Abstract
Objectives
To compare the costs and cost-effectiveness of managing patients with uncontrolled blood pressure (BP) using telemonitoring versus usual care from the perspective of the National Health Service (NHS).
Design
Within trial post hoc economic evaluation of data from a pragmatic randomised controlled trial using an intention-to-treat approach.
Setting
20 socioeconomically diverse general practices in Lothian, Scotland.
Participants
401 primary care patients aged 29–95 with uncontrolled daytime ambulatory blood pressure (ABP) (≥135/85, but <210/135 mm Hg).
Intervention
Participants were centrally randomised to 6 months of a telemonitoring service comprising of self-monitoring of BP transmitted to a secure website for review by the attending nurse/doctor and patient, with optional automated patient decision-support by text/email (n=200) or usual care (n-201). Randomisation was undertaken with minimisation for age, sex, family practice, use of three or more hypertension drugs and self-monitoring history.
Main outcome measures
Mean difference in total NHS costs between trial arms and blinded assessment of mean cost per 1 mm Hg systolic BP point reduced.
Results
Home telemonitoring of BP costs significantly more than usual care (mean difference per patient £115.32 (95% CI £83.49 to £146.63; p<0.001)). Increased costs were due to telemonitoring service costs, patient training and additional general practitioner and nurse consultations. The mean cost of systolic BP reduction was £25.56/mm Hg (95% CI £16.06 to £46.89) per patient.
Conclusions
Over the 6-month trial period, supported telemonitoring was more effective at reducing BP than usual care but also more expensive. If clinical gains are maintained, these additional costs would be very likely to be compensated for by reductions in the cost of future cardiovascular events. Longer-term modelling of costs and outcomes is required to fully examine the cost-effectiveness implications.
Trial registration
International Standard Randomised Controlled Trials, number ISRCTN72614272.
Keywords: Health Economics, Health Services Administration & Management
Article summary.
Article focus
Poor blood pressure (BP) control contributes a substantial financial burden on the National Health Service (NHS) via increased risk of stroke and heart disease; however, it is often difficult to control.
Home telemonitoring offers a potentially attractive means of overcoming issues leading to poor BP control such as infrequent monitoring and clinician and patient reluctance to intensify therapy.
This paper presents a within-trial economic evaluation from an NHS perspective comparing the costs and cost-effectiveness of home telemonitoring of BP versus usual care for patients with uncontrolled BP.
Key messages
Over the 6 months of trial period observed, home telemonitoring was significantly more expensive and more effective than usual care at £25.56/mm Hg reduced/patient.
If clinical gains are maintained, the additional costs are likely to be compensated for by reductions in the cost of future cardiovascular events; however, longer term modelling of costs and outcomes is required to fully examine this.
Strengths and limitations of this study
The trial benefited from a pragmatic setting, intention-to-treat analysis, blinding of outcome assessment and a broad socioeconomic spectrum of participants.
The main limitations were due to the relatively short follow-up period of 6 months. This restricted the potential to examine long-term effects on cardiovascular events or estimate outcomes in the preferred terms of quality adjusted life years.
Introduction
Hypertension is a major reversible risk factor for stroke and heart disease. It was estimated in 2001 that uncontrolled high blood pressure (BP) costs $370 billion globally (£256 billion, €413 billion) with a potential cost of $3.6 trillion (£2.5 trillion, €4.0 trillion) over a 10-year period in indirect costs.1 Despite effective medications, BP is difficult to control for many people.2 This is due in part to infrequent monitoring,3 a reluctance on the part of clinicians to intensify treatment4 and pharmacological interventions by patients.5 Telemonitoring of BP involves patients regularly taking their own readings with onward transmission in almost real time to a website which can be accessed by themselves or by their doctor or nurse and can provide patients with decision-support, in terms of when to contact a doctor or nurse for advice, which is then sent by text or email.
This paper presents a within trial, economic evaluation from the perspective of the National Health Service (NHS) of data collected during the HITS trial.6 This was a trial of a telemonitoring-based service redesign compared with usual care for the management of uncontrolled hypertension which was powered to detect differences in mean systolic BP but also collected resource use data as a secondary outcome. The analysis presented here, while not part of the trial protocol, was conceived prior to completion of the primary clinical analysis for the trial.
Methods
Overview of the HITS trial
This was a 6-month pragmatic, prospective, parallel-group randomised controlled trial with blinded outcome assessments. In total, 401 patients were recruited from 20 practices representing a range of socioeconomic diversity including the fifth most deprived and second most affluent in Lothian, Scotland.
Participants were included in the study if their daytime ambulatory BP averaged ≥135/85 and <210/135 mm Hg measured by the Spacelabs 90207 Ambulatory Blood Pressure Monitor (ABPM).7 Exclusion criteria were the inability to consent, atrial fibrillation, being on the stroke or diabetes registers (as these patients would be invited to other trials in our portfolio of trials investigating the role of telemonitoring in the management of long-term conditions), treatment for cardiac event or other life-threatening illness within the past 6 months, major surgery within the last 3 months, renal failure or hypertension not managed in primary care. A full list of baseline measurements can be found in table 1.
Table 1.
Baseline characteristics for full dataset
| Variable | Monitored (N=200) | Control (N=201) |
|---|---|---|
| Age (years) mean (SD) | 60.5 (11.8) | 60.8 (10.7) |
| Male N (%) | 117 (58.5) | 120 (59.7) |
| Blood pressure self-monitoring history N (%) | ||
| Never | 128 (64.0) | 126 (62.7) |
| Occasionally | 56 (28.0) | 56 (27.9) |
| Regularly | 16 (8.0) | 19 (9.5) |
| Body mass index (kg/m2) mean (SD) | 30.1 (5.7) | 30.2 (6.2) |
| Smoking N (%) | ||
| Yes | 23 (11.5) | 20 (10.0) |
| Mean (SD) (cigarettes/day) | 17.6 (9.2) | 14.9 (10.4) |
| No | 177 (88.5) | 181 (90.0) |
| Alcohol use* N (%) | ||
| Yes | 158 (79.0) | 159 (79.1) |
| Median (first, third quartile)(units of alcohol(10 ml)/day) | 1.7 (0.9, 2.9) | 2.0 (0.7, 4.0) |
| No | 37 (18.5) | 41 (20.4) |
| Exhaled carbon monoxide category N (%) | ||
| Non-smoker (1–6) | 177 (88.5) | 179 (89.1) |
| Light smoker (7–10) | 0 (0.0) | 3 (1.5) |
| Moderate smoker (11–20) | 8 (4.0) | 11 (5.5) |
| Heavy smoker (20+) | 15 (7.5) | 8 (4.0) |
| Cholesterol level (mmol/l)† mean (SD) | 5.5 (1.0) | 5.3 (1.0) |
| HbA1c level (mmol/mol)‡ mean (SD) | 37.7 (6.5) | 37.7 (5.4) |
| Urinary sodium/creatine ratio§ mean (SD) | 9.7 (5.4) | 10.9 (8.7) |
| Surgery measured systolic BP (mm Hg) mean (SD) | 152.9 (15.1) | 152.4 (14.3) |
| Surgery measured diastolic BP (mm Hg) mean (SD) | 92.1 (11.5) | 89.9 (11.3) |
| Daytime ambulatory systolic BP (mm Hg) mean (SD) | 146.2 (10.6) | 146.2 (10.5) |
| Daytime ambulatory diastolic BP (mm Hg) mean (SD) | 87.1 (10.0) | 85.4 (9.6) |
| HADS29 anxiety score¶ mean (SD) | 5.0 (2.9) | 5.1 (3.6) |
| HADS depression score¶ mean (SD) | 2.8 (2.4) | 2.9 (2.5) |
| Exercise tolerance score35** mean (SD) | 7.8 (2.9) | 7.6 (3.0) |
| Stanford self efficacy questionnaire (short version)36†† | ||
| Mean (SD) | 8.7 (1.4) | 8.5 (1.4) |
| Morisky medication adherence scale37 N (%) | ||
| Sometimes forgets to take medication‡‡ | ||
| Yes | 61 (30.5) | 63 (31.3) |
| No | 132 (66.0) | 132 (65.7) |
| Sometimes careless about taking medication§§ | ||
| Yes | 24 (12.0) | 23 (11.4) |
| No | 169 (84.5) | 173 (86.1) |
| Sometimes stops taking medication when feels better¶¶ | ||
| Yes | 11 (5.5) | 15 (7.5) |
| No | 181 (90.5) | 180 (89.6) |
| Sometimes stops taking medication when feels worse*** | ||
| Yes | 18 (9.0) | 22 (10.9) |
| No | 170 (85.0) | 173 (86.1) |
| Number of defined daily doses of hypertension drugs | ||
| Median (first, third quartile) | 1.5 (1, 3) | 1.7 (1, 3) |
| EuroQol-5D23††† mean (SD) | 0.875 (0.177) | 0.857 (0.220) |
Missing data.
*Five in monitored and one in control group.
†Five in monitored and eight in control group.
‡Seven in monitored and nine in control group.
§Four in monitored and two in control group.
¶Two missing in each group.
**One in monitored and two in control group.
††Six in monitored and one in control group.
‡‡Six in monitored and seven in control group.
§§Five in monitored and seven in control.
¶¶Six in monitored and eight in control.
***Six in monitored and 12 in control group.
†††Five in monitored and six in control group.
HADS, hospital anxiety and depression scale.
Patients were randomised in a 1:1 ratio either to the telemonitoring intervention or usual care using a secure randomisation system provided by the Edinburgh Clinical Trials Unit with minimisation on the basis of age, sex, family practice, use of three or more hypertension drugs and self-monitoring history. As simple minimisation within centres can lead to the alternation of treatment allocation and potential loss of allocation concealment, a degree of random allocation was also incorporated.
Research nurses gave patients assigned to the intervention a training session on how to use the telemonitoring equipment. As the intervention comprised providing telemetric equipment, neither the participants nor the investigators could be masked to group assignment.
Participants were asked to monitor their own BP twice each morning and twice each evening for the first week and then at least weekly thereafter or as often as they wished. They used a validated automated sphygmomanometer (Stabil-O-Graph mobil, IEM, Germany).8 This linked via Bluetooth connection to a mobile phone, which automatically transmitted readings to a central server managed by IEM Ltd (Stuttgart, Germany). Patients and clinicians could log on to a website to see the data and automated SMS texts/emails could be sent to patients informing them of the level of their control (see box 1 for a fuller description of the process). Patients could contact clinicians if they were concerned about their BP control and clinicians could contact patients if needed to arrange modification of therapy where required. The target home-monitored BP was <135/85 mm Hg based on the contemporaneous UK guidelines,9 subsequently endorsed by the National Institute for Health and Clinical Excellence (NICE).10
Box 1. Description of the telemonitoring intervention (see web supplemental files for illustrations).
The intervention: The practices and participants were asked to use a system which comprised a validated electronic home blood pressure (BP) monitor and mobile phone technology that enabled the transfer of BP readings via SMS to a secure website which was accessible to the user and their doctor or nurse, and also provided automated feedback to the patient. The BP monitor linked to a mobile phone wirelessly, via Bluetooth. The components of the intervention were
Home BP monitoring: Participants were asked to record their BP as agreed with the healthcare team, or more frequently as they wished. Guidance was initially to record BP twice in the morning and twice in the evening for a week in line with the European guideline on BP monitoring,36 to build a baseline average. Thereafter, they were asked to take weekly measurements preferably at different times of day if their average BP was within the recommended range; however, if they had made any lifestyle or medication change which would impact on their BP, they were asked to measure their BP for a more intensive period of monitoring to allow the rolling average to change and to more quickly assess the effect.
Transmission of data: This simply required the phone to be switched on and to have a signal when the BP measurement was taken. Participants just had to apply the cuff and press a button on the BP monitor. The reading and transmission occurred automatically. Mobile phone problems did not lead to the loss of data because all readings were stored in the monitor and any untransmitted readings were sent when the next reading was taken.
Feedback to patient participants (closed loop feedback): In addition to optionally accessing their BP record on-line, participants could also opt to receive reports via text message or email. These gave advice on the current status of their BP based on the average of the last 10 readings, and whether they should contact their doctor or nurse. Reports were generated every 10 readings or weekly, whichever was sooner, with a reminder to check BP if this had not been done. These reports could reassure them that their average BP was within target (<135/85 mm Hg) or tell them that their BP average was improved on the last report, but not yet to target and to maintain current therapy, or that their BP was not at target and that they should contact their clinician. If an individual BP reading was very high (>220/120 mm Hg) an immediate text or email report was generated reinforcing the written advice in the patient information leaflet to rest for 30 min, check again and contact the practice if BP remained very high.
Sharing the readings with the healthcare team: Members of the healthcare team were able to access the records of their patients online via a secure login to a summary screen which listed their patients, their average BP over the last 10 readings and the date of their last reading. Average BPs outside the recommended limits (set at 135/85 mm Hg for the study) were highlighted. Clicking on the each individual patient led to lists or graphs of all their readings. Clinicians could then check their patients' electronic general practitioner (GP) record to see if there had been recent advice regarding medication or lifestyle change and if not, could contact the patient to make a change. Clinicians were recommended to check the website weekly, but the frequency of log-on could be chosen by them.
Usual care: Participants allocated to the usual care group were asked to continue to attend the practice for BP checks according to the usual routine of the practice. If they were already home monitoring they were not discouraged from continuing.
All participants: For all participants, the GP/practice nurse were informed that the ambulatory monitoring used to screen for eligibility for the HITS trial had shown that their average BP was above the target range, but they were not given the actual reading. All participants were given an information pack containing a range of publicly available leaflets on hypertension management and lifestyle modification.
Patients allocated to the usual care arm were told that the ABPM showed that their BP was uncontrolled and that they should see their general practitioner (GP)/practice nurse for further management, otherwise they should receive standard care for hypertension from their GP or nurse who were asked to aim for a target surgery BP of <140/90 mm Hg based on UK guidelines (current at the time).9
In order to maintain blinding of outcome assessment, the patients were asked not to reveal their treatment group allocation to the research nurse undertaking the assessment; however, it is not possible to rule out unblinding where the patients did not adhere to this.
Cost estimation
Mean costs per patient were estimated from an NHS perspective. The trial collected the data of the number of consultations with a GP, practice nurse or district nurse (separately for practice, telephone or home visits), NHS24 (emergency out-of-hours telephone helpline) contacts, out-of-office consultations with the Lothian Unscheduled Care Service and accident and emergency visits. These were collected at follow-up by a research nurse with access to patient records. If the patient did not attend the follow-up, but agreed for the data to be collected, the research nurses completed the data from the records in their absence. Data on each drug issued to each patient, the dose per day and the number of days issued were taken from GP records from randomisation until 6 months after randomisation. Drugs were assumed to be the lowest cost generic treatment which matched the daily dosing structure recommended in the British National Formulary11 unless a specific brand was stated. Assumptions were made on an ad hoc basis blind-to-treatment allocation for the 2.4% of drug entries where doses and drug combinations failed to match perfectly to the recommended dosing structure.
Unit costs were applied to each item. Where possible, these were taken from recognised national sources.12–18 The base year for costs was the financial year 2009/2010. Any estimates from different years were inflated/deflated using an appropriate inflation index (see table 2). With the exception of equipment costs (see table 3), discounting was not required as the trial was less than 1 year in duration.
Table 2.
Price weights, calculations and sources
| Variable | Value | Unit | Source(s)/notes | |
|---|---|---|---|---|
| General Practitioner | ||||
| Surgery | £36.00 | Per | Consultation | 12 |
| Home | £120.00 | Per | Consultation | 12 |
| Phone | £22.00 | Per | Consultation | 12 |
| Practice nurse | ||||
| Surgery | £12.00 | Per | Consultation | 12 |
| Home | £20.00 | Per | Consultation | 12 |
| Phone | £4.74 | Per | Consultation | Cost per hour12×average call length13 |
| District nurse | ||||
| Surgery | £18.86 | Per | Consultation | Cost per hour12×average consultation length.13 Consultation length assumed to be equal to that of a practice nurse |
| Home | £27.00 | Per | Consultation | 12 |
| Phone | £10.46 | Per | Consultation | Cost per hour12×average Call length.13 Call length assumed to be equal to that of a practice nurse |
| NHS 24 contact | £41.71 | Per | Contact | £35.6914 inflated to 2009/2010 prices using Hospital and Community Health Services (HCHS) pay and price inflation index12) |
| LUCS consultation | £64.82 | Per | Consultation | Number of LUCS contacts divided by total budget, obtained private communication with NHS Lothian. Information on cost per consultation was not available |
| A&E visit | £95.00 | Per | Visit | 15 |
| Medication | All medication use recorded was priced individually using the 2011 prices from the MIMS data base16 deflated to 2009 prices using the Pharmaceutical Inflation component of the CPI37 with adjustments made for 10.5% claw back17 and container costs18 | |||
| HBPM service and device | £70.77 | For | 6 months | Per patient (see table 3) |
A&E, accident and emergency; HBPM, home blood pressure monitor; LUCS, Lothian Unscheduled Care Service; NHS, National Health Service.
Table 3.
Price estimation and components for cost of intervention over 6 months (per patient)
| Variable | Value | Unit | Source(s)/notes | |
|---|---|---|---|---|
| Home blood pressure monitor (HBPM): | ||||
| Initial training of patient in device use | £12.00 | Per | Patient | One off patient training in use of device. Priced as an assumed 20 min of practice nurse time (£36/h client contact12) based on the trial's pilot work |
| HBPM device | £53.11 £1.20 | Per | Each month* | Local pricing from manufacturer invoice (60 Euro converted to GBP using average exchange rate 2009/201038) |
| Mobile phone | £48.48 £1.44 | Per | Each month* | Local pricing from internal communications with NHS Lothian telecoms (£49)) deflated from 2011 prices to 2009/2010 using medical products component of CPI37 |
| Server hosting | £0.42 | Per | Month | Local pricing from Supplier Invoice (£1000/year for all patients, divided by 200 patients over 12 months) |
| Web hosting | £2.59 | Per | Month | Local pricing from Supplier Invoice (3.10 Euro converted to GBP using average exchange rate 2009/201038) |
| Sim card | £1.98 | Per | Month | Local pricing from internal communications with NHS Lothian telecoms (£2 deflated from 2011 prices to 2009/2010 using medical products component of CPI37) |
| Nurse time | £2.17 | Per | Month | Assumption of 1 min/week of practice Nurse time spent checking incoming HBPM data (£30/h non-specific work12) based on anecdotal information |
| Total† | £70.77 | For | 6 months |
A detailed breakdown of the interventions costs of 6 months of BP telemonitoring, assumptions made in their estimation, price weights applied, inflation indices used and their sources is given in table 3. The price of the full 6 months of intervention was applied uniformly to all patients in the monitored group, regardless of whether or not they completed the trial.
Although data were also collected on the number of hospital admissions attended by each patient during the trial, the cost of hospital admissions can vary substantially depending on the nature of the admission15 19 and specific details of the nature of each admission were not recorded. Instead, reported admissions were matched with entries in the adverse events log for the trial to generate verbal descriptions of each event. BM viewed the extracted descriptions of each event blind-to-randomisation allocation, assigned Healthcare Resource Group (HRG4) codes based on the descriptions and assessed whether the event could be at least be possibly related to BP management. HRG4 codes are used in the NHS to group procedures into categories of hospital care which incur similar resource use.
Of the 28 admissions recorded in the adverse events log, 7 (25%) were for cardiovascular-related diagnoses and as such were deemed indirectly related to high BP or related to dizziness or falls for which BP could not be ruled out as a contributing factor. None could specifically be related to telemonitoring itself. The decision therefore was made not to include these costs in the base-case analysis as there was a risk of overwhelming the more robust estimates of other cost factors with unreliable, and likely unrelated, admission costs. However, a sensitivity analysis was undertaken including the costs of hospital admissions where price weights were applied from the Scottish National Tariff19 based on the HRG4 code selected.
Effect variable
The effect variable for the cost-effectiveness analysis was mean daytime systolic ambulatory blood pressure (SABP). For both groups, this was measured using a Spacelabs 90207 ABPM. We therefore calculated the cost per mm Hg systolic BP reduced over the 6 months intervention period.
Analysis
All analyses were undertaken on an intention-to-treat basis.
Missing data
Primary outcome data were missing for 11.5% of patients including 20 participants (6 in the intervention group and 14 in the usual care group) were either lost to follow-up or who withdrew consent. Economic variables were missing for 0–8.7%. In total, 21.9% of patients had missing data for at least one variable of interest. Multiple imputation by chained equations20 was used to create 10 imputed datasets by imputing incomplete variables under fully conditional specification. This was based on age, sex, body mass index, BP (systolic and diastolic), number of hypertension drugs, cholesterol, exhaled per cent carbon monoxide, blood glycated haemoglobin (HbA1c), Euroqol-5D (EQ-5D) responses and all healthcare resource use variables. Calculations were undertaken in STATA 12 using the ‘mi ice’ command. Normally distributed parameters (including primary outcome data) were imputed using multiple regression by ordinary least squares; ordered categorical variables were imputed using ordinal logistic regression and other non-normal variables imputed using predictive mean matching. Model parameters were then estimated using the respective regressions techniques described below. These estimates and their SEs were combined using Rubin's rules.21
Cost analysis
Univariate analysis was undertaken of differences between trial arms in terms of total costs and each cost subelement. As the cost data were non-normally distributed with a heavy right skew and long tail, testing was performed using non-parametric bootstrap of differences in mean patient costs between trial arms and bias corrected CIs and p values (two-tailed) were reported for each cost item with significance set at the 5% level.
Cost-effectiveness analysis
Baseline resource use was not recorded. We would expect randomisation to balance out baseline costs between groups. However, to counteract any baseline imbalances, point estimates for incremental costs were estimated using a generalised linear model (GLM) controlling for age, sex, baseline systolic BP and baseline health-related quality of life (calculated by baseline EQ-5D index score22). GLMs allow adjustments to be made for heteroscedasticity and skew by the adoption of a ‘family’ and link function.23 24
Family function was selected by Modified-Parks test24 and a power function for the link was selected on the balance of p values from three tests of fit as recommended by Glick et al23 These tests were the Modified Hosmer & Lemeshow test (tests for systematic bias in fit on raw scale), the Pregibon link test (tests for linearity of response on scale of estimation) and Pearson correlation test (tests for systematic bias in fit on raw scale). The Gaussian family was selected and a power of 0.5343 was selected for the link function.
For incremental BP point reduction, multiple regression (by ordinary least squares) was used controlling for baseline SABP and all minimisation variables, namely age, sex, general practice, use of three or more hypertension drugs and self-monitoring history. This was selected for its equivalence to the analysis used for the variable in the primary analysis.6
Incremental cost-effectiveness ratios (ICERs) were expressed as cost/1 mm Hg systolic BP point reduced. Bias-corrected CIs25 for the ICERs were estimated from the bootstrapped data generated using the ‘recycled predictions’ method as described by Glick et al.23 This technique generates a large number of bootstrapped samples (10 000 replications were used). The chosen regressions for each variable were used to estimate incremental costs, BP and their respective ICERS within each sample.
The proportion of samples in which the intervention is shown to be cost-effective to the NHS at a given price per mean systolic mm Hg (using the net benefit technique26) is used to estimate the probability that the intervention is cost-effective at that price. The process was repeated varying the price over a range of £0 to £100/systolic mm Hg to plot cost-effectiveness acceptability curves (CEACs). CEACs show the probability that the treatment is cost-effective at varying costs (willingness on behalf of the NHS to pay) per unit of outcome (1 mm Hg systolic BP reduced) to a decision-maker.
As several assumptions were made in the intervention costs (see table 3), as a sensitivity analysis, CEACs were calculated with the total cost of 6 months of intervention varied in increments of 25% to ± 100% of the base case price (see figure 1).
Figure 1.
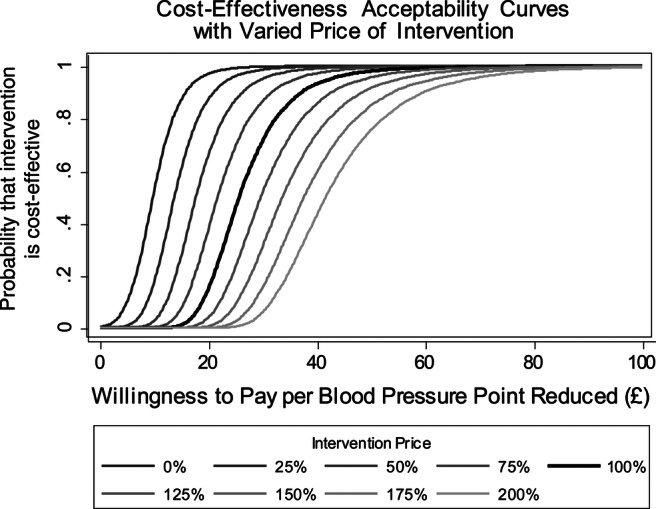
Cost effectiveness acceptability curves with varied price of intervention.
Results
Analysis of costs
Table 4 details the results of the univariate analysis of costs and resource use per patient associated with each trial arm. The mean total estimated healthcare cost per patient was £287.18 in the intervention group and £177.95 in the usual care group (mean difference £109.23, 95% CI £76.36 to £140.63) in univariate analysis. Controlling for baseline characteristics in the multivariate analysis gave similar results with a mean total costs of £290.13 in the intervention group and £174.81 in the usual care group (mean difference £115.32, 95% CI £83.49 to £146.63).
Table 4.
Estimated mean (SE) healthcare service resources used and associated costs per patient by factor
| Monitored group (N=200) |
Control group (n=201) |
|||||
|---|---|---|---|---|---|---|
| Number used | Cost, £ | Number used | Cost, £ | Mean cost Difference, £ (95% CI*) | p Value* | |
| GP consultations | ||||||
| Surgery consultations | 3.61 (0.19) | 130.00 (7.00) | 2.70 (0.21) | 97.11 (7.46) | 32.89 (14.55 to 51.04) | <0.001 |
| Phone consultations | 0.57 (0.08) | 12.43 (1.78) | 0.49 (0.09) | 10.69 (1.98) | 1.74 (−2.74 to 6.09) | 0.447 |
| Home consultations | 0.06 (0.03) | 7.74 (3.24) | 0.09 (0.04) | 10.39 (4.52) | −2.65 (−11.91 to 5.27) | 0.553 |
| Total consultations | 4.24 (0.23) | 150.17 (8.90) | 3.27 (0.27) | 118.19 (10.52) | 31.97 (8.38 to 54.22) | 0.004 |
| Practice nurse consultations | ||||||
| Surgery consultations | 1.90 (0.18) | 22.75 (2.11) | 1.41 (0.14) | 16.88 (1.71) | 5.86 (1.14 to 11.00) | 0.016 |
| Phone consultations | 0.69 (0.09) | 3.28 (0.42) | 0.15 (0.05) | 0.71 (0.25) | 2.57 (1.75 to 3.45) | <0.001 |
| Home consultations | 0.02 (0.01) | 0.41 (0.28) | 0.01 (0.01) | 0.30 (0.27) | 0.11 (−0.38 to 0.77) | 0.704 |
| Total consultations | 2.61 (0.21) | 26.43 (2.27) | 1.57 (0.17) | 17.89 (1.88) | 8.54 (3.46 to 14.15) | 0.002 |
| District nurse consultations | 0.04 (0.02) | 0.67 (0.41) | 0.15 (0.11) | 3.94 (3.05) | −3.26 (−11.94 to 0.39) | 0.249 |
| NHS24 consultations | 0.10 (0.03) | 4.03 (1.39) | 0.05 (0.02) | 2.12 (0.79) | 1.91 (−0.42 to 4.95) | 0.139 |
| LUCS consultations | 0.07 (0.02) | 4.34 (1.39) | 0.04 (0.02) | 2.48 (1.16) | 1.86 (−0.89 to 4.83) | 0.193 |
| Medication | 24.07 (2.12) | 23.59 (2.20) | 0.48 (−5.83 to 6.40) | 0.868 | ||
| Accident and emergency visits | 0.07 (0.02) | 6.70 (2.24) | 0.10 (0.03) | 9.74 (2.98) | −3.04 (−8.87 to 2.47) | 0.286 |
| Subtotal excluding tele-monitoring | 216.41 (11.66) | 177.95 (15.15) | 38.46 (5.59 to 69.87) | 0.019 | ||
| Tele-monitoring service and device | 70.77 | 70.77 | ||||
| Total healthcare costs | 287.18 (11.66) | 177.95 (15.15) | 109.23 (76.36 to 140.63) | <0.001 | ||
*p Values (two-tailed) for significant difference from zero and Bias corrected CI estimated by non-parametric bootstrap (10 000 replications).
GP, general practitioner; LUCS, Lothian Unscheduled Care Service (out of hours GP or nurse consultations); NHS, National Health Service.
The difference in total costs remained significant with the cost of the telemonitoring technology excluded from the analysis demonstrating that NHS costs rose outside of the cost of the intervention itself. This was driven largely by a significant increase in mean costs arising from approximately one additional GP surgery consultation and half a practice nurse surgery consultation per person in the intervention group compared with the usual care group. The only other significant cost element difference was approximately half an additional practice nurse phone consultation. No other cost element was significantly different between the groups. This included the cost of the prescribed medication. Despite a significantly greater increase in the doses of the prescribed medication in the telemonitored group over that of the usual care group,6 the rise in cost was relatively trivial as often higher strength medications were priced similarly to lower strengths.
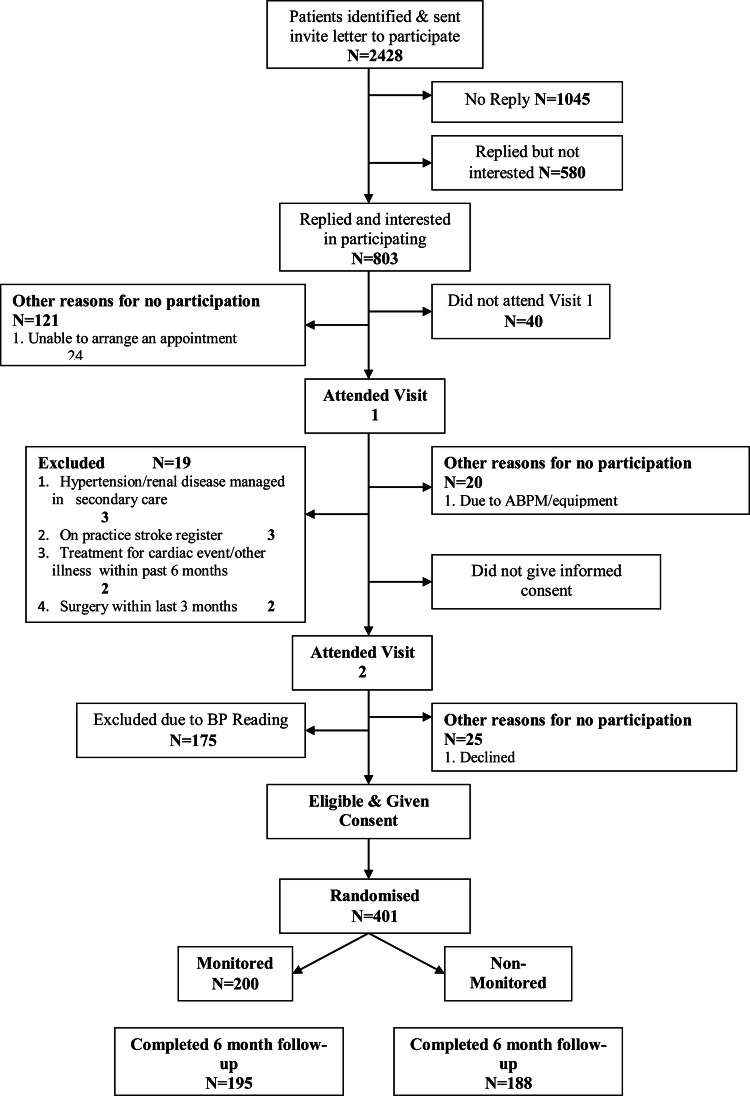
Figure 2.
Consort diagram.
In sensitivity analysis, the mean cost of hospital admissions in the intervention arm was £287.01 compared with £181.54 in the control arm (mean difference £105.47, 95% CI £−123.16 to £402.40; p=0.424) which raises the mean difference in total NHS costs to £214.70 (95% CI £−23.71 to £526.65; p=0.098). However, this estimate was dominated by one patient in the intervention arm with admissions costing over £17 000, none of which were assessed to be possibly related to BP management. When the costs of these admissions of this patient were excluded, the equivalent mean differences in hospital costs fell to £16.56 (95% CI £−188.04 to £202.17; p=0.846) and total costs to £125.79 (95% CI £−88.85 to £318.40; p=0.223).
Analysis of BP point reduction
Following imputation, the mean daytime SABP fell in both groups, from 146.20 to 140.15 mm Hg in the telemonitoring arm and 146.22 to 144.50 mm Hg in the usual care arm. The difference in mean daytime SABP at 6 months between the two arms (ie, control-telemonitoring) was 4.51 mm Hg (95% CI 2.49 to 6.61; p<0.001), adjusted for baseline mean daytime SABP and minimisation factors.
Cost-effectiveness analysis
Figure 3 shows the joint distribution of incremental costs and incremental systolic BP point reduction generated by the bootstrap replicates. In all replicates, costs per patient were higher and mean SABP per patient was lower in the monitored group than the control (p<0.001 for both variables). This indicates that the telemonitoring was both more costly and more effective than usual care in all replicates. The ICER was £25.60/mm Hg (95% CI £16.05 to £46.69).
Figure 3.

Scatter plot: incremental costs against incremental blood pressure reduction.
Figure 1 shows the probability of telemonitoring being cost-effective at varied NHS willingness to pay per BP point reduction. The 100% line represents the base case analysis with intervention costs at £70.77 and the other lines showing how the CEAC would change if intervention costs were higher or lower.
Discussion
Over the 6 months of the trial, the intervention was significantly more effective than usual care but also significantly more costly on average lowering SABP by 4.51 mm Hg and raising the total cost by £115.32. The increase in costs was predominantly driven by the estimated intervention costs (£70.77) and increased costs associated with telemonitored patients using on average approximately one additional GP surgery consultation and half a practice nurse surgery consultation. Although telephone consultations with the practice nurse and their costs also significantly rose (by approximately half a call on average), the costs for these were relatively small and had little impact on total cost.
The trial found a significant increase in the dosages of medication issued6 which may explain some of the additional consultations as they were likely to have been required for prescribing and monitoring of patients during transition to a new drug/dose. Interestingly, however, medication costs did not rise significantly in spite of this intensification. This is due to higher dosage pills often costing less per dose than lower dosage pills when costs of generic treatments are used for these estimates.16 There is a risk that the way in which the medication costs were estimated (selecting a generic where available and selecting the lowest cost option that matched dosing recommendations11) could have contributed substantially to this finding. However, such an approach does at least attempt to estimate the difference in costs attainable under best practice assuming this includes the selection of the lowest cost drug based on active agent.
It should be noted that our accompanying qualitative study27 suggests that over the trial, clinicians found face-to-face communication with patients was not necessary to support BP telemonitoring and that they substituted some of these forms of consultation with other modes of communication: mainly telephone and on two occasions email. Both patients and clinicians thought that in the longer term, BP telemonitoring would reduce the need for surgery visits. Thus, a reduction in the GP consultations element of the overall costs may be realised in the longer term. This has been found in previous studies of telemonitoring where consultation costs were lower.28–30
The remaining cost elements were also non-significantly different between groups and played relatively minor roles in the overall total cost differences.
In sensitivity analysis, hospital costs were non-significantly higher on average in the intervention group than in the control by £105.47 raising differences in mean total costs to £214.70. However, the differences in costs of hospital admissions were exaggerated by the inclusion of an outlier patient in the intervention arm unrelated to BP management. With these excluded, hospital costs were similar in both arms with an insignificant difference of £16.56. In both cases, uncertainty surrounding hospital cost dominated uncertainty surrounding total cost figures. Hence, when hospital admissions are included in total costs estimates, we are no longer confident that the cost of resource use outside of the intervention service itself was higher or lower in the intervention group because secondary care costs have the potential for the largest financial impact on the NHS. However, there are strong reasons to doubt that any of the hospital admissions observed during the trial were related to the patient's current BP, even those of cardiovascular nature. This is because it is possible that the events resulting in hospital admission may well have been set in motion prior to the onset of the study though we lack data to confirm this either way. A recent meta-analysis also found no link between home BP telemonitoring and short-term rates of adverse events.28
Dividing per patient mean differences in costs by per patient mean differences in blood-pressure reduction yields an ICER of £25.56/mm Hg. While on the face of it modest, there are to our knowledge no criteria available to assess the cost-effectiveness of the value of a BP-point reduction, hence no formal assessment of whether this constitutes evidence that the intervention is cost-effective or not can be offered. It should also be recognised that £25.56/mm Hg is a ratio rather than a tariff. It is perhaps more accurate to say that over the first 6 months of the intervention we estimate that BP will reduce on average by 4.51 mm Hg at a cost of around £115.32/patient.
It is not known if the improved BP control found in the trial would be sustained once telemonitoring ceased. However, if sustained over 10 years, this type of reduction would be expected to lead to a >15% reduction in risk of stroke and >10% reduction in risk of coronary heart disease.31 The costs incurred in the intervention period were low relative to the several thousand pounds likely to be spent on a cardiovascular event.15 19 32 For example, Youman et al32 calculated the cost of a stroke to the NHS in 2001 to be £15 306 over 5 years. Should the BP-point reduction be sustained beyond the observed 6 months examined here, the expected reduction in cardiovascular events31 may mean that the intervention is dominant over usual care in the long term that is to say, both more effective and cost saving. Estimating this would require a study with a much longer follow-up or, perhaps more realistically, mathematical modelling of longer term health costs and benefits. Longer term follow-up of the participants is planned to determine the extent to which the difference in systolic BP persists after the end of the trial, which will be vital data to underpin such modelling.
Strengths and limitations
Unlike many previous studies, we used ABPM to measure BP. This is a considerable strength as ABPM is considered the gold standard for BP measurement and lends greater generalisability to the results as it is now a recommended practice in the UK to diagnose high BP with ABPM.10 The generalisability is further strengthened by the pragmatic setting, intention to treat analysis, the broad socioeconomic profile of participants and the absence of restrictions on participant age (oldest patient was 95) or exclusion on the basis of maximal treatment. The results may be less generalisable outside of a UK context as this is beyond the scope of this analysis.
The analysis was not part of the original trial protocol. It was, however, conceived prior to the start of the primary clinical analysis; the usual limitations of post hoc analysis are less relevant. This did, however, mean that the cost analysis was restricted by the variables available in the dataset and that some cost elements have not have been accounted for, most notably outpatient visits. On the other hand, the variables that were collected were similar to those used in other trials and are likely to be robust as surveys were completed with access to medical records.
It was not possible to control for baseline cost in multivariate analysis as these were not recorded. Instead, the analysis relies on baseline SABP and health-related quality of life and on the randomisation process in its place. While it is possible that a different result may have been observed had baseline costs been available for use, we do not anticipate that this would have differed considerably from the results presented here as it is not unreasonable to expect both of these factors to be highly correlated with baseline costs. Selection bias is very unlikely to have occurred during the randomisation/minimisation process as this service was provided remotely by clinical trials unit.
It was not possible to determine if £25.56/mm Hg reduced would be considered cost-effective or not. Using the NICE criteria for cost-effectiveness, the value of interventions is interpreted in terms of long-term cost per quality-adjusted life-year (QALY) gained.33 34 The EuroQol EQ-5D survey from which QALYs can be calculated22 was included in the trial.6 However, without sufficient power or follow-up to detect major cardiovascular events, differences in quality of life observed in the trial period would be unlikely to manifest themselves in an asymptomatic condition. Moreover, given that the participants were not blind to the intervention, this might be open to bias. Hence, QALYs could not reliably be estimated in this context. They are arguably better left to be determined by longer term modelling.
Comparisons to similar studies
Caution is advised when comparing studies of telemonitoring as the services within which the telemonitoring is nested often vary substantially and it is the combined effect of the telemonitoring and other interrelated services which are observed.
Two recent systematic reviews of BP telemonitoring found few studies which included measures of healthcare utilisation and/or cost. Of those which did, office visits are frequently the only healthcare resource considered outside of the direct cost of the technology issued28 35 and none were based in a UK setting, though a UK study by McManus et al29 suggests that an accompanying cost-effectiveness analysis is forthcoming.
Meta-analyses of home BP telemonitoring versus usual care by Omboni et al28 find home BP telemonitoring to be associated with increased medication use, reduced office visits and increased overall healthcare costs, though medication use and overall healthcare casts suffered from heterogeneity between studies. While the increased prescribing is in line with our own findings, the decreased office visits are not. As a result, Omboni et al28 attribute the rise in healthcare costs to the cost savings in terms of office visits being more than offset by equipment costs where our findings suggest an increase in both.
An explanation for this disparity may come from the heterogeneity of the services being delivered alongside the telemonitoring. For example, McManus et al29 showed that adding a medication self-titration plan to BP telemonitoring produced similar reductions in BP to our study, but found no increase in face-to-face consultations with physicians. This lends strength to the possibility that many of the increased GP surgery visits observed in this trial were required for prescribing.
Comparisons of healthcare costs with studies outside of the UK can also be problematic as different social insurance systems jeopardise cross-border generalisability; indeed, Omboni et al28 attribute the heterogeneity in their analysis of healthcare costs to this issue.
Madsen et al30 compared the cost-effectiveness of a similar intervention with usual care from a Danish health service perspective. In contrast to our findings, they found higher consultation and medication costs in their control arm. Again, these were more than offset by equipment costs leaving total costs significantly higher in the intervention arm; however, SABP was non-significantly higher in the intervention arm by 2.8 mm Hg. The authors attribute the raised medication costs to significantly increased prescribing of AT2-antagonists in the control arm. This intensification in prescribing in the usual care group rather than the intervention group as in our trial may go some way to explaining the lower reduction in BP observed. However, the fact that point estimates for SAPB improvement in Madsen et al's study were still in favour of the intervention suggests that medication prescribing may not be the only factor influencing BP.
Conclusions
In conclusion, although more expensive to the NHS than usual care, telemonitoring of BP in primary care was more effective at reducing BP during the 6 months of intervention. These costs may be recuperated in the long term as a result of prevention of future cardiovascular events if the reduction in BP is maintained. Further research is required to determine if the BP improvement is sustained and, if so, what impact this has on cost-effectiveness.
Supplementary Material
Footnotes
Contributors: BMcK, JH, SW, CP and PP designed the trial. JH and BMcK led the research. MP was trial manager, SL planned and supervised the statistical analysis, AK carried out the statistical analysis, AS carried out the economic analysis and wrote the first and subsequent drafts of the paper, AS provided advice throughout the trial. All authors critically revised the drafts and have approved the submission of the final paper.
Funding: This study was funded by the BUPA Foundation with additional support from the High Blood pressure Foundation and NHS Lothian. Brian McKinstry and Janet Hanley were supported by the Scottish Chief Scientist Office during the course of the trial. Andrew Stoddart is supported by the Edinburgh Health Services Research Unit.
Competing interests: None.
Ethics approval: The study was approved by Lothian Research Ethics Committee REC reference number: 08/S1101/38. Written informed consent was obtained from all participants.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We are happy to share deidentified data with other researchers on application to the corresponding author. In addition to the data reported in this article, we have patient acquired blood pressure data during the period of the study.
References
- 1.Gaziano TA, Bitton A, Anand S, et al. The global cost of non-optimal blood pressure. J Hypertens 2009;27:1472–7 [DOI] [PubMed] [Google Scholar]
- 2.Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension 2008;52:10–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serumanga B, Ross-Degnan D, Avery AJ, et al. Effect of pay for performance on the management and outcomes of hypertension in the United Kingdom: interrupted time series study. BMJ 2011;342:d108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okonofua EC, Simpson KN, Jesri A, et al. Therapeutic inertia is an impediment to achieving the healthy people 2010 blood pressure control goals. Hypertension 2006;47:345–51 [DOI] [PubMed] [Google Scholar]
- 5.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97 [DOI] [PubMed] [Google Scholar]
- 6.McKinstry B, Hanley J, Wild S, et al. Telemonitoring-based service redesign for the management of uncontrolled hypertension (HITS): a multi-centre randomised controlled trial. BMJ. In press [DOI] [PMC free article] [PubMed]
- 7.O'Brien E, Mee F, Atkins N, et al. Accuracy of the SpaceLabs 90207 determined by the British hypertension society protocol. J Hypertens 1991;9(Suppl 5):S25–31 [DOI] [PubMed] [Google Scholar]
- 8.Westhoff TH, Schmidt S, Zidek W, et al. Validation of the Stabil-O-Graph blood pressure self-measurement device. J Hum Hypertens 2008;22:233–5 [DOI] [PubMed] [Google Scholar]
- 9.Williams B, Poulter NR, Brown MJ, et al. The BHS guidelines working party. British hypertension society guidelines for hypertension management, 2004—BHS IV: summary. BMJ 2004;328:634–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence NICE guideline CG127: management of hypertension in adults in primary care. London: NICE, 2011 [Google Scholar]
- 11.Joint Formulary Committee The British National Formulary (BNF). London: BMJ Group and Pharmaceutical Press, 2011 [Google Scholar]
- 12.Curtis L. Unit costs of health & social care 2010. Kent: Personal Social Services Research Unit, 2010 [Google Scholar]
- 13.The Information Centre 2006/07 UK general practice workload survey, primary care statistics. Leeds: The Information Centre, 2007 [Google Scholar]
- 14.Heaney D, O'Donnell C, Wood A, et al. Evaluation of the introduction of NHS24 in Scotland, Final Report. Report to the Scottish Executive 2011. http://www.abdn.ac.uk/crh/uploads/files/National%20Evaluation%20of%20the%20introduction%20of%20NHS%2024%20in%20Scotland.pdf (accessed 7 Jul 2011).
- 15.Department of Health The reference costs 2009–10 publication. London: Department of Health, 2011 [Google Scholar]
- 16.Haymarket Medical Media The Monthly Index of Medical Specialities (MIMS). Haymarket Publications, 2011. http://www.mims.co.uk/ (accessed 12 Sep 2011). [Google Scholar]
- 17.Hughes DA, Tilson L, Drummond M. Estimating drug costs in economic evaluations in Ireland and the UK an analysis of practice and research recommendations. Pharmacoeconomics 2009;27:635–43 [DOI] [PubMed] [Google Scholar]
- 18.NHS Prescriptions Services The Drugs Tariff. http://www.ppa.org.uk/ppa/edt_intro.htm (accessed 26 Aug 2011).
- 19.ISD Scotland The Scottish National Tariff 2011/12. http://www.isdscotland.org/Health-Topics/Finance/Publications/2011-10-25/1112ScotTariffs.xls (accessed 10 Jan 2012). [Google Scholar]
- 20.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2010;30:377–99 [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons, 1987 [Google Scholar]
- 22.Dolan P. Modelling valuations for EuroQol health states. Med Care 1997;35:1095–108 [DOI] [PubMed] [Google Scholar]
- 23.Glick HA, Doshi JA, Sonnad AA, et al. Economic evaluation in clinical trials. Oxford: Oxford University Press, 2007 [Google Scholar]
- 24.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ 2001;20:461–94 [DOI] [PubMed] [Google Scholar]
- 25.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327–40 [DOI] [PubMed] [Google Scholar]
- 26.Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press, 2006 [Google Scholar]
- 27.Hanley J, Ure J, Pagliari C, et al. “You can't cheat the machine”: embedded multi- faceted qualitative exploration of the experiences of patients and professionals participating in the HITS home blood pressure telemonitoring trial. BMJ Open. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omboni S, Gazzola T, Carabelli G, et al. Clinical usefulness and cost-effectiveness of home blood pressure telemonitoring: meta-analysis of randomised controlled studies. J Hypertens 2012;31:455–68 [DOI] [PubMed] [Google Scholar]
- 29.McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomized controlled trial. Lancet 2010;376:163–72 [DOI] [PubMed] [Google Scholar]
- 30.Madsen LB, Kirkegaard P, Pedersen EB. Blood pressure control during telemonitoring of home blood pressure. A randomized controlled trial during 6 months. Blood Press 2008;17:78–86 [DOI] [PubMed] [Google Scholar]
- 31.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youman P, Wilson K, Harraf F, et al. The economic burden of stroke in the United Kingdom. Pharmacoeconomics 2003;21:43–50 [DOI] [PubMed] [Google Scholar]
- 33.National Institute for Health and Clinical Excellence (NICE) Guide to the methods of technology appraisal. London: NICE Publications, 2000 [PubMed] [Google Scholar]
- 34.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold what it is and what that means. Pharmacoeconomics 2008;26:733–44 [DOI] [PubMed] [Google Scholar]
- 35.AbuDagga A, Resnick HE, Alwan M. Impact of blood pressure telemonitoring on hypertension outcomes: a literature review. Telemed e-Health 2010;16:830–8 [DOI] [PubMed] [Google Scholar]
- 36.Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension Practice Guidelines for home blood pressure monitoring. J Hum Hypertens 2010;24:779–85 [DOI] [PubMed] [Google Scholar]
- 37.Office of National Statistics The Consumer Price Indices, 2011. http://www.ons.gov.uk/ons/datasets-and-tables/data-selector.html?dataset=mm23&table-id=1.1 (accessed 24 Oct 2011).
- 38.HMRC Exchange Rates—Yearly Lists. HMRC 2011. http://www.hmrc.gov.uk/exrate/yearly_rates.htm (accessed 28 Sep 2011).
- 39.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes, 3rd edn Oxford: Oxford University Press, 2005 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.