Google Scholar]
- 19.Deadwyler, S. A., Bunn, T. & Hampson, R. E. (1996) J. Neurosci. 16, 354-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolls, E. T., Cahusac, P. M., Feigenbaum, J. D. & Miyashita, Y. (1993) Exp. Brain Res. 93, 299-306. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka, K. (1997) Curr. Opin. Neurobiol. 7, 523-529. [DOI] [PubMed] [Google Scholar]
- 22.Tarr, M. J. & Bulthoff, H. H. (1998) Object Recognition in Man, Monkey, and Machine (MIT Press, Cambridge, MA). [DOI] [PubMed]
- 23.Newell, F. N. & Bulthoff, H. H. (2002) Cognition 85, 113-143. [DOI] [PubMed] [Google Scholar]
- 24.Freedman, D. J., Riesenhuber, M., Poggio, T. & Miller, E. K. (2001) Science 291, 312-316. [DOI] [PubMed] [Google Scholar]
- 25.Sigala, N. & Logothetis, N. K. (2002) Nature 415, 318-320. [DOI] [PubMed] [Google Scholar]
- 26.Vogels, R. (1999) Eur. J. Neurosci. 11, 1223-1238. [DOI] [PubMed] [Google Scholar]
- 27.Eichenbaum, H. (1993) Science 261, 993-994. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe, T. & Niki, H. (1985) Brain Res. 325, 241-254. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos, G., Huang, X. F. & Toga, A. W. (2003) The Rhesus Monkey Brain in Stereotaxic Coordinates (Academic, San Diego).
- 30.Hampson, R. E., Simeral, J. D. & Deadwyler, S. A. (2002) J. Neurosci. 22, RC198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuster, J. M., Bodner, M. & Kroger, J. K. (2000) Nature 405, 347-351. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura, K. & Kubota, K. (1995) J. Neurophysiol. 74, 162-178. [DOI] [PubMed] [Google Scholar]
- 33.Wachsmuth, E., Oram, M. W. & Perrett, D. I. (1994) Cereb. Cortex 4, 509-522. [DOI] [PubMed] [Google Scholar]
- 34.Miller, E. K., Nieder, A., Freedman, D. J. & Wallis, J. D. (2003) Curr. Opin. Neurobiol. 13, 198-203. [DOI] [PubMed] [Google Scholar]
- 35.Miller, E. K., Erickson, C. A. & Desimone, R. (1996) J. Neurosci. 16, 5154-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desimone, R. (1998) Philos. Trans. R. Soc. London B 353, 1245-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto, K., Suzuki, W. & Tanaka, K. (2003) Science 301, 229-232. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, W. A., Miller, E. K. & Desimone, R. (1997) J. Neurophysiol. 78, 1062-1081. [DOI] [PubMed] [Google Scholar]
- 39.Scalaidhe, S. P., Wilson, F. A. & Goldman-Rakic, P. S. (1999) Cereb. Cortex 9, 459-475. [DOI] [PubMed] [Google Scholar]
- 40.Squire, L. R. (1998) C. R. Acad. Sci. Ser. III 321, 153-156. [DOI] [PubMed] [Google Scholar]
- 41.Rudy, J. W., Barrientos, R. M. & O'Reilly, R. C. (2002) Behav. Neurosci. 116, 530-538. [DOI] [PubMed] [Google Scholar]
- 42.Tulving, E. & Markowitsch, H. J. (1998) Hippocampus 8, 198-204. [DOI] [PubMed] [Google Scholar]
- 43.Parker, A., Wilding, E. & Akerman, C. (1998) J. Cognit. Neurosci. 10, 691-703. [DOI] [PubMed] [Google Scholar]
- 44.Kreiman, G., Koch, C. & Fried, I. (2000) Nature 408, 357-361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.