Abstract
Binge drinking during adolescence may perturb the maturing neuroenvironment and increase susceptibility of developing an alcohol use disorder later in life. In the present series of experiments, we utilized a modified version of the drinking in the dark-multiple scheduled access (DID-MSA) procedure to study how heavy binge drinking during adolescence alters responsivity to ethanol later in adulthood. Adult and adolescent C57BL/6J (B6) and DBA/2J (D2) males and females were given access to a 20% ethanol solution for 3 hourly periods, each separated by 2 h of free water access. B6 adults and adolescents consumed 2 to 3.5 g/kg ethanol an hour and displayed significant intoxication and binge-like blood ethanol concentrations. There was an interaction of sex and age, however, driven by high intakes in adult B6 females, who peaked at 11.01 g/kg. Adolescents of both sexes and adult males never consumed more than 9.3 g/kg. D2 mice consumed negligible amounts of alcohol and showed no evidence of intoxication. B6 mice were abstinent for one month and were retested on the balance beam 10 min following 1.75 g/kg ethanol challenge (20%v/v; i.p). They were also tested for changes in home cage locomotion immediately following the 1.75 g/kg dose (for 10 min prior to balance beam). Although there was no effect of age of exposure, all mice with a binge drinking history demonstrated a significantly dampened ataxic response to an ethanol challenge. Female mice that binge drank during adulthood showed a significantly augmented locomotor response to ethanol when compared to their water drinking controls. This alteration was not noted for males or for females that binge drank during adolescence. These results highlight the importance of biological sex, and its interaction with age, in the development of behavioral adaptation following binge drinking.
Keywords: Adolescence, DBA/2J, C5BL/6J, Ethanol, Motor impairment, Drinking in the dark, Intermittent access, Mouse, Alcohol, Sex differences
1. Introduction
Adolescence is a major stepping-stone inmammalian development. It is a period characterized by substantial changes in brain structure, systems and connectivity, and includes reorganization of neurochemical networks, and increases in synaptic pruning and myelination (Bava and Tapert, 2010; Giedd, 2004; Spear and Brake, 1983; Tamnes et al., 2011). The dramatic brain changes that occur at this time period may leave the central nervous system especially vulnerable to adulteration by drugs and alcohol. Consequently, the high rate of binge alcohol consumption in this age group elicits concern (Johnston et al., 2007). Alcohol use during this time period may not only perturb the neuroenvironment, but may also stunt maturation and increase susceptibility to the development of dependence and abuse (Crews et al., 2007; Witt, 2010). Indeed, there is a strong relationship between age of first drink and rate of alcohol dependence (Dawson et al., 2008; Hingson et al., 2006; Pitkänen et al., 2005). Our research team has previously shown a positive relationship between binge alcohol consumption during adolescence and higher than average consumption of the drug during adulthood (Moore et al., 2010). Interestingly, we have also shown that both sensitivity to alcohol during adolescence, and the effects of adolescent alcohol exposure on adult receptivity to the drug, may be modulated by genetic background (Melón and Boehm, 2011; Moore et al., 2010). This is not surprising, as a substantial body of literature supports a role for genetic background in the progression from recreational drug use or social drinking to abuse and addiction. Furthermore, though most alcohol consumers initiate use prior to the end of adolescence, only a small percentage of those go on to develop an alcohol use disorder. However, little is known about how the interaction between genetics and ontogeny alters the effect of adolescent exposure on the risk of developing addiction during adulthood.
Given the ethical limitations of human research, animal models are crucial to our ability to clarify the independent and/or synergistic roles of genetics and ontogeny with respect to the vulnerability to develop alcohol use problems (Zucker et al., 2008). Unfortunately, many animal models of voluntary alcohol consumption yield higher alcohol intake among adolescents than adults (Doremus et al., 2005; García-Burgos et al., 2009; Maldonado et al., 2008; Moore et al., 2010; Vetter et al., 2007). Although this highlights the face and ecological validity of these animal models in representing alcohol related behaviors seen in human adolescents, it makes it difficult to isolate the importance of age of exposure from the general pathological effects of high alcohol intake. Put another way, in experimental models where adolescent rodents actually consume more alcohol than their adult conspecifics, it is impossible to infer whether the effects seen following this early pre-exposure were due to the age at which the animals were drinking, or to the amount of alcohol to which the animals were exposed. With this in mind, we adapted the recently characterized drinking in the dark-multiple scheduled access (DID-MSA) paradigm (Bell et al., 2006, 2011) in order to induce home cage binge drinking in mice. Like the drinking-in-the-dark (DID) paradigm (Rhodes et al., 2005, 2007), this procedure is an oral self-administration protocol that takes place in the animal's home environment. Although the original DID-MSA protocol has been shown to induce age-dependent binge drinking behavior in rats (Bell et al., 2011), preliminary evidence from our laboratory suggested that this adapted access schedule could produce similar alcohol consumption across adolescent and adult mice.
The goals of the present series of experiments were threefold: 1) to characterize the level of consumption and intoxication achieved using the DID-MSA procedure in adolescent and adult C57Bl/6J (B6) mice; 2) to assess whether age of exposure moderates the development of functional tolerance to intoxication following multiple binge sessions and 3) to evaluate whether age of exposure affects later sensitivity to alcohol. We hypothesized that this modified DID-MSA protocol would initiate high but comparable levels of intake in B6 adults and adolescents and that later sensitivity to alcohol would be affected by age of exposure in this strain. Given our ultimate interest in exploring the interaction of ontogeny and genetics in moderating the effects of alcohol exposure, we also included the alcohol non-preferring, DBA/2J inbred mouse strain to see whether this type of scheduled drinking procedure could induce any level of relevant alcohol intake in these mice.
2. Methods
2.1. Subjects
Male and female DBA/2J (D2) and C57BL/6J (B6) adult (PD 60±3) and adolescent (PD 30±3) mice were purchased from Jackson Laboratory (N=251 mice). Animals arrived at the Indiana University-Purdue University Indianapolis School of Science animal facility at PD 21±3 or PD 56±3. Animals were singly housed in standard shoebox cages and were habituated to the facility for seven days. Mice were maintained across two holding rooms, each kept at 21±1 °C and approximately 50% humidity. An anteroom, where all mice were moved for daily weights, separated the holding rooms. Behavioral testing and blood retrieval also occurred in this anteroom. Food and water were available ad libitum, except during alcohol access periods. All procedures were approved by the Indiana University-Purdue University Indianapolis School of Science Institutional Animal Care and Use Committee and were consistent with the Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011).
2.2. Drugs and drinking solution
For drinking, 95% ethanol (Ethanol; Pharmco Products Inc., Brook-field, CT) was diluted with tap water to a 20% v/v solution. For intraperitoneal injections, 95% ethanol was diluted with 0.9% physiological saline to a 20% v/v solution and administered by varying injection volume for a 1.75 g/kg dose.
2.3. Experiment 1: alcohol pre-exposure using the “drinking in the dark-multiple scheduled access” (DID-MSA) protocol
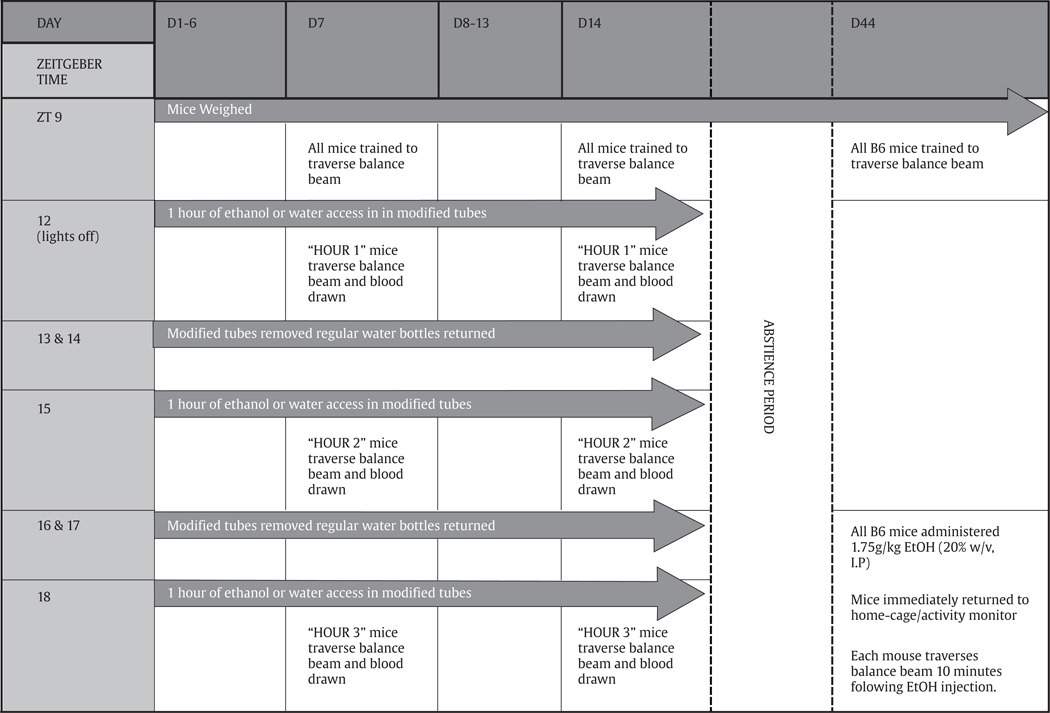
The drinking protocol was adapted from Bell et al. (2011) and is outlined in Table 1. Each day, mice received access to water or a 20% unsweetened ethanol solution during three, 1-hour access periods. Each access period was separated by 2 h, during which all mice had ad libitum access to water. Immediately following lights-out, regular water bottles were removed from all cages and replaced with a 10 mL plastic Mohr pipette affixed to a ball bearing sipper. This modified drinking tube contained either water or the ethanol solution and volumes were recorded before and after each hourly access period. The regular heavy duty glass water bottles (16 mL) were placed atop the modified tubes. This helped to reduced leakage by keeping the modified tubes in place. Additionally, two leak cages (one with a modified tube containing water, and one with a modified tube containing the ethanol solution) were maintained on each animal rack, and were read at the end of each access period. An average hourly leak was calculated for each solution (water or 20% ethanol), for the entire experiment. These constants were subtracted from all respective intake values.
Table 1.
Diagrammatic representation of experimental methods.
 |
2.4. Experiment 2: assessment of intoxication and blood ethanol concentration during DID-MSA
We were interested in evaluating the level of intoxication achieved using this DID-MSA procedure with B6 and D2 mice. Additionally, we wanted to assess the degree of functional tolerance seen following multiple binges using this DID-MSA procedure. Therefore, mice were assessed for signs of motor incoordination immediately following either the first (1H), second (2H) or third (3H) hour of access to ethanol (or water) on days 7 and 14 of drinking. Mice were pseudorandomly assigned to either group 1H, 2H or 3H. Motor incoordination was measured using the balance beam apparatus. Given the potential confound due to size differences between the adults and adolescents (Doremus et al., 2006; Moore et al., 2011; Linsenbardt et al., 2009) we used one hardwood balance beam for adults (122 cm long×2 cm wide×4 cm tall) and a second hardwood balance beam for adolescents, scaled to 3/4 the size of the adult beam (91.5 cm long×1.5 cm wide×3 cm tall). Each beam was affixed atop two 48 cm tall ring stands. Approximately 2 h before lights out on days 7 and 14, adolescents and adults were trained on their respective balance beam apparatus. During this training, a mouse was placed onto the starting edge of the balance beam to traverse the length of the beam, to and fro. The eraser end of a pencil was used to nudge mice that paused, or attempted to turn prematurely, along the beam. During the balance beam test, hind foot-slips were counted by the same experimenter that performed the training earlier that morning. Immediately after the mouse traversed the balance beam, a retro-orbital sinus blood sample was collected (25 µL).
2.5. Experiment 3: effect of alcohol intake during adolescence on alcohol-induced motor in-coordination and stimulation during adulthood in B6 mice
Only B6 mice were maintained for this portion of the study. Exactly one month following the fourteen days of DID-MSA ethanol access, the same B6 mice from Experiments 1 and 2 were intraperitoneally administered a 1.75 g/kg dose of ethanol (20% v/v). Animals who formerly consumed ethanol as adolescents were PD 73±3 and those who consumed ethanol as adults were PD 102±3. Prior to lights out on this test day, all mice were trained on the adult sized balance beam. Training proceeded as described earlier. Immediately following the 1.75 g/kg ethanol administration, mice were returned to their home cages. The home cages were placed onto a rack containing home cage activity monitoring systems (Columbus Instruments, Columbus, OH) in order to assess locomotor activity following the 1.75 g/kg ethanol administration. The activity monitor sampled activity in ten separate, one-minute time bins. Immediately following the home cage activity monitoring, mice were made to traverse the length of the balance beam and hind footslips were recorded. At the end of this test, a retro-orbital sinus blood sample was collected (25 µL).
2.6. Blood ethanol concentration analyses
Blood samples collected following days 7 and 14 of DID-MSA ethanol consumption, and following the 1.75 g/kg I.P administration of ethanol, were centrifuged immediately following collection, and plasma supernatant stored at −80 °C. Samples were later analyzed for alcohol content using an Analox Ethanol Analyzer (Analox Instruments, Lunenburg, MA) and blood ethanol concentration (BEC) recorded as mg/dL.
2.7. Statistical analyses
DID-MSA ethanol consumption was separately analyzed for B6 and D2 mice using a three-way mixed factor ANOVA, with age (adolescent vs. adult), sex (males vs. females), and day (days 1 through 14; within-subjects variable) as the variables of interest. Pilot data from our laboratory (unpublished results) using a replicate of the high alcohol preferring selected mouse lines (HAP1, Grahame et al., 1999) suggested that this scheduled access procedure increased consumption significantly by the seventh session of drinking. Therefore, an a priori decision was made to assess whether B6 or D2 mice showed a similar escalation of intake by comparing the average daily intake during the first and second weeks of access using a two-way mixed factor ANOVA (age*sex*week). We also analyzed data for B6 separately from D2, as the B6 mice continued on to Experiment 3, whereas D2 mice were only included in Experiments 1 and 2. Intake on days 7 and 14 (the balance beam test days) and hind footslips were assessed separately using a three-way ANOVA with age, sex, and solution as factors. Home cage locomotor activity and motor intoxication (balance beam hind footslips) following the 1.75 g/kg I.P. administration of ethanol were analyzed using a three-way mixed factor ANOVAs, with age of exposure (adolescent vs. adult), sex (males vs. females), and solution consumed (ethanol vs. water) as independent variables. Dunnett's or Tukey post hoc tests were used, as appropriate, to explore significant interactions. Simple linear regressions were used to evaluate the relationship between BEC and hourly ethanol intake. All statistical analyses were carried out using IBM SPSS Statistics, Version 19. Results were considered significant at p<0.05.
3. Results
3.1. Experiment 1: assessment of intake during the drinking in the dark-multiple scheduled access (DID-MSA) alcohol pre-exposure
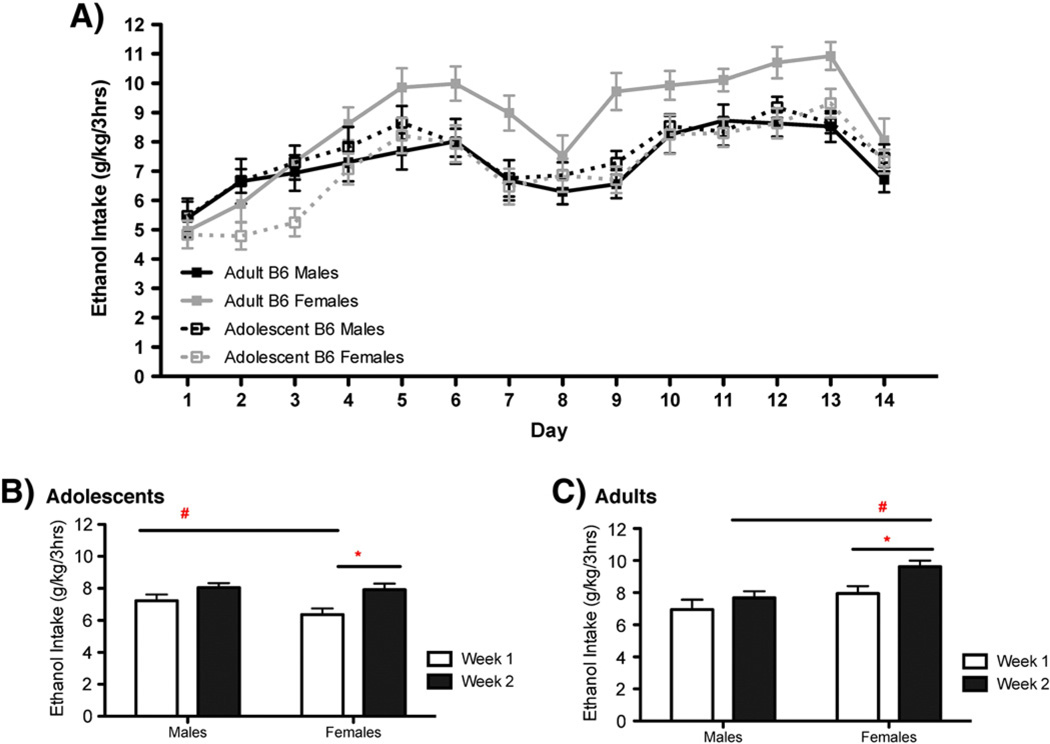
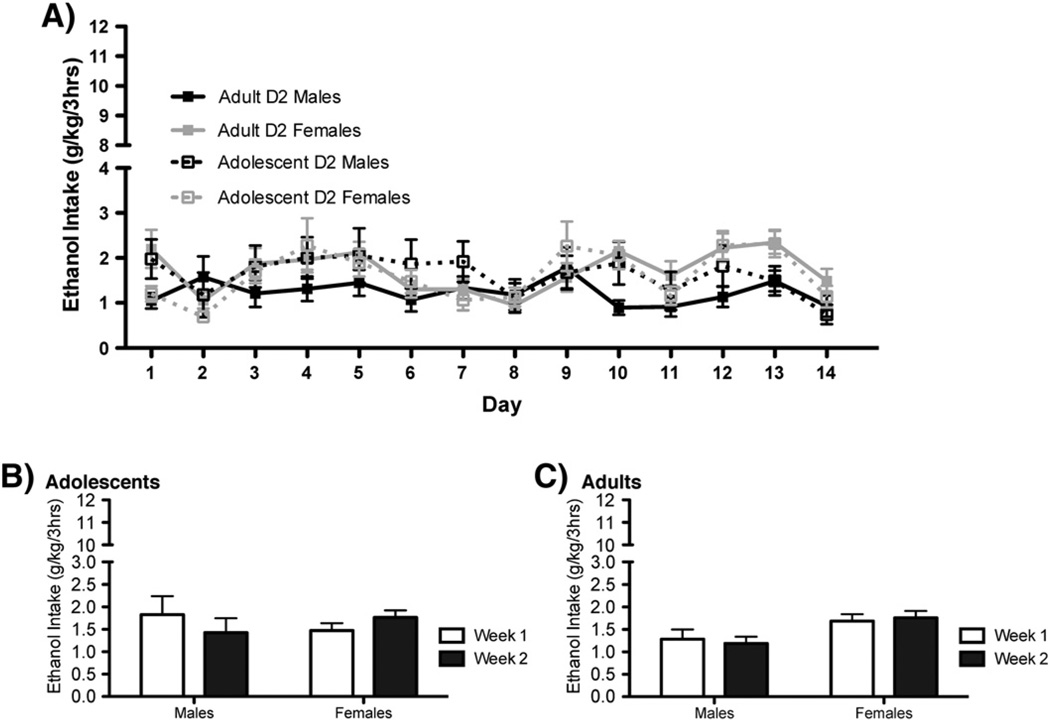
The total amount of ethanol consumed across the three 1-hour time bins can be seen for B6 mice in Fig. 1 and for D2 mice in Fig. 2. Data were analyzed separately for each genotype. The variables of interest in the initial analyses were age (2), sex (2) and day (14). These data violated the assumption of sphericity; therefore, the Greenhouse–Geisser correction was used when assessing the significance of the F statistic. As such, the degrees of freedom reported reflect this correction. B6 mice showed significant changes in their pattern of drinking across the 14 days of access [Fig. 1A; F(9, 822)=37.315, p<0.0001]. Planned comparisons support a linear trend (p<0.0001) as these mice significantly increased their drinking over time. Ethanol consumption for B6 mice also showed a significant quadratic trend, suggesting that this drinking was sensitive to environmental/procedural changes associated with the behavioral test days. Changes in the pattern of ethanol intake across the 14 days was also dependent upon sex [F(9, 822)=4.133, p<0.0001]. Pairwise comparisons reveal that, when comparing intake on day 1 to successive days of drinking, B6 males do not show any significant increases in drinking until the 10th day of access. In contrast, B6 females begin to show a significant increase in intake by day 5. Daily ethanol drinking for D2 mice also showed a significant effect of day [Fig. 2A; F(9.8, 853.8)=5.704, p<0.0001]. There was, however, no significant linear or quadratic trend to the drinking pattern. Instead, this main effect represents general inconsistencies in the pattern of intake across various days. Drinking data for D2 mice also revealed a significant interaction of day and sex [F(9.8, 853.8)=2.4, p<0.01], as the day to day variation in drinking was slightly different across male and female D2mice.
Fig. 1.
Females, but not males, increase binge-like ethanol consumption following limited access using DID-MSA. A) Total daily intake across the three hourly binge access periods for adolescent (23–24/sex) and adult (23–25/sex) B6 mice. B) Adolescent females showed an increase in intake across the two weeks (*, p<0.05), though they consumed less than adolescent males overall (#, p<0.05). C) Adult females consumed more than adult males (#, p<0.01) and had greater ethanol intake during the second week of access when compared to the first (*, p<0.05).
Fig. 2.
D2 mice maintain their alcohol avoiding phenotype when given limited access to alcohol using DID-MSA. A) Total daily intake across three hourly binge access periods for D2 adolescents (n=21–23) and adults (23–24). B) D2 adolescent males and females did not alter their intake across the two weeks of access. C) For D2 adults, neither males nor females showed a change in alcohol intake during the second week of access when compared to the first.
Drinking data were also analyzed by comparing the average intake from the first week, to that from the second week, using a mixed-3-way ANOVA (age*sex*week). For B6 mice, this analysis revealed a significant effect of week [F(1,92)=42.5, p<0.0001], as all mice consumed more during their 2nd week of access than their 1st (Fig. 1B and C). There was also a significant week*sex interaction [F(1,92)=5.4, p<0.05]. Pairwise comparison clarified that a significant sex difference in intake was only supported during the second week of drinking (p<0.05). For D2 mice, ethanol intake did not show a significant effect of week [F(1,87)=0.143, p=n.s], but there was a significant interaction of sex*week [Fig. 2A–B; F(1,87)=6.05, p<0.05]. This interaction appears to be driven by a marginal decrease in drinking seen for D2 males during the second week of access (p=0.060) that resulted in a marginal sex difference in intake during this week (p=0.056).
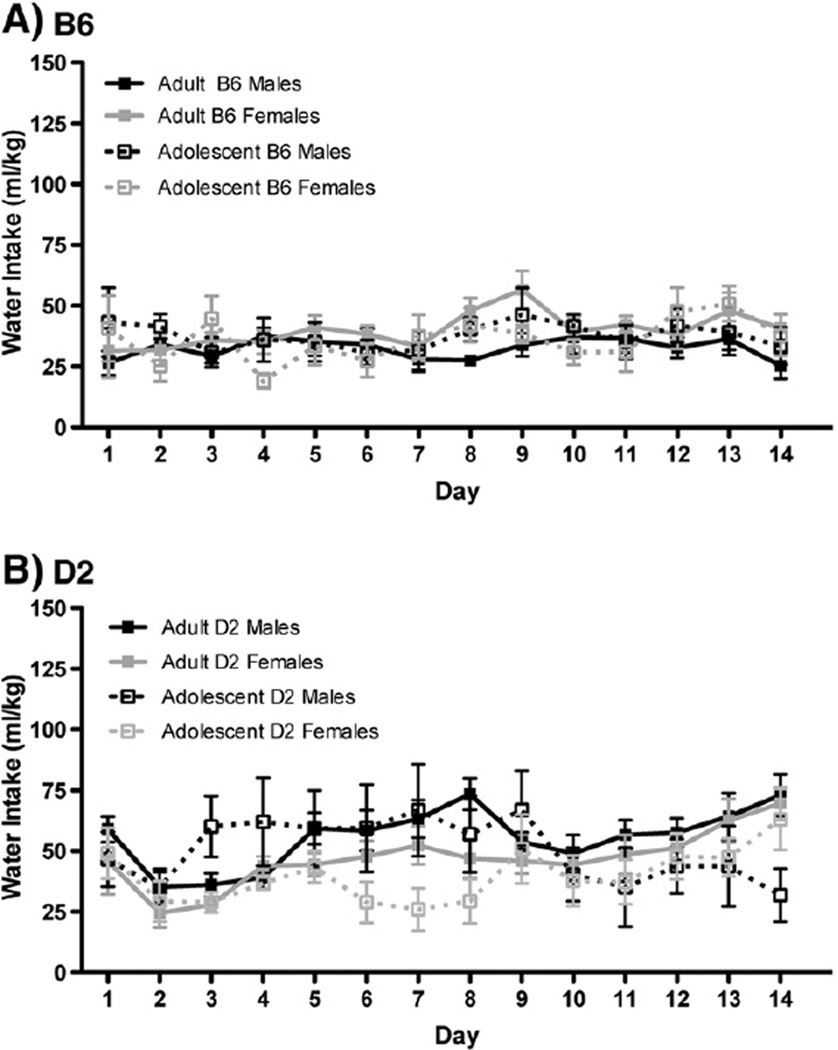
The total amount consumed by control animals, who had access to water in the modified tubes using the DID-MSA protocol, can be seen for B6 mice in Fig. 3A and D2 mice in Fig. 3B. These data were also analyzed separately for each genotype. Neither B6 nor D2 mice showed any relationship between sex, age or day on the pattern of water consumption.
Fig. 3.
Intermittent fluid access using DID-MSA does not alter water intake in inbred strains. A) B6 adults (n=23 males and 25 females) and adolescents (n=23 males and 24 females) did not show different patterns of water consumption in this paradigm. B) D2 adults and adolescents showed no significant differences in their water intake across the 14 days.
3.2. Experiment 2: assessment of intoxication and blood ethanol concentration during DID-MSA
3.2.1. Experiment 2A: intoxication (post-drinking balance beam performance)
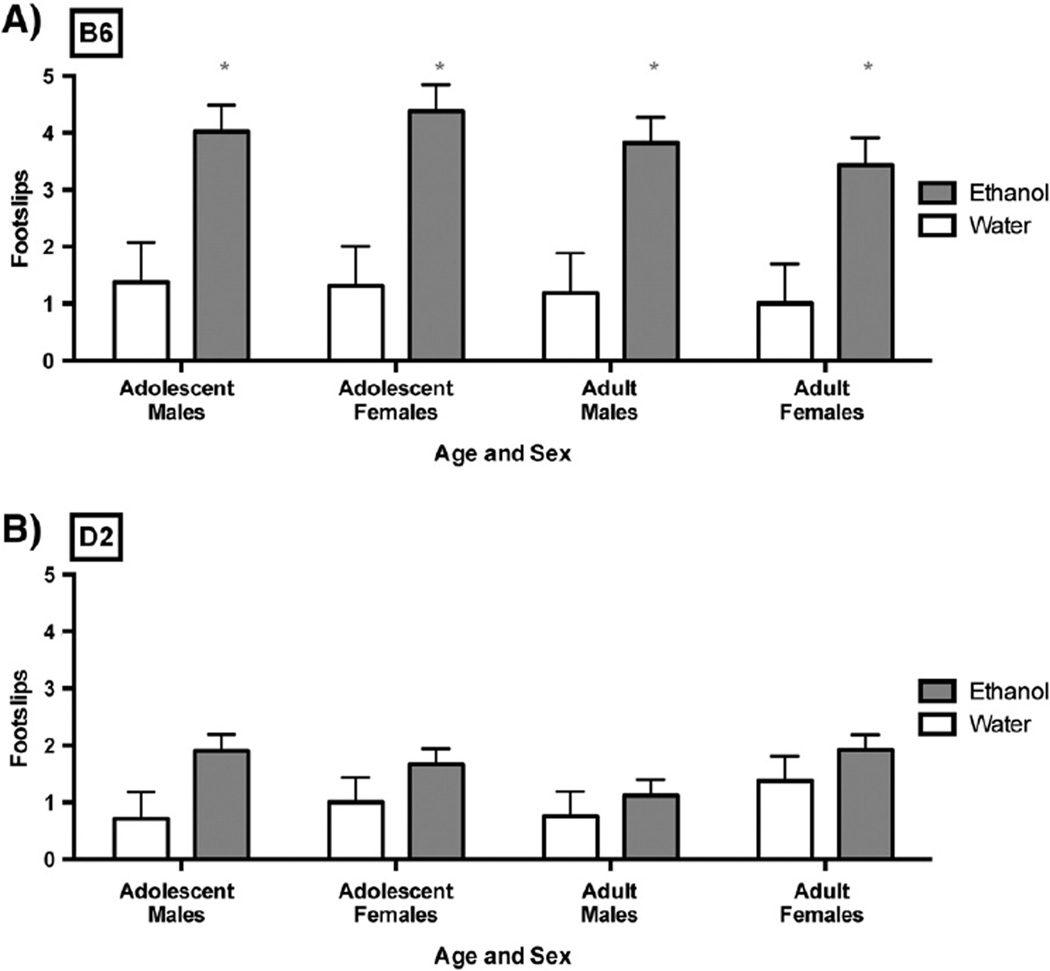
The degree of intoxication, assessed as hind footslips on a balance beam, achieved each hour was monitored on day 7 and day 14. These data are illustrated in Fig. 4. Separate groups of animals were used for each time point and are referred to as 1H (tested following their first hour of ethanol access), 2H (tested following their second hourly session of ethanol access), and 3H (tested following their third hourly session of ethanol access). Water animals were randomly tested following the first, second or third hour of access, but are presented and assessed as one group. Data were analyzed using a 4-factor RM-ANOVA comparing day (7 vs. 14; repeated measures variable), sex, age and group (1H, 2H, 3H or water). For B6 mice, alcohol intake in the DID-MSA paradigm resulted in significant intoxication, but the mice failed to demonstrate tolerance to this intoxication across the 14 days of drinking. Specifically, there was no significant effect of day, nor was there a significant interaction between day and any other factor. There was a significant effect of group [F(3,110)=19.23; p<0.0001]. Dunnett's post hoc comparing each ethanol drinking group (1H, 2H and 3H) to water drinking mice showed that mice with access to ethanol exhibited intoxication after the binge drinking sessions (Fig. 4A; ps<0.01; data shown collapsed across day). B6 adolescents and adults displayed the same level of intoxication following binge drinking in this paradigm, as there was no main effect of age or an interaction of this variable with any other factor. Similarly, although adult B6 females consumed greater amounts of ethanol than all other groups, there was no main effect of sex or an interaction of this variable with age or group. For D2 mice, drinking did not lead to intoxication, as there was no main effect of group (Fig. 4B). Furthermore, performance on the balance beam for these mice did not differ across sex or age. There was a significant effect of day, as all D2 mice showed a decrease in footslips with subsequent exposures to the balance beam.
Fig. 4.
B6 (A) but not D2 (B) mice show significant intoxication following binge drinking using DID-MSA. All ethanol drinking animals performed significantly worse on the balance beam than water drinking controls (ps<0.05). A) B6 mice that drank ethanol (n=22–25/age/sex) performed significantly worse on the balance beam than water drinking controls (n=8/age/sex). This intoxication did not vary across hour of consumption or across day 7 and day 14 and data are collapsed across these variables. B) D2 mice that drank ethanol (n=21–24/age/sex) showed no difference in balance beam performance when compared to those that consumed water (n=8/age/sex).
3.2.2. Experiment 2B: blood ethanol concentration
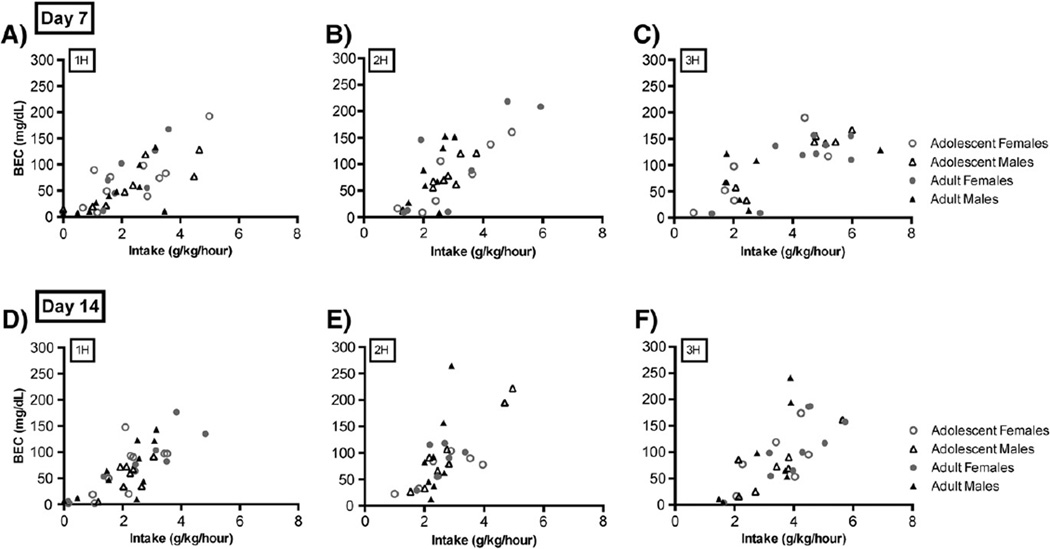
Blood ethanol concentrations achieved each hour were monitored on day 7 and day 14 and are detailed in Fig. 5. Ultimately, we wanted to determine whether this DID-MSA protocol could produce equivalent levels of heavy/binge ethanol consumption across adolescents and adults, in order to facilitate our investigations on the interactive effects that genotype and early/adolescent alcohol consumption has on later sensitivity to the drug (without the confound of disparate drinking histories across the age groups). For this, we analyzed the intake and blood ethanol concentration data from day 7 and day 14 using a mixed 4-factor ANOVA with day, sex, age and group (1H, 2H, 3H) as the independent variables. These data for B6 mice are shown in Fig. 5 and are detailed in Table 2. For this high drinking genotype, there was no main effect of day, sex or age. Our analysis did reveal a significant effect of group [F(2,83)=3.40; p<0.05], as mice consumed different amounts of alcohol during the 1st, 2nd and 3rd hourly access periods. Turkey's post hoc test confirmed that intakes measured during the 3rd hour of access were significantly greater than those from the 1st hour of access (p<0.05), but not the 2nd. BECs achieved by B6 mice were not significantly different across these hourly sessions. Relatedly, we found no evidence supporting an effect of age or sex on the BEC achieved by these mice. D2 mice maintained their alcohol avoiding phenotype in this drinking protocol, with hourly BECs all below 21 mg/dL (data not shown). This average and its variability were not significantly affected by day of intake, sex, age or hour of access. All groups of B6 mice had an average BEC above 80 mg/dL–the National Institute on Alcohol Abuse and Alcoholism standard for binge drinking—in at least one of the three hourly binge sessions.
Fig. 5.
For B6 mice, ethanol drinking in DID-MSA continues to predict BEC and results in significant behavioral intoxication in B6 mice on day 7 and day 14. Levels of ethanol intake during the 1st hour of drinking (A and D), 2nd hour of drinking (B and E) and 3rd hour of drinking (C and F) significantly correlate with respective BECs (n=6–11/age/sex/h).
Table 2.
Relationship between hourly ethanol intake and BEC for B6 mice.
| Adolescent |
Adults |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Males |
Females |
Males |
Females |
||||||
| D7 | D14 | D7 | D14 | D7 | D14 | D7 | D14 | ||
| 1HR | G/KG ETOH | 2.36 ±0.57 | 2.19 ±0.20 | 2.13 ±0.44 | 2.0 ±0.31 | 1.77 ±0.37 | 1.99 ±0.34 | 2.18 ±0.32 | 2.53 ±0.56 |
| BEC | 61.2 ±15.66 | 54.78 ± 9.89 | 67.1 ±15.92 | 62.21 ±14.28 | 44.2 ±18.58 | 66.1 ±16.01 | 82.77 ±22.20 | 76.19 ±20.68 | |
| R2 | 0.7** | 0.47† | 0.64** | 0.49* | 0.38† | 0.52* | 0.64* | 0.77* | |
| Sig. (2-tailed) | 0.009 | 0.061 | 0.003 | 0.016 | 0.056 | 0.019 | 0.031 | 0.01 | |
| Sample | 8 | 8 | 11 | 11 | 10 | 10 | 8 | 8 | |
| 2HR | G/KG ETOH | 2.7±0.26 | 2.92± 0.44 | 2.99± 0.51 | 2.56±0.38 | 2.37±0.17 | 2.40±0.17 | 3.13±0.66 | 2.52±0.19 |
| BEC | 73.2±12.49 | 102.33±25.18 | 77.2± 22.87 | 66.63±11.50 | 85.7±20.77 | 94.12±28.82 | 98.9±22.2 | 58.8± 16.64 | |
| R2 | 0.83** | 0.95*** | 0.81** | 0.59* | 0.43ns | 0.56* | 0.63* | 0.33 | |
| Sig. (2 tailed) | 0.002 | 0.0001 | 0.006 | 0.044 | 0.077 | 0.032 | 0.03 | 0.236 | |
| Sample size | 8 | 8 | 7 | 7 | 8 | 8 | 7 | 7 | |
| 3HR | G/KG ETOH | 4.37±0.57 | 3.39 ± 0.46 | 2.67 ±0.71 | 3.42 ± 0.42 | 2.82 ± 0.71 | 3.02 ±0.41 | 4.27 ±0.57 | 4.02 ± 0.4 |
| BEC | 120.5 ±19.84 | 74.49 ±18.12 | 83.1 ± 26.8 | 89.23 ±22.26 | 77.5 ± 22.2 | 95.57±34.19 | 106.1 ±19.58 | 107.88 ±20.61 | |
| R2 | 0.91*** | 0.69** | 0.67* | 0.34ns | 0.22ns | 0.51ns | 0.62* | 0.63* | |
| Sig. (2-tailed) | 0.001 | 0.021 | 0.045 | 0.221 | 0.291 | 0.072 | 0.012 | 0.011 | |
| Sample size | 7 | 7 | 6 | 6 | 8 | 8 | 9 | 9 | |
significant;
marginally significant;
not significant.
significant, p<0.05.
significant, p<0.01.
significant, p<0.001.
As seen in Fig. 5A–F, BECs for B6 mice on both day 7 and day 14 were positively associated with the amount of alcohol consumed in each hourly session. Intakes significantly predicted BEC on days 7 (R2=0.58; p<0.0001) and 14 (R2=0.42; p<0.0001). Separate correlation coefficients for each group (by age, sex and hour) are presented in Table 2.
3.3. Experiment 3: effect of adolescent alcohol intake on alcohol-induced motor in-coordination and stimulation during adulthood
3.3.1. Experiment 3A: balance beam performance following ethanol challenge
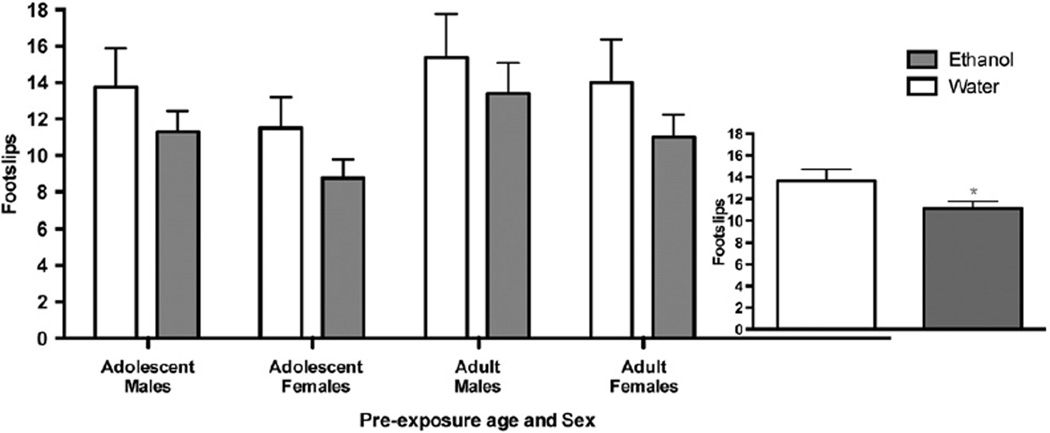
The motor-incoordinating and stimulant responses to an ethanol challenge (1.75 g/kg; I.P.) following one month of abstinence in B6 mice are illustrated in Figs. 6 and 7, respectively. For motor-incoordination, the number of hind footslips made on the balance beam was analyzed using a three-way ANOVA, with sex, age during binge drinking pre-exposure and solution consumed (during binge pre-exposure) as independent variables. There was a significant main effect of solution [F(1,126)=3.945; p<0.05], as mice with a history of binge alcohol consumption displayed a dampened ataxic response to this ethanol challenge, when compared to water drinking controls (Fig. 6). This relationship was not altered by sex, or the age at which the binge drinking occurred.
Fig. 6.
B6 mice with a history of binge drinking show attenuated ataxic response to ethanol, even following 30 days of abstinence. There was a significant effect of solution consumed on the level of impairment following 1.75 g/kg EtOH (p<0.05; inset). There was no effect of age or sex on this display of reduced sensitivity to an ethanol challenge (n=8/age/sex for water and n=23–24/age/sex for ethanol).
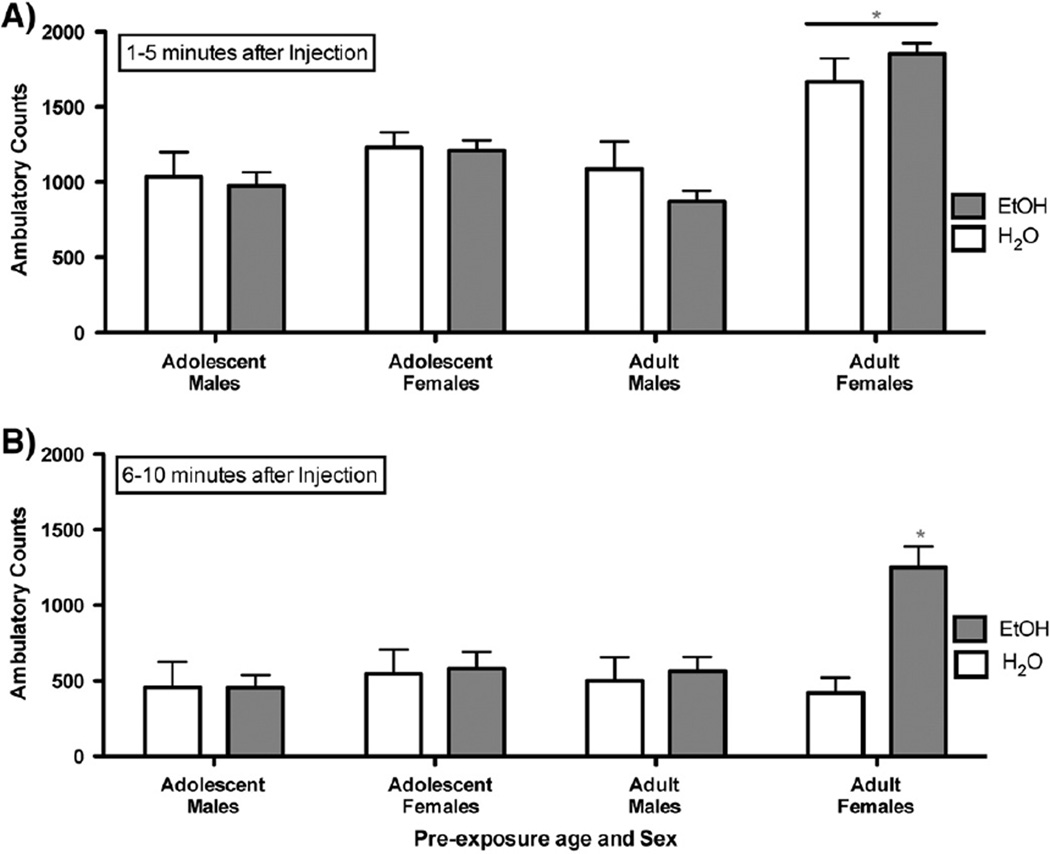
Fig. 7.
Binge drinking using DID-MSA significantly alters locomotor response to ethanol for female mice that binged during adulthood. A) During the first 5 min following injection, adult B6 females, regardless of binge drinking history, display greater locomotor response to the 1.75 g/kg ethanol challenge compared to all other groups (p<0.001). B) During the final 5 min of the ten minute test, adult females with a history of DID-MSA ethanol consumption showed a significantly greater locomotor response to this ethanol challenge, as compared to their water consuming controls (p<0.01). This effect was not noted for males or females that binged as adolescents (n=6–7/age/sex for water and n=15–16/age/ sex for ethanol).
3.3.2. Experiment 3A: home-cage activity following ethanol challenge
A number of laboratories have demonstrated that B6 mice display a complex, biphasic locomotor response to low dose ethanol (Crabbe et al., 1982; Melón and Boehm, 2011; Tarragón et al., 2012). In particular, these mice often show stimulation 1–5 min following ethanol administration and hypolocomotion by 10 min post injection. For this reason, we chose to assess the activity data as two separate 5 min time bins. These data were subject to a three-way ANOVA with sex, age during binge drinking pre-exposure and solution consumed as independent variables. The analysis of the first 5 min following injection revealed a significant interaction of sex and age [F(1,80)=13.92; p<0.0001], as females exposed to alcohol or water as adults, all had a greater locomotor response to ethanol than all other groups (Fig. 7A). This effect did not depend on the binge drinking history of the females. Interestingly, females who were exposed to water during DID-MSA as adolescents, showed a locomotor response more similar to that of males than to females exposed to water during DID-MSA as adults. Analysis of the second 5 min following injection revealed a significant three way interaction of sex, age of binge exposure and solution [F(1,80)=4.67; p<0.05]. Turkey's post hoc analysis clarified that females who binge drank during adulthood demonstrated significantly higher activity following the ethanol challenge when compared to control females (Fig. 7B; p<0.01). These females actually had ambulatory counts significantly higher than all other groups (ps<0.01). Post hoc analysis also clarified that control females, during this time bin (6–10 min post injection), no longer demonstrated greater activity when compared to other B6 mice.
4. Discussion
The present series of experiments have yielded three main demonstrations. First, we established that the DID-MSA procedure yields intoxicating levels of binge-like alcohol consumption in C57BL/6J (B6) and not DBA/2J (D2) mice. Second, we found that adult B6 females are particularly sensitive to this type of scheduled access, displaying a 120% increase in their intake over the 14 days of drinking. Third, we provide evidence supporting long term changes to ethanol responsivity following binge drinking using this protocol.
4.1. Behavioral intoxication and binge drinking during adolescence
The alcohol intake levels noted during this DID-MSA procedure are comparable to those using other limited access drinking paradigms for all groups except B6 adult females. Similar to other models, we found binge drinking using this procedure to be genotype specific, as D2 mice consumed negligible amounts of alcohol and showed no evidence for intoxication when assessed using the balance beam. In contrast to what has been demonstrated using the DID procedure (Linsenbardt et al., 2011), binge drinking using DID-MSA did not result in the development of functional tolerance across the 14 days of drinking for B6 mice. Although this was surprising, the significant drop in intake noted on the final day of drinking suggests that data from this day should be interpreted with care, as stress from the experimental procedures may have affected the animal's behavior on this day. Moreover, given that mice were tested at the end of their hourly binge session, we do not know if differences in the rate of consumption on day 7 vs day 14 obfuscate our ability to detect changes in the degree of intoxication measured across the two days. We do attempt to disentangle this potential confound by measuring BECs following the balance beam test and hour of drinking that preceded it, and note statistically comparable BECs achieved following drinking on day 7 vs day 14. Still, we contend that there are notable shifts in the correlation between ataxia and BEC on day 7 vs. day 14. Specifically, there is a predictable relationship between BEC and ataxia following the first session of access on day 7 only (data not shown). We are unaware of any published studies showing a significant correlation between BEC and ataxia following drinking. Moreover, those that do report their findings usually see significant ataxia in ethanol drinking mice but no significant correlation between the degree of ataxia and BEC following drinking (Sharpe et al., 2005). The fact that we do note a significant predictable relationship between these factors and that the correlation wanes following multiple presentations of alcohol (within day 7 and across days 7 to 14), suggests that some form of tolerance may be developing that we do not tap into with our crude measure. Thus, we must still conclude that we fail to support the development of behavioral tolerance to the intoxicating effects of binge drinking within the 14 days of access to alcohol administered using DID-MSA.
We were also unable to find differences across age or sex in the degree of intoxication noted following each binge session. This finding adds to the currently conflicting body of literature on age differences in sensitivity to the motor impairing effects of ethanol. Studies in rats have generally found adolescents to be less sensitive to ethanol-induced ataxia (Silveri and Spear, 2001; Ramirez et al., 2010; Broadwater et al., 2011). In mice, this relationship has been shown to be dependent upon genotype and sex. Additionally, given their fast metabolic rate, dose significantly moderates the relationship between age and sensitivity to alcohol induced ataxia for mice. B6 adolescents have shown greater sensitivity to ethanol induced ataxia at moderate alcohol doses (1.75 g/kg to 2.5 g/kg; Hefner and Holmes, 2007; Linsenbardt et al., 2009). However, at the 1.5 g/kg dose, our lab has been unable to find evidence for significant differences in sensitivity to this response across B6 adults and adolescents. As this dose better approximates the high end of the BEC range achieved during our binge drinking procedure, we believe that our collective efforts suggest that this genotype does not show evidence for age-related differences in sensitivity to ethanol induced ataxia at doses relevant to binge intoxication.
4.2. Alcohol responsivity following abstinence in adolescent or adult binge drinking
Our efforts herein suggest that binge alcohol consumption perturbs the neurobiological systems that mediate ethanol-induced hyper- and hypo-locomotion, as well as motor incoordination, in a sex- and age-specific manner. For example, B6 mice with binge drinking histories demonstrated dampened sensitivities to the motor incoordinating effect of an ethanol challenge. However, expression of this reduced sensitivity did not depend upon the age of the animal at the time of the binge alcohol exposure. Still, we were able to find evidence of tolerance long after the cessation of binge drinking in both adolescent pre-exposed mice and adult pre-exposed mice. On its own, this is a substantial finding. Though functional tolerance following voluntary consumption has been demonstrated in rats (Gatto et al., 1987; Darbra et al., 2002) and mice (Cronise et al., 2005; Linsenbardt et al., 2011), few have been able to demonstrate long-lasting changes to ethanol induced motor-incoordination as a function of voluntary oral preexposure. Recently, Rimondini et al. (2008) demonstrated long-lasting tolerance that persists into protracted abstinence (3 weeks post alcohol cessation) in rats that had 7 weeks of intermittent ethanol vapor. The alcohol exposure paradigm used by those authors is a well established model of dependence, producing persistent increases in voluntary intake and documented neurobiological effects (Roberts et al., 2000; Rimondini et al., 2003), thus we are hesitant to believe that ethanol intakes achieved using this DID-MSA paradigm could approach those necessary to induce comparable persistent changes. It is possible that our demonstration of persistent tolerance may be due to intoxicated practice, which has been shown to prolong demonstration of tolerance in rats up to two weeks post chronic alcohol administration (32 daily doses of 2 or 4 g/kg i.p; Lê et al., 1989). However, it may also be argued that the mice in the present study did not have enough intoxicated exposure to the balance beam (2 times prior to the post-abstinence test; each 1 week apart) to support the development of intoxicated practice, which is shown following extensive intoxicated experience with the testing apparatus. Future studies should clarify the duration of tolerance following abstinence from voluntary binge-like drinking and determine whether binge consumption using DID-MSA may induce persistent altered preference for alcohol and/or increased consumption of the drug in unlimited/free choice paradigms (i.e. shift “too much to fast” drinking to “too much to often”; Leeman et al., 2010).
Regarding the failure to find a specific effect of adolescent binge drinking on the degree of tolerance demonstrated following abstinence, it is possible that the level of alcohol exposure achieved during DID-MSA was high enough to induce adaptation in both adults and adolescents. An alternative explanation is that the neurochemical systems important for the expression of ethanol induced ataxia at this dose range are already developed by PD 30 (when binge drinking was initiated), such that alcohol exposure at this period would result in an adult-like pattern of behavioral adaptation. Indeed, during the binge drinking phase, adolescent mice showed no difference in sensitivity to ethanol induced motor incoordination as compared to adults. Moreover, the results add to inconsistent findings from previous works showing that the development of chronic tolerance may be greater during adolescence (Swartzwelder et al., 1998), reduced during adolescence (Matthews et al., 2008) or not different across adolescence and adulthood (Varlinskaya and Spear, 2007). We have previously evaluated tolerance to the ataxic effects of alcohol following injection in mice (Linsenbardt et al., 2009) and found that adolescents developed tolerance with higher (1.75 g/kg) but not lower (1.5 g/kg) doses. It is therefore possible that in the present studies, our adolescents were consuming alcohol at a level that surpassed the threshold for capturing their reduced ability to develop chronic tolerance.
The initial (first 5 min) locomotor response to an alcohol challenge (Fig. 7A) suggests that females show unique differences in their response to the experimental procedures depending upon their age at the start of the experiment. Among the water-drinking females (drug naive), mice that were initiated into the experiment during adolescence do not show the same heightened locomotor response to ethanol as females that were initiated as adults. This is a peculiar finding, as these females are all adults at the time of the ethanol challenge injection. However, these naive water drinkers were subjected to unique experiences associated with the experimental design (e.g., limited access to the ball-bearing sipper tubes) and possible stressors, at different developmental stages. Although it was not our intention to model adolescent stress in our experiment, we do concede that the chronic isolation required to administer alcohol and appropriately record intake may be interpreted as a chronic stressor. Additionally, the acute stress experienced following the retro-orbital blood sampling could have worked synergistically with the isolation stress to produce a dampened locomotor response to ethanol noted for females that drank either water or ethanol as adolescents when compared to those that drank as adults. Interestingly, males who started in the experiment as adolescents show a similar locomotor response to males who started as adults, regardless of the solution consumed. Therefore, it is possible that age and sex interact to modify the effects of early life stress on adult responsivity to an ethanol challenge. Indeed, McCormick and colleagues have demonstrated that adolescent stress results in an augmented expression of locomotor sensitization following repeated exposure to nicotine (McCormick et al., 2004) or amphetamine (McCormick et al., 2005) later in life, and that this occurs only in females. Although the directionality of our effect is opposite that seen by McCormick and colleagues, the fact that we only note a difference for females is similar and adds to the body of evidence supporting sex differences in the effect of adolescent stress on adult responsivity to drugs of abuse.
4.3. Sex differences in binge drinking and its long-term effects
Among B6 adults, there was a clear effect of sex on the escalation of binge drinking using this paradigm. Although not the goal of this particular series of experiments, we believe that this DID-MSA model offers an important opportunity to study sex differences in the acquisition of oral alcohol self-administration. For other drugs of abuse, like cocaine and amphetamine, differences across males and females in the acquisition and maintenance of rewarding compounds have revealed important dimorphic mechanisms underlying the development of addiction (Carroll and Anker, 2010). For alcohol, we have long accepted that female rodents often consume greater amounts of the compound than males, and have made important strides in understanding what underlies this difference. Yet, aside from the heroic efforts of a few investigators, there has been little attention paid to biological sex as an important variable in the acquisition of alcohol consumption (Roth et al., 2004). The data here (Fig. 1) indicates that female B6 mice may acquire heavy alcohol self-administration faster than males, when given limited access to the drug. Of course, a number of factors unrelated to addiction vulnerability may underlie these differences. For example, females may show stronger habituation to the novel, ball-bearing sipper tubes used in this procedure. Given that females do not show dramatic changes in their water consumption using the same procedures, this is unlikely a major factor in the diergic escalation of alcohol self-administration. Still, as alcohol access (in this protocol) initiates at the onset of lights out, females may better adapt their activity patterns to match access to this calorie rich ethanol solution. Indeed, mice have shown evidence for sex differences in their circadian response to zeitgebers (Lee et al., 2004), and food anticipatory activity (FAA) has been demonstrated for drugs of abuse, including limited-access to alcohol (Kosobud et al., 2007). However, a notable sex difference in FAA has not been demonstrated for B6 mice (Feillet et al., 2006). Lastly, although adolescent females show a significant increase in their consumption across weeks (Fig. 1), they never consume more alcohol than males from either age group. Instead, the increased intake demonstrated by the adolescent females is more a function of their low intakes during the first 5 days of access. Therefore, the escalation noted for adult B6 females may be said to occur following adolescence. Given the important hormonal changes that occur around this time period (i.e. puberty), it is possible that sex differences in the escalation of alcohol consumption for B6 mice reflect an interaction between the effects of alcohol and the activational effects of hormones that increase their synthesis drastically following puberty (i.e. progesterone and its neuroactive metabolites).
In addition to a sex difference in the escalation of intake, we found a marked sex difference in the effect of binge drinking on the locomotor response to ethanol (1.75 g/kg; i.p) after one month of abstinence (Fig. 7). There are a number of alternative explanations for the heightened locomotor response noted for adult pre-exposed females. For example, it is possible that the level of alcohol exposure achieved for B6 adult females was enough to cause unique perturbations not seen for the adult pre-exposed males or pre-exposed adolescents. Though, it should be noted that these females did not achieve significantly higher BECs at their level of drinking. Another interpretation concerns a true sex difference in vulnerability to adaptation following binge drinking. Clinical studies suggest that women show a telescoped development of alcohol addiction, progressing through the landmark events associated with the development of alcohol use disorders faster than men (Piazza et al., 1989; Randall et al., 1999). Preclinical studies have also demonstrated sex differences in the development of ethanol dependence (Devaud et al., 1999, 2003, 2006; Kuhn, 2011; Wiren et al., 2006). Preclinical studies also suggest that adult females are more susceptible to the development of psychomotor sensitization following repeated exposure to a variety of compounds including cocaine (Cailhol and Mormède, 1999; Hu and Becker, 2003), nicotine (McCormick et al., 2004) and alcohol (Grahame et al., 2000). Though we did not set out to model the development of psychomotor sensitization to ethanol in the classical sense, there is evidence that alcohol consumption in B6 mice (24 hour, 2-bottle choice) can increase the stimulant effects of an acute alcohol injection (Lessov et al., 2001). Therefore, we may interpret the heightened locomotor response to the ethanol challenge noted for binge drinking females as compared to the naive mice as an example of a between-group sensitized response. This would suggest a sex difference in the development or expression of sensitization following binge drinking in these mice. Further, the “sensitization” noted following this DID-MSA binge drinking regiment appears less vulnerable to decay that demonstrated following injection, which degrades by 17 days following the cessation of ethanol treatment (Lessov and Phillips, 1998). Future studies should clarify whether dose, pharmacokinetics, or genuine dimorphic adaptations to alcohol exposure underlie sex differences in the effect of pre-exposure to alcohol on later responsivity to the drug following abstinence.
4.4. DID-MSA as a protocol to induce binge drinking in mice
There have been a growing number of drinking protocols with the common goal of inducing high alcohol consumption in animal models of oral alcohol self-administration. Although this redundancy may seem unnecessary to some, these procedures offer opportunities to study unique aspects and consequences of alcohol consumption, a surprisingly complex behavioral phenomenon. The DID-MSA drinking procedure (adapted from Bell et al.,2011) provides a number of advantages over currently used protocols, depending upon the investigator's experimental design and variables of interest. Clearly, this drinking protocol results in binge-like consumption and intoxicating levels of alcohol intake in B6 mice (Figs. 4 and 5). However, one may use the simpler and well characterized DID protocol if these are the experimental goals (Rhodes et al., 2005, 2007; Moore et al., 2007). Given the results presented here, the DID-MSA procedure may be useful to investigate the varied consequences specific to adolescent binge drinking (as the procedure equates adolescent binge-drinking with adult male intake levels) and the mechanisms underlying those effects. Additionally, the procedure provides a unique opportunity to study sex differences in the escalation and/or effects of binge consumption, given the dimorphic response to the protocol by B6 adult males and females.
5. Conclusion
To conclude, the experiments presented above support an interaction of sex and age on the effect that binge alcohol intake has on later sensitivity to the drug. These data also support the utility of the DID-MSA paradigm for studying the isolable influence of these two important variables.
Acknowledgments
The authors are grateful to the National Institute on Alcohol Abuse and Alcoholism for supporting the work presented herein by grant #'s AA016789 (SLB), AA07462 (LCM) and AA18910 (EMM). The authors would like to thank Brandy Shneider, Robert Forrest IV and Zachary Nolan for their invaluable technical assistance.
References
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol Rev. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Kimpel MW, Rodd ZA, Strother W, Bai F, Peper CL, et al. Protein expression changes in the nucleus accumbens and amygdala of inbred alcohol-preferring rats given either continuous or scheduled access to ethanol. Alcohol. 2006;40:3–17. doi: 10.1016/j.alcohol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, McBride WJ. Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol Biochem Behav. 2011;100:90–97. doi: 10.1016/j.pbb.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Different chronic ethanol exposure regimens in adolescent and adult male rats: effects on tolerance to ethanol-induced motor impairment. Behav Brain Res. 2011;225:358–362. doi: 10.1016/j.bbr.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhol S, Mormède P. Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Res. 1999;842:200–205. doi: 10.1016/s0006-8993(99)01742-4. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Johnson NA, Gry DK, Kosobud A, Young ER. Biphasic effects of ethanol on open-field activity: sensitivity and tolerance in C57BL/6N and DBA/2N mice. J Comp Physiol Psychol. 1982;96:440–451. doi: 10.1037/h0077898. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronise K, Finn DA, Metten P, Crabbe JC. Scheduled access to ethanol results in motor impairment and tolerance in female C57BL/6J mice. Pharmacol Biochem Behav. 2005;81:943–953. doi: 10.1016/j.pbb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Darbra S, Prat G, Pallares M, Ferre N. Tolerance and sensitization to the hypnotic effects of alcohol induced by chronic voluntary alcohol intake in rats. J Psychopharmacol. 2002;16:79–83. doi: 10.1177/026988110201600107. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Patricia Chou S, June Ruan W, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Matthews DB, Morrow AL. Gender impacts behavioral and neurochemical adaptations in ethanol-dependent rats. Pharmacol Biochem Behav. 1999;64:841–849. doi: 10.1016/s0091-3057(99)00164-1. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Alele P, Ritu C. Sex differences in the central nervous system actions of ethanol. Crit Rev Neurobiol. 2003;15:41–59. doi: 10.1615/critrevneurobiol.v15.i1.20. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Risinger FO, Selvage D. Impact of the hormonal milieu on the neurobiology of alcohol dependence and withdrawal. J Gen Psychol. 2006;133:337–356. doi: 10.3200/GENP.133.4.337-356. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya EI, Spear LP. Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83:570–577. doi: 10.1016/j.pbb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Feillet CA, Ripperger JA, Magnone MC, Dulloo A, Albrecht U, Challet E. Lack of food anticipation in Per2 mutant mice. Curr Biol. 2006;16:2016–2022. doi: 10.1016/j.cub.2006.08.053. [DOI] [PubMed] [Google Scholar]
- García-Burgos D, González F, Manrique T, Gallo M. Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse-like conditions. Alcohol Clin Exp Res. 2009;33:722–728. doi: 10.1111/j.1530-0277.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Murphy JM, Waller MB, McBride WJ, Lumeng L, Li T-K. Chronic ethanol tolerance through free-choice drinking in the P line of alcohol-preferring rats. Pharmacol Biochem Behav. 1987;28:111–115. doi: 10.1016/0091-3057(87)90021-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li T-K, Lumeng L. Selective breeding for high and low alcohol preference in mice. Behav Genet. 1999;29:47–57. doi: 10.1023/a:1021489922751. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Rodd-Henricks K, Li T-K, Lumeng L. Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high- and low-alcohol preferring mice. Psychopharmacology. 2000;151:252–260. doi: 10.1007/s002130000388. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology. 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L, O'Malley P, Bachman J, Shulenberg J. The monitoring the future national survey results on adolescent drug use: overview of key findings. 2007 [Google Scholar]
- Kosobud AEK, Gillman AG, Leffel JK, Pecoraro NC, Rebec GV, Timberlake W. Drugs of abuse can entrain circadian rhythms. ScientificWorldJournal. 2007;7:203–212. doi: 10.1100/tsw.2007.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn CM. Alcohol and women: what is the role of biologic factors? Alcoholism Treat Q. 2011;29:479–504. [Google Scholar]
- Lê AD, Kalant H, Khanna JM. Roles of intoxicated practice in the development of ethanol tolerance. Psychopharmacology. 1989;99:366–370. doi: 10.1007/BF00445559. [DOI] [PubMed] [Google Scholar]
- Lee TM, Hummer DL, Jechura TJ, Mahoney MM. Pubertal development of sex differences in circadian function: an animal model. Ann N Y Acad Sci. 2004;1021:262–275. doi: 10.1196/annals.1308.031. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O'Malley SS. Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:109–124. doi: 10.1111/j.1369-1600.2009.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessov CN, Phillips TJ. Duration of sensitization to the locomotor stimulant effects of ethanol in mice. Psychopharmacology. 1998;135:374–382. doi: 10.1007/s002130050525. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Palmer AA, Quick EA, Phillips TJ. Voluntary ethanol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacology. 2001;155:91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm IISL. Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL. Tolerance to ethanol's ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model. Alcohol Clin Exp Res. 2011;35:1246–1255. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado AM, Finkbeiner LM, Alipour KK, Kirstein CL. Voluntary ethanol consumption differs in adolescent and adult male rats using a modified sucrose-fading paradigm. Alcohol Clin Exp Res. 2008;32:1574–1582. doi: 10.1111/j.1530-0277.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Tinsley KL, Diaz-Granados JL, Tokunaga S, Silvers JA. Chronic intermittent exposure to ethanol during adolescence produces tolerance to the hypnotic effects of ethanol in male rats: a dose-dependent analysis. Alcohol. 2008;42:617–621. doi: 10.1016/j.alcohol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Gleason E, Kelsey JE. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Horm Behav. 2004;46:458–466. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stress on corticosterone responses to restraint and locomotor responses to psychostimulants in rats. Horm Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Melón LC, Boehm IISL. Role of genotype in the development of locomotor sensitization to alcohol in adult and adolescent mice: comparison of the DBA/2J and C57BL/6J inbred mouse strains. Alcohol Clin Exp Res. 2011;35:1351–1360. doi: 10.1111/j.1530-0277.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL. GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melón L, Boehm IISL. Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol Clin Exp Res. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Linsenbardt DN, Melón LC, Boehm IISL. Ontogenetic differences in adolescent and adult C57BL/6J and DBA/2J mice: anxiety-like, locomotor, and consummatory behaviors. Dev Psychobiol. 2011;53:141–156. doi: 10.1002/dev.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- Piazza NJ, Vrbka JL, Yeager RD. Telescoping of alcoholism in women alcoholics. Int J Addict. 1989;24:19–28. doi: 10.3109/10826088909047272. [DOI] [PubMed] [Google Scholar]
- Pitkänen T, Lyyra A-L, Pulkkinen L. Age of onset of drinking and the use of alcohol in adulthood: a follow-up study from age 8–42 for females and males. Addiction. 2005;100:652–661. doi: 10.1111/j.1360-0443.2005.01053.x. [DOI] [PubMed] [Google Scholar]
- Ramirez LR, Varlinskaya EI, Spear LP. Effect of the selective NMDA NR2B antagonist, Ifenprodil, on acute tolerance to ethanol-induced motor impairment in adolescent and adult rats. Alcohol Clin Exp Res. 2010;35:1–11. doi: 10.1111/j.1530-0277.2011.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60:252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol. 2003;64:445–449. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer WH, Dall'Olio R, Heilig M. Long-lasting tolerance to alcohol following a history of dependence. Addict Biol. 2008;13:26–30. doi: 10.1111/j.1369-1600.2007.00079.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Tsivkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin Exp Res. 2005;29:1419–1426. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Fjell AM, Østby Y, Westlye LT, Due-Tønnessen P, Bjørnerud A, et al. The brain dynamics of intellectual development: waxing and waning white and gray matter. Neuropsychologia. 2011;49:3605–3611. doi: 10.1016/j.neuropsychologia.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Tarragón E, Baliño P, Aragon CMG, Pastor R. Ethanol drinking-in-the-dark facilitates behavioral sensitization to ethanol in C57BL/6J, BALB/cByJ, but not in mu-opioid receptor deficient CXBK mice. Pharmacol Biochem Behav. 2012;101:14–23. doi: 10.1016/j.pbb.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague–Dawley rats. Neurotoxicol Teratol. 2007;29(1):23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren KM, Hashimoto JG, Alele PE, Devaud LL, Price KL, Middaugh LD, et al. Impact of sex: determination of alcohol neuroadaptation and reinforcement. Alcohol Clin Exp Res. 2006;30:233–242. doi: 10.1111/j.1530-0277.2006.00032.x. [DOI] [PubMed] [Google Scholar]
- Witt ED. Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol. 2010;44:119–124. doi: 10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Donovan JE, Masten AS, Mattson ME, Moss HB. Early developmental processes the continuity of risk for underage drinking and problem drinking. Pediatrics. 2008;121(Suppl. 4):S252–S272. doi: 10.1542/peds.2007-2243B. [DOI] [PMC free article] [PubMed] [Google Scholar]