Abstract
Differentiated cells can be experimentally reprogrammed back to pluripotency by nuclear transfer, cell fusion or induced pluripotent stem cell technology. Nuclear transfer and cell fusion can lead to efficient reprogramming of gene expression. The egg and oocyte reprogramming process includes the exchange of somatic proteins for oocyte proteins, the post-translational modification of histones and the demethylation of DNA. These events occur in an ordered manner and on a defined timescale, indicating that reprogramming by nuclear transfer and by cell fusion rely on deterministic processes.
The remarkable stability of cell differentiation under normal conditions can be reversed experimentally by nuclear transfer, cell fusion and induced pluripotent stem (iPS) cell technology1-5. This provides an opportunity to generate pluripotent embryonic cells from adult cells of the same individual and hence opens the possibilit y of cell replacement without the need for immunosuppression.
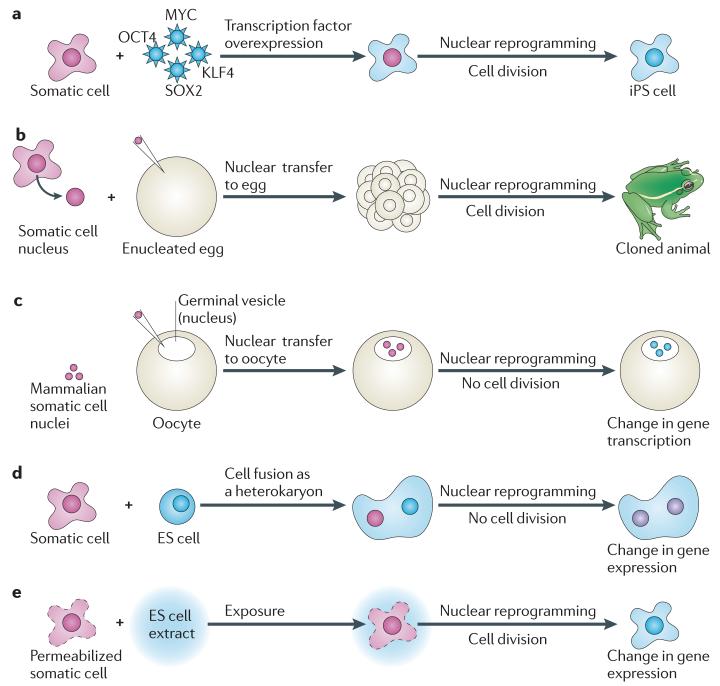
iPS cell technology makes use of the overexpression of transcription factors (FIG. 1a) and has been extensively reviewed, so it is not discussed in detail6-9. To generate entirely unrelated cell types by iPS cell technology, treated cells must be grown for multiple cell divisions over a long period of time (up to 3 weeks). By contrast, nuclear transfer and cell fusion do not involve the overexpression of new genes, and instead make use of natura components present in eggs and some early embryos to initiate new transcription.
Figure 1. Different experimental approaches to nuclear reprogramming.
a | Induced pluripotency. The expression of four transcription factors (Krüppel-like factor 4 (KLF4), MYC, OCT4 and Sry-box containing 2 (SOX2)) can reprogramme somatic cells to a state that is similar to that of embryonic stem (ES) cells, and these cells are called induced pluripotent stem (iPS) cells. This process involves many cell divisions and can occur at various times after expression of the transcription factors2,8. b | Nuclear transfer to eggs. A single Xenopus laevis (or mammalian) somatic cell nucleus is transplanted to an enucleated X. laevis (or mammalian) egg. In this experimental setting, a large number of cell divisions take place before new gene transcription is initiated. Eventually new cell types and new organisms are generated1,5. c | Nuclear transfer to X. laevis oocytes. Up to several hundred mammalian somatic cell nuclei are transplanted to the germinal vesicle (the nucleus) of a X. laevis oocyte. In these experimental conditions, the nuclei do not undergo cell division and new cell types are not generated. Instead, direct reprogramming of gene expression is triggered by exposure to the oocyte components10,11,16. d | Cell fusion. A differentiated cell is fused to another cell, such as an ES cell. In the resulting heterokaryon, the nucleus of the differentiated cell is exposed to ES cell factors and is reprogrammed to express stem cell-specific genes12,19,20. e | Reversible permeabilization and exposure to ES cell extract. Somatic cells permeabilized with streptolysin O can be briefly exposed to ES cell extract and then resealed. After culture of such treated cells, changes in gene expression can be detected14.
There are two kinds of nuclear transfer experiments: egg-NT involves the transfer of a single somatic nucleus to an unfertilize d enucleated egg (in both mammals and amphibians)1 (FIG. 1b); and ooc-NT involves the transplantation of multiple somatic cell nuclei into the germinal vesicle (the nucleus) of a growing meiotic prophase amphibian oocyte (an immature egg)10 (FIG. 1c). Note that the terms egg and oocyte refer to different developmental stages in amphibians and mammals: amphibian eggs are in metaphase II of meiosis, which is equivalent to mouse metaphase II stage oocytes, whereas their immediate precursors, oocytes, are blocked in meiotic prophase I, which is equivalent to mouse germinal vesicle stage oocytes.
There are important differences between the two types of nuclear transfer experimen t. Extensive cell division takes place in egg-NT experiments, and functional new cell types appear as the nuclear transplant embryo develops. By contrast, in ooc-NT experiments, no new cell types are formed, and neither the oocyte nor the introduced nuclei divide, but there is a direct transition from nuclei of differentiated cells to reprogrammed nuclei that transcribe pluripotency genes. Analysis of the mechanism of reprogramming (which involves transcription of pluripotency and other genes) in egg-NT experiments is complicated owing to rapid DNA replication and numerous cell divisions, with little or no transcription at first. By contrast, the only activity of first meiotic prophase oocytes is transcription, with no DNA replication or cell division. The same somatic cell chromatin is directly reprogrammed to express pluripotency genes within a day. Because of this complexity, in this Opinion article we focus primarily on ooc-NT experiments in amphibians10,11.
There are also two kinds of cell fusion experiments. In one, the two nuclei remain separate in the fused cell (a heterokaryon) and cell division is inhibited12 (FIG. 1d). In the other, the chromosomes of the fused cells assemble together on a shared mitotic spindle and form a fused nucleus (a synkaryon)13. Similarly to egg-NT, reprogramming in synkaryons is hard to analyse because of the cell divisions that follow the fusion of nuclei and cytoplasms. By contrast, heterokaryons allow the description and analysis of the events that accompany the reprogramming of a somatic cell nucleus in the absence of cell division.
An alternative to cell fusion is the exposure of somatic cells to embryonic stem (ES) cell extract14. Under these conditions, cell division-dependent reprogramming of gene expression is also observed (FIG. 1e). This type of experiment is not discussed furthe r in this Opinion article.
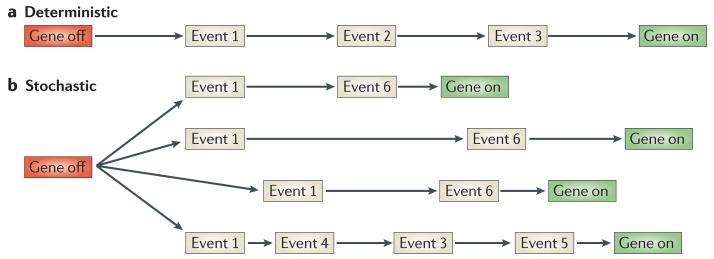
Reprogramming can be achieved by stochastic and deterministic mechanisms. What is the difference between the two? We regard a deterministic mechanism as that in which one or more steps are temporally and causally connected with a known initial stimulus (FIG. 2). By contrast, a stochasti c process is one that is unrelated in any simple way to the timing or sequence of events that cause it to take place. It has been proposed that re programming of somatic cells to iPS cells is stochastic, or at least includes stochastic events6,15. In this Opinion article, we summarize the reasons why we believe that nuclear reprogramming by nuclear transfer and cell fusion experiments depends mainly on deterministic steps.
Figure 2. Deterministic and stochastic events in transcriptional reprogramming.
A diagram to show a theoretical change in gene expression (in this case, the reactivation of a gene) that takes place during reprogramming. Several steps are required to allow the shift of a gene from an inactive to an active state. a | In the deterministic model, the inducer triggers an ordered series of determined events that lead from the original state to that of the reprogrammed state. In this case, the timescale is defined, and nearly all cells enter a fixed sequence of events in unison. b | In the stochastic model, some of the changes that take place are random in both nature and timing. Stochastic events during reprogramming can be characterized by an irregular sequence of events and a variable timescale. Only the inducer and final state are fixed.
Mechanisms of reprogramming
Below, we summarize what is now known about the events that accompany reprogramming in nuclear transfer to eggs and oocytes and in heterokaryon cell fusion experiments. Most of these events are necess ary steps towards successful transcriptional reprogramming.
Early morphological changes
In egg-NT and ooc-NT, and in cell fusion experiments, one of the first manifestations of reprogramming is the extensive swelling of nuclei. For example, mammalian nuclei transplanted into Xenopus laevis oocytes undergo up to a 30-fold increase in volume16. This change is reminiscent of what happens to sperm chromatin at fertilization. Chromatin decondensation seems to be a hallmark of reprogramming, as it is observed following nuclear transfer to eggs17 and oocytes18, in cell fusion experiments19,20, and in somatic nuclei exposed to egg extract21.
Because nuclear swelling directly correlates with RNA synthesis, these morphological changes reflect increased levels of transcription and successful reprogramming22. Indeed, the decondensation of chromatin is followed by an increase in transcriptio n, as measured by active polymerase II loading, bromine-labelled UTP (BrUTP) incorporation or histone H3 trimethylation on Lys4 (H3K4me3), and these changes are seen in almost all nuclei transplanted into oocytes18,23. Nucleoplasmin-mediated decondensation of nuclei before ooc-NT improves gene reactivation, demonstrating the importance of this process for successful transcriptional reprogramming21. Similarly, chromatin decondensation precedes gene reactivation following cell fusion20.
Changes in chromosomal proteins
The exposure of somatic nuclei to the egg cytoplasm or oocyte nucleus leads to marked changes in nuclear composition. Components of the transplanted somatic nuclear chromatin are replaced by egg or oocyte components. It has been shown that nuclear components of the egg or oocyte are necessary for successful reprogramming following nuclear transfer in both mammalian and amphibian species11,16,24,25.
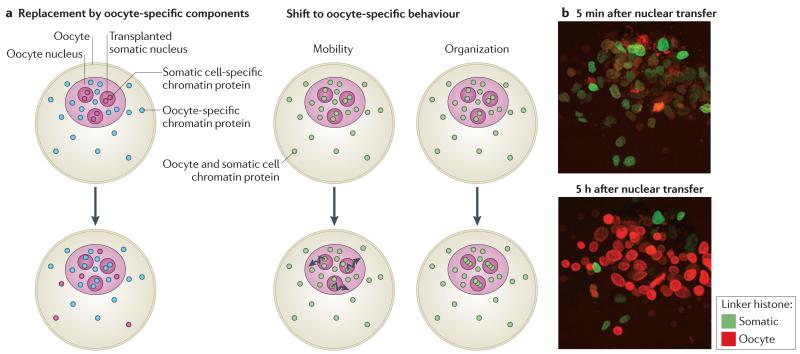
Two kinds of replacement can be distinguished. First, oocyte-specific components can displace their somatic counterparts, resulting in a change in the qualitative composition of chromatin (FIG. 3a). For example, the oocyte-specific linker histone H1foo in mice, or B4 in X. laevis, replaces somatic linker histone types present in somatic nuclei before transplantation11,26,27 (FIG. 3b). In nuclear transfer to X. laevis oocyte experiments, this replacement is required for the reactivation of the pluripotency genes oct4 (also known as pou5f1) and Sry-box containing 2 (sox2)11. It is likely that other oocyte-specific components also outcompete their somatic counterparts. Of particular importance might be the oocyte-specific basal transcription machinery, which is made up of TATA-box-binding protein 2 (TBP2) and TFIIAα/β-like factor (ALF), among other proteins28,29. Indeed, components of the basal transcription machinery start to emerge as important regulators of cell type-specific transcriptional programmes30.
Figure 3. Reprogramming of transplanted nuclei through the exchange of chromatin components.
a | Nuclear transfer is accompanied by an ordered series of chromatin component exchanges between the transplanted nucleus and the surrounding egg or oocyte environment. In some cases (left), chromatin factors of the transplanted nuclei are replaced by oocyte-specific components; for example, histone H1 is replaced with histone B4 (in Xenopus laevis), and TATA-box-binding protein (TBP) is replaced with TBP2. Alternatively, nuclear transfer results in the exchange of the same components between the somatic nucleus and the egg or oocyte (right). The oocyte environment modifies the behaviour of these chromatin proteins in transplanted chromatin so that, for example, their mobility (as with linker histone) or their organization (as with actin) shifts to that of an oocyte type. This series of events is a prerequisite for successful transcriptional reprogramming, and interfering with parts of this process impairs proper gene reactivation. b | Nuclei of NIH3T3 cells expressing somatic linker histone H1C tagged with green fluorescent protein were transplanted into Xenopus laevis oocytes expressing oocyte linker histone B4 tagged with red fluorescent protein. Images were taken 5 minutes and 5 hours after nuclear transfer. Images in part b are reproduced, with permission, from REF. 11 ©(2010) National Academy of Sciences.
The second type of replacement is one in which a somatic component is supplemented by the same component from the oocyte, resulting in a quantitative but not qualitative change (FIG. 3a). This is observed, for example, for chromatin proteins involved in repression, such as heterochromatin protein 1 (HP1; also known as CBX) and BMI1. These are rapidly loaded onto chromatin from the oocyte stock, replacing and supplementing their somatic counter parts. These changes might seem to be functionally neutral, as the same protein will occupy the same site on chromatin. However, the exchange rate of these proteins is changed, as shown by fluorescence recovery after photobleaching (FRAP) analysis11,27, indicating an increase in chromatin mobility similar to what is observed in pluripotent cells31.
Oocytes also contain a high concentration of nuclear components that are already present in somatic cells, albeit at lower levels. This is the case for nuclear actin, the polymerized form of which is increased in nuclei transplanted into X. laevis oocytes32. These changes in nuclear composition are required for gene reactivation following ooc-NT and contribute to nuclear reprogramming on a defined timescale.
Core histones do not show high levels of exchange during the first few hours following ooc-NT. In general, core histones are relatively immobile in the chromatin of cultured cells when compared with other chromatin components (such as linker histones, high mobility group (HMG) proteins and HP1)33-36. In the case of the core histone H3, only the variant H3.3 is loaded onto chromatin within the first day following nuclear transfer. This process depends on the histone chaperone HIRA and is a necessary step towards transcriptional reprogramming (J.J., C. Astrand, J.B.G. and G. Almouzni, unpublished observations).
The exchange of proteins between nuclei can also be observed in heterokaryon experiments. For example, in human lymphocyte-mouse ES cell heterokaryons, the ES cell transcription factor OCT4 is loaded onto lymphocyte chromatin37, and this is required for transcriptional reprogramming of the lymphocyte. Similarly, structural components of chromatin, such as HMG proteins, are efficiently exchanged between the nuclei of a heterokaryon38. Of note, the highly mobile histone H1 is not exchanged between the nuclei of a heterokaryon39; this is in sharp contrast to the highly efficient loading of this component onto nuclei transplanted to eggs and oocytes11,26,27. One explanation for this discrepancy is that, despite their high mobility and abundance, chromosomal proteins such as linker histone H1 do not shuttle between the cytoplasm and nuclei of a heterokaryon, and this reduces their ability to travel between nuclei39.
Histone and DNA modifications
Oocytes and eggs are characterized by the presence of high levels of enzymatic activity directed towards the oocyte and sperm genome to prepare the embryonic chromatin for development40. This high enzymatic activity towards chromatin is recapitulated after nuclear transfer. Therefore, reprogramming by nuclear transfer uses the developmental programme that is normally used after fertilization.
For example, the core histones H3 and H4, although not extensively replaced after nuclear transfer, undergo extensive modification, including phosphorylation, methyl ation and acetylation23,41. The timing of H3K4 methylation correlates with pluripotency gene activation. These modifications of histone tails are likely to be the result of the high levels of chromatin-modifying enzymes stored in oocytes40.
Eggs and oocytes also possess active DNA demethylation activity, which is a requirement for the transcriptional reactivation of silenced genes (for example, OCT4); this activity is observed after nuclear transfer41-44, as well as in cell fusion to ES cells45. Two examples of enzymes that are thought to play a part in active DNA demethylation are growth arrest and DNA damage-inducible 45α (GADD45α)42 and activation-induced deaminase (AID)45. More recently, in mouse eggs and early embryos, the TET enzymes have been shown to help active DNA demethylation by hydroxylating of methylated cytosine46-48. This active process leads to replication-independent changes in the levels of methylated cytosine in DNA.
These numerous enzymatic activities of eggs and oocytes that are directed against chromatin are likely to orchestrate the reprogramming events following nuclear transfer.
Transcription
In nuclear transfer experiments, the direct transcriptional reprogramming of pluripotency genes is observed after the initial phase of nuclear swelling and chromatin protein exchange. Typically, reactivation of previously silent genes is detected between 24 and 48 hours after nuclear transplantation10,11,16,43. Similar kinetics are observed following mouse ES cell fusion to human lympho cytes37 or fibroblasts45. The oocyte exerts its transcriptional reprogramming activity on a very large scale, not only reactivating pluripotency genes, such as OCT4, SOX2 and NANOG, but also activating, to a certain extent, many different genes that are not actively transcribed in the oocyte49. This general increase in transcriptional activity is reminiscent of the genome-wide increase in transcription that is observed in ES cells50.
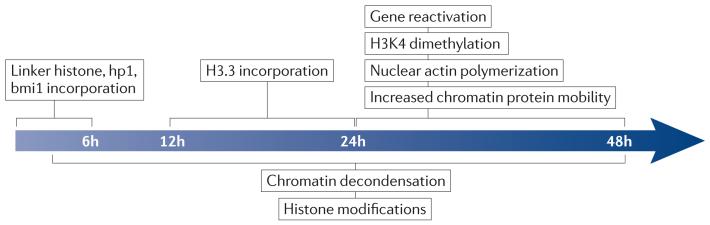
The occurrence of these transcriptional events is predictable in both timing and extent (all nuclei show changes), supporting a deterministic nature of the underlying mechanism (FIG. 4). Moreover, it has now been shown that some of these events are necessary steps towards successful transcriptional reprogramming11,32.
Figure 4. A temporal sequence of events in transcriptional reprogramming following nuclear transfer to Xenopus laevis oocytes.
Specific nuclear reprogramming events that happen following transplantation of nuclei to Xenopus laevis oocytes are shown. The first identified events following transplantation are the rapid exchange of mobile chromatin components, such as linker histone, heterochromatin protein 1 (hp1) and bmi1. The next step is the incorporation of histone variant H3.3, followed by increased chromatin protein mobility, nuclear actin polymerization, histone H3 Lys4 (H3K4) dimethylation of promoters and reactivation of previously silent genes. The decondensation of chromatin and additional modifications of histone tails (such as phosphorylation) take place throughout the reprogramming period. The mechanistic link between the events of this determined sequence in the reprogramming process is not known.
Reprogramming efficiency
Nuclear reprogramming can be measured according to several criteria51. Its efficiency can be judged by the frequency with which a reprogramming event occurs in a cell population, as well as by the timing of the occurrence of this event. From the use of these particular parameters we can infer a stochastic versus deterministic nature of the process (FIG. 2), with stochastic and deterministic processes generally having low and high efficiency, respectively.
The efficiency of reprogramming in iPS cells is regarded as low, and therefore more difficult to analyse, because many cell divisions over a long time are required for a minority of cells to show a change in the expression of pluripotency genes52. By contrast, in ooc-NT experiments10,11 and in some egg-NT studies53-55, the transferred somatic cell nuclei seem to be reprogrammed with high efficiency and on a defined timescale. This view is based on two criteria. First, in ooc-NT experiments, nearly all transplanted nuclei always show incorporation of oocyte chromatin proteins11,26,27 and new transcription after nuclear transfer (see below). Second, differentiated cell nuclei transplanted to oocytes reactivate Sox2 to the level of undifferentiated cell nuclei11. This suggests that all the transplanted nuclei are transcriptionally reprogrammed to reexpress this gene. Similarly, in some types of egg-NT experiments, up to 100% of the reconstructed embryos show reactivation of an Oct4 reporter53-55.
On the basis of these criteria, it seems that nuclei transplanted to oocytes and eggs are efficiently reprogrammed on a defined timescale and that the changes undergone by each transplanted nucleus are relevant to the reprogramming process. These are characteristics of a deterministic process. Nevertheless, defects in gene expression following nuclear transfer to oocytes and eggs have also been reported in both mice and amphibians, indicating the existence of mechanisms that restrict transcriptional reprogramming of transplanted nuclei56-58. In this case, reprogramming in egg-NT experiments may depend on stochastic events to release restriction and allow reprogrammin g to occur.
Cell fusion experiments also show high reprogramming efficiency, which is indicative of a deterministic process. In human lymphocyte-mouse muscle cell heterokaryons, nearly all of the human nuclei that have been successfully incorporated into a heterokaryon show active transcription of a muscle marker gene20, indicating high reprogramming efficiency. Similarly, heterokaryons formed by the fusion of human cells with mouse ES cells show a high efficiency of reprogramming (as high as 70%), as judged by the percentage both of nuclei showing pluripotency gene reactivation and of the demethylation of the OCT4 promoter37,45; the time course suggests a defined timescale.
Deterministic or stochastic?
Most of the processes that we understand in normal development are usually thought to depend on deterministic mechanisms. For example, early cell fate decisions, including the induction of mesoderm by vegetal cells (known as Nieuwkoop signalling) and of the neural tube by the mesoderm (known as Spemann signalling), result from the release of signal factors and their binding by receptors on nearby cells. These events take place in a defined sequence, on a precise timescale and with complete efficiency. By contrast, the iPS cell route to reprogramming affects only a small proportion of similarly treated cells, and the derived pluripotent cells appear at irregular time intervals and in an undefined sequence. On the basis of these criteri a, the iPS cell route to reprogramming is thought to include stochastic steps.
Nonetheless, recent data suggest that the initiation of reprogramming through the expression of the Yamanaka factors (Krüppe-l-like factor 4 (KLF4), MYC, OCT4 and SOX2) includes some chromatin changes that are characteristic of a deterministic process. For example, efficient downregulation of somatic gene expression was observed, as was widespread post-translational modification of histones on the regulatory regions of many of the target genes of Yamanaka factors59-61.
We have summarized here the steps so far known to take place during reprogramming by nuclear transfer and cell fusion, focusing on amph ibian ooc-NT studies (FIG. 4). Reprogramming through ooc-NT in amphibians seems to take place in a defined sequence, and on a relatively short timescale at 17°C (a temperature at which the X. laevis adult, embryo and isolated oocyte can be maintained). We have also emphasized that most of the steps that have been identified are achieved with high efficiency, so that nearly all transplanted nuclei undergo the same change at the same time. We therefore propose that reprogramming by ooc-NT and egg-NT is achieved by a mostly deterministic mechanism. We suggest that this may also be true of hetero karyon cell fusion experiments.
The deterministic nature of the events for oocyte and egg reprogramming probably depends on two characteristics of these cells. One is that oocytes are endowed with an exceptionally high concentration of components such as B4 histone, H3.3 histone and nucleoplasmin. This accumulation, coupled with a fast exchange rate for chromosomal proteins (in the order of minutes or hours, not days), would account for the observed global-scale exchange of nuclear components following nuclear transfer. This event includes the accumulation of oocyte and egg components that are very permissive to the activity of chromatin-remodelling complexes and that therefore facilitate transcription factor access to their binding sites62. Oocytes also have special enzymatic activities, such as histone-modifying and DNA-demethylating enzymes. These activities of oocyte components are likely to be related to the natural reprogramming activities of the egg, which reprogrammes incomin g sperm after fertilization.
It is not yet clear how the reprogramming mechanisms at work in the various available experimental settings are related. In particular, it is not known whether the transcription factors involved in all reprogramming experiments (such as OCT4 and SOX2, which accumulate in eggs, oocytes and ES cells and are exogenously provided to iPS cells) are used in the same way. The stochastic steps of reprogramming through the iPS cell route may depend on random access of these transcription factors to their many targets scattered throughout the genome. This could reflect the decrease in accessibilit y of repressed chromatin that accompanies cell differentiation (BOX 1). The higher reprogramming efficiency observed through nuclear transfer may rely on the additional activities present in eggs and oocytes that allow better access of these transcription factor s to chromatin.
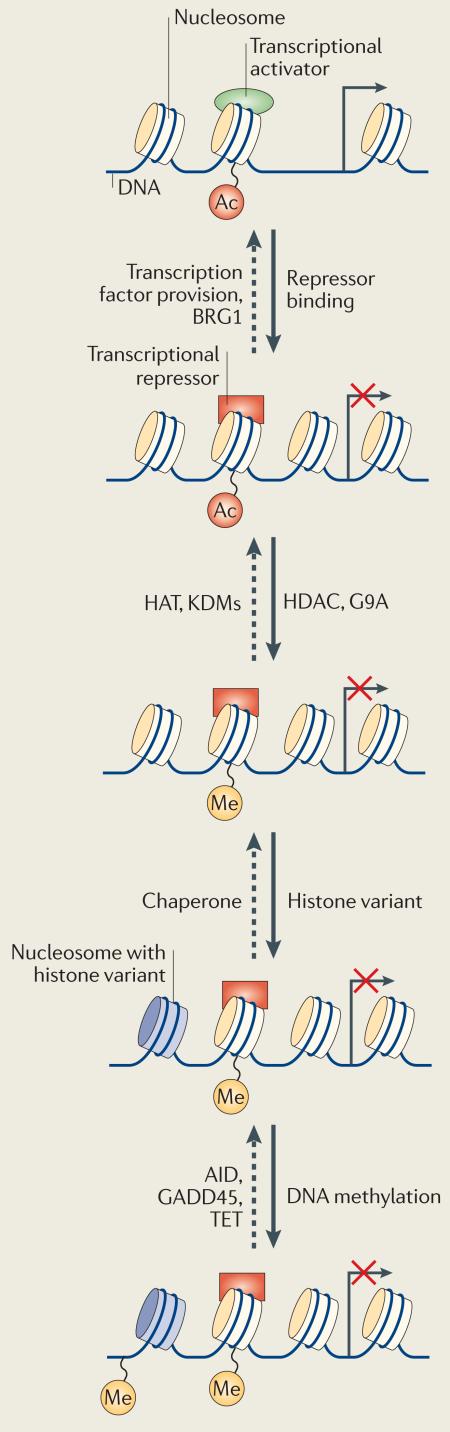
Box 1. Combinatorial control of gene status and resistance to reprogramming.
In general, the more differentiated a cell is, the more resistant it is to the reprogramming process10,12,66-68. What is responsible for this resistance to reprogramming? Differences between cell types in resistance to reprogramming could be explained by the presence of different layers of regulation in different cells, which could control the status of a gene. The various repressed states through which the pluripotency gene OCT4 (also known as POU5F1) transits during differentiation of embryonic stem (ES) cells exemplify this phenomenon (see the figure). During retinoic acid-mediated differentiation of mouse ES cells, Oct4 transcription is first shut down by the replacement of the activator LHR1 with the repressor germ cell nuclear factor (GCNF) on the proximal promoter of the gene69,70 and by the remodelling of nucleosome positions71. Next, the recruitment of chromatin modifiers, such as histone deacetylase (HDAC) and G9A, contributes to a shift from acetylation to methylation on Lys9 of histone H3 (REFS 72,73). Incorporation of repressive histone variants can also take place. Finally, recruitment of DNA methyltransferase 3 (DNMT3) leads to DNA methylation of the gene. Interfering with any of these events during differentiation leads to partial reversibility of Oct4 silencing72,73. As differentiation of a cell proceeds, the gene states are sealed by combinatorial mechanisms (such as changes in histone modifications, histone variants and DNA methylation). Increasing resistance to nuclear reprogramming may depend on the existence of such combinatorial mechanisms of control74. The eggs and oocytes contain components (transcription factors, histone modifiers and chaperone and DNA demethylation complexes) that can, in theory, revert each of the steps involved in gene silencing during differentiation (see the figure; indicated by dashed arrows). The progressive combination of elements added during development to control the status of a gene could be responsible for the overall low efficiency of the reversal process. Alternatively, resistance to reprogramming may be the result of a single event in gene regulation, happening late in the differentiation process, and for which eggs and oocytes lack efficient means of reversal.
AID, activation-induced deaminase; GADD45, growth arrest and DNA damage-inducible 45; HAT, histone acetyl transferase; KDMs, Lys-specific histone demethylases.
Nonetheless, some aspects of reprogramming by nuclear transfer are not fully efficient. This is because epigenetic mechanisms ensuring the stability of the differentiated state confer a resistance to these reprogramming activities of the egg and oocyte74. Indeed, nuclear transfer can be improved by interfering with the epigenetic state of the donor nuclei, for example their DNA methylation and histone acetylation levels or their expression of non-coding RNAs63-65. One can speculate that the eggs and oocytes are not fully equipped to reverse the effects of specific components that restrict nuclear plasticit y, especially in highly differentiated cells (BOX 1). In this case, stochastic events may be required to release such a blockage and allow full reprogramming. A deeper understanding of the characteristics of stochastic versus deterministic reprogramming modes may help to design more efficient ways of reprogramming somatic cells to pluripotency. Of particular importance will be the characterization of the mechanisms associated with cell differentiation that restrict the reprogramming process (BOX 1). Finally, identification of the key steps involved in reprogramming will be facilitated by the deterministic nature of reprogramming by eggs and oocytes. In particular, a better understanding of the mechanistic link between the reprogramming events that are characteristic of nuclear transfer (FIG. 4) will help to define the key steps underlying the reprogramming process.
Acknowledgements
V.P. is supported by a Wallonia-Brussels International Excellence Grant. R.P.H.-S. is supported by the National Research Foundation of South Africa and the Cambridge Commonwealth Trust, UK. K.M. is supported by the Japan Society for the Promotion of Science (International Research Fellowship Program). This work was also supported by the Wellcome Trust (RHG54943).
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
J. B. Gurdon’s homepage: http://www.gurdon.cam.ac.uk/gurdon.html
References
- 1.Gurdon JB, Elsdale TR, Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature. 1958;182:64–65. doi: 10.1038/182064a0. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Blau HM, Chiu CP, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- 4.Eggan K, et al. Mice cloned from olfactory sensory neurons. Nature. 2004;428:44–49. doi: 10.1038/nature02375. [DOI] [PubMed] [Google Scholar]
- 5.Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- 6.Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460:49–52. doi: 10.1038/nature08180. [DOI] [PubMed] [Google Scholar]
- 7.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143:508–525. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halley-Stott RP, et al. Mammalian nuclear transplantation to germinal vesicle stage Xenopus oocytes — a method for quantitative transcriptional reprogramming. Methods. 2010;51:56–65. doi: 10.1016/j.ymeth.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jullien J, Astrand C, Halley-Stott RP, Garrett N, Gurdon JB. Characterization of somatic cell nuclear reprogramming by oocytes in which a linker histone is required for pluripotency gene reactivation. Proc. Natl Acad. Sci. USA. 2010;107:5483–5488. doi: 10.1073/pnas.1000599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blau HM, et al. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- 13.Kimura H, Tada M, Nakatsuji N, Tada T. Histone code modifications on pluripotential nuclei of reprogrammed somatic cells. Mol. Cell. Biol. 2004;24:5710–5720. doi: 10.1128/MCB.24.13.5710-5720.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taranger CK, et al. Induction of dedifferentiation, genomewide transcriptional programming, and epigenetic reprogramming by extracts of carcinoma and embryonic stem cells. Mol. Biol. Cell. 2005;16:5719–5735. doi: 10.1091/mbc.E05-06-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna J, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne JA, Simonsson S, Western PS, Gurdon JB. Nuclei of adult mammalian somatic cells are directly reprogrammed to oct-4 stem cell gene expression by amphibian oocytes. Curr. Biol. 2003;13:1206–1213. doi: 10.1016/s0960-9822(03)00462-7. [DOI] [PubMed] [Google Scholar]
- 17.Gao T, et al. Nuclear reprogramming: the strategy used in normal development is also used in somatic cell nuclear transfer and parthenogenesis. Cell Res. 2007;17:135–150. doi: 10.1038/cr.2007.2. [DOI] [PubMed] [Google Scholar]
- 18.Gurdon JB. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J. Embryol. Exp. Morphol. 1968;20:401–414. [PubMed] [Google Scholar]
- 19.Harris H. The reactivation of the red cell nucleus. J. Cell Sci. 1967;2:23–32. doi: 10.1242/jcs.2.1.23. [DOI] [PubMed] [Google Scholar]
- 20.Terranova R, Pereira CF, Du Roure C, Merkenschlager M, Fisher AG. Acquisition and extinction of gene expression programs are separable events in heterokaryon reprogramming. J. Cell Sci. 2006;119:2065–2072. doi: 10.1242/jcs.02945. [DOI] [PubMed] [Google Scholar]
- 21.Tamada H, et al. Chromatin decondensation and nuclear reprogramming by nucleoplasmin. Mol. Cell. Biol. 2006;26:1259–1271. doi: 10.1128/MCB.26.4.1259-1271.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurdon JB, Partington GA, De Robertis EM. Injected nuclei in frog oocytes: RNA synthesis and protein exchange. J. Embryol. Exp. Morphol. 1976;36:541–553. [PubMed] [Google Scholar]
- 23.Murata K, Kouzarides T, Bannister AJ, Gurdon JB. Histone H3 lysine 4 methylation is associated with the transcriptional reprogramming efficiency of somatic nuclei by oocytes. Epigenetics Chromatin. 2010;3:4. doi: 10.1186/1756-8935-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egli D, Rosains J, Birkhoff G, Eggan K. Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature. 2007;447:679–685. doi: 10.1038/nature05879. [DOI] [PubMed] [Google Scholar]
- 25.Greda P, Karasiewicz J, Modlinski JA. Mouse zygotes as recipients in embryo cloning. Reproduction. 2006;132:741–748. doi: 10.1530/rep.1.01204. [DOI] [PubMed] [Google Scholar]
- 26.Gao S, et al. Rapid H1 linker histone transitions following fertilization or somatic cell nuclear transfer: evidence for a uniform developmental program in mice. Dev. Biol. 2004;266:62–75. doi: 10.1016/j.ydbio.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Teranishi T, et al. Rapid replacement of somatic linker histones with the oocyte-specific linker histone H1foo in nuclear transfer. Dev. Biol. 2004;266:76–86. doi: 10.1016/j.ydbio.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 28.D’Alessio JA, Wright KJ, Tjian R. Shifting players and paradigms in cell-specific transcription. Mol. Cell. 2009;36:924–931. doi: 10.1016/j.molcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikyo N, Wade PA, Guschin D, Ge H, Wolffe AP. Active remodeling of somatic nuclei in egg cytoplasm by the nucleosomal ATPase ISWI. Science. 2000;289:2360–2362. doi: 10.1126/science.289.5488.2360. [DOI] [PubMed] [Google Scholar]
- 30.Goodrich JA, Tjian R. Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation. Nature Rev. Genet. 2010;11:549–558. doi: 10.1038/nrg2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meshorer E, et al. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamoto K, Pasque V, Jullien J, Gurdon JB. Nuclear actin polymerization is required for transcriptional reprogramming of Oct4 by oocytes. Genes Dev. 2011;25:946–958. doi: 10.1101/gad.615211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura H, Cook PR. Kinetics of core histones in living human cells: little exchange of H3 and H4 and some rapid exchange of H2B. J. Cell Biol. 2001;153:1341–1353. doi: 10.1083/jcb.153.7.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phair RD, et al. Global nature of dynamic protein-chromatin interactions in vivo : three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol. Cell. Biol. 2004;24:6393–6402. doi: 10.1128/MCB.24.14.6393-6402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misteli T, Gunjan A, Hock R, Bustin M, Brown DT. Dynamic binding of histone H1 to chromatin in living cells. Nature. 2000;408:877–881. doi: 10.1038/35048610. [DOI] [PubMed] [Google Scholar]
- 36.Schmiedeberg L, Weisshart K, Diekmann S, Meyer Zu Hoerste G, Hemmerich P. High- and low-mobility populations of HP1 in heterochromatin of mammalian cells. Mol. Biol. Cell. 2004;15:2819–2833. doi: 10.1091/mbc.E03-11-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira CF, et al. Heterokaryon-based reprogramming of human B lymphocytes for pluripotency requires Oct4 but not Sox2. PLoS Genet. 2008;4:e1000170. doi: 10.1371/journal.pgen.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rechsteiner M, Kuehl L. Microinjection of the nonhistone chromosomal protein HMG1 into bovine fibroblasts and HeLa cells. Cell. 1979;16:901–908. doi: 10.1016/0092-8674(79)90105-3. [DOI] [PubMed] [Google Scholar]
- 39.Wu LH, Kuehl L, Rechsteiner M. Dynamic behavior of histone H1 microinjected into HeLa cells. J. Cell Biol. 1986;103:465–474. doi: 10.1083/jcb.103.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boiani M, et al. Proteomic analysis of mouse oocytes reveals 28 candidate factors of the ‘reprogrammome’. J. Proteome Res. 2011;10:2140–2153. doi: 10.1021/pr100706k. [DOI] [PubMed] [Google Scholar]
- 41.Bian Y, Alberio R, Allegrucci C, Campbell KH, Johnson AD. Epigenetic marks in somatic chromatin are remodelled to resemble pluripotent nuclei by amphibian oocyte extracts. Epigenetics. 2009;4:194–202. doi: 10.4161/epi.4.3.8787. [DOI] [PubMed] [Google Scholar]
- 42.Barreto G, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–675. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- 43.Simonsson S, Gurdon J. DNA demethylation is necessary for the epigenetic reprogramming of somatic cell nuclei. Nature Cell Biol. 2004;6:984–990. doi: 10.1038/ncb1176. [DOI] [PubMed] [Google Scholar]
- 44.Wossidlo M, et al. Dynamic link of DNA demethylation, DNA strand breaks and repair in mouse zygotes. EMBO J. 2010;29:1877–1888. doi: 10.1038/emboj.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhutani N, et al. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature. 2010;463:1042–1047. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wossidlo M, et al. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nature Commun. 2011;2:241. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- 47.Ficz G, et al. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011;473:398–402. doi: 10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- 48.Ito S, et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biddle A, Simeoni I, Gurdon JB. Xenopus oocytes reactivate muscle gene transcription in transplanted somatic nuclei independently of myogenic factors. Development. 2009;136:2695–2703. doi: 10.1242/dev.036327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Efroni S, et al. Global transcription in pluripotent embryonic stem cells. Cell Stem Cell. 2008;2:437–447. doi: 10.1016/j.stem.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasque V, Miyamoto K, Gurdon JB. Efficiencies and mechanisms of nuclear reprogramming. Cold Spring Harb. Symp. Quant. Biol. 2010;75:189–200. doi: 10.1101/sqb.2010.75.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith ZD, Nachman I, Regev A, Meissner A. Dynamic single-cell imaging of direct reprogramming reveals an early specifying event. Nature Biotech. 2010;28:521–526. doi: 10.1038/nbt.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith C, et al. Simultaneous gene quantitation of multiple genes in individual bovine nuclear transfer blastocysts. Reproduction. 2007;133:231–242. doi: 10.1530/rep.1.0966. [DOI] [PubMed] [Google Scholar]
- 54.Smith SL, et al. Global gene expression profiles reveal significant nuclear reprogramming by the blastocyst stage after cloning. Proc. Natl Acad. Sci. USA. 2005;102:17582–17587. doi: 10.1073/pnas.0508952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egli D, Sandler VM, Shinohara ML, Cantor H, Eggan K. Reprogramming after chromosome transfer into mouse blastomeres. Curr. Biol. 2009;19:1403–1409. doi: 10.1016/j.cub.2009.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boiani M, Eckardt S, Scholer HR, McLaughlin KJ. Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev. 2002;16:1209–1219. doi: 10.1101/gad.966002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bortvin A, et al. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development. 2003;130:1673–1680. doi: 10.1242/dev.00366. [DOI] [PubMed] [Google Scholar]
- 58.Ng RK, Gurdon JB. Epigenetic memory of active gene transcription is inherited through somatic cell nuclear transfer. Proc. Natl Acad. Sci. USA. 2005;102:1957–1962. doi: 10.1073/pnas.0409813102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koche RP, et al. Reprogramming factor expression initiates widespread targeted chromatin remodeling. Cell Stem Cell. 2011;8:96–105. doi: 10.1016/j.stem.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sridharan R, et al. Role of the murine reprogramming factors in the induction of pluripotency. Cell. 2009;136:364–377. doi: 10.1016/j.cell.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saeki H, et al. Linker histone variants control chromatin dynamics during early embryogenesis. Proc. Natl Acad. Sci. USA. 2005;102:5697–5702. doi: 10.1073/pnas.0409824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blelloch R, et al. Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells. 2006;24:2007–2013. doi: 10.1634/stemcells.2006-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kishigami S, et al. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem. Biophys. Res. Commun. 2006;340:183–189. doi: 10.1016/j.bbrc.2005.11.164. [DOI] [PubMed] [Google Scholar]
- 65.Inoue K, et al. Impeding Xist expression from the active X chromosome improves mouse somatic cell nuclear transfer. Science. 2010;330:496–499. doi: 10.1126/science.1194174. [DOI] [PubMed] [Google Scholar]
- 66.Gurdon JB. The developmental capacity of nuclei taken from differentiating endoderm cells of Xenopus laevis. J. Embryol. Exp. Morphol. 1960;8:505–526. [PubMed] [Google Scholar]
- 67.Hiiragi T, Solter D. Reprogramming is essential in nuclear transfer. Mol. Reprod. Dev. 2005;70:417–421. doi: 10.1002/mrd.20126. [DOI] [PubMed] [Google Scholar]
- 68.Hanna J, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu P, et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol. Cell. Biol. 2005;25:3492–3505. doi: 10.1128/MCB.25.9.3492-3505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gu P, et al. Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Mol. Cell. Biol. 2005;25:8507–8519. doi: 10.1128/MCB.25.19.8507-8519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 71.Chuang YS, et al. Promyelocytic leukemia protein in retinoic acid-induced chromatin remodeling of Oct4 gene promoter. Stem Cells. 2011;29:660–669. doi: 10.1002/stem.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Epsztejn-Litman S, et al. De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nature Struct. Mol. Biol. 2008;15:1176–1183. doi: 10.1038/nsmb.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feldman N, et al. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nature Cell Biol. 2006;8:188–194. doi: 10.1038/ncb1353. [DOI] [PubMed] [Google Scholar]
- 74.Pasque V, Gillich A, Garrett N, Gurdon JB. Histone variant macroH2A confers resistance to nuclear reprogramming. EMBO J. 2011 May 6; doi: 10.1038/emboj.2011.144. doi:10.1038/emboj.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]