Abstract
Endogenous cannabinoid ligands (endocannabinoids) produced by neurons, astrocytes, and microglial cells activate cannabinoid receptors, the molecular target for marijuana's bioactive ingredient Δ9-tetrahydrocannabinol. The molecular mechanism underlying the production of the most abundant endocannabinoid, 2-arachidonoylglycerol (2-AG), is unclear. A prevalent hypothesis proposes that activation of metabotropic receptors coupled to the phosphatidylinositol-specific phospholipase C and diacylglycerol (DG) lipase pathway will systematically lead to increases in 2-AG production. Here, we show that ATP increases 2-AG production by cultured microglial cells in a phosphatidylinositol-specific phospholipase C and DG lipase-dependent manner. However, efficacious activation of metabotropic P2Y purinergic receptors coupled to phosphatidylinositol-specific phospholipase C does not increase 2-AG production. This suggests that ionotropic, and not metabotropic, purinergic receptors control 2-AG production at an unexpected enzymatic step of its metabolic pathway. We show that activation of P2X7 ionotropic receptors, which are highly permeable to calcium, is necessary and sufficient to increase 2-AG production in microglial cells. We also show that the sustained rise in intracellular calcium induced by activation of P2X7 receptors directly increases DG lipase activity while inhibiting the activity of monoacylglycerol lipase, the enzyme that degrades 2-AG. This inverse sensitivity of DG lipase and monoacylglycerol lipase to calcium constitutes an original and efficient modality for sustained accumulation of 2-AG. Because prolonged increases in 2-AG amounts in brain parenchyma are thought to orchestrate neuroinflammation, the enzymatic steps involved in 2-AG synthesis and degradation by microglial cells constitute appealing targets for therapy aimed at controlling exacerbated neuroinflammation.
Microglial cells, the macrophages of the brain, express membrane receptors that sense signals produced by brain injury. For example, microglial cells express purinergic receptors that sense ATP released by reactive astrocytes and lysed cells (1). At micromolar concentrations, ATP activates metabotropic P2Y receptors coupled to phosphatidylinositol-specific phospholipase C (PI-PLC) and ionotropic P2X receptors that modulate membrane potential (2). As ATP reaches millimolar concentrations, a subset of purinergic receptors, P2X7, is activated, becoming highly permeable to cations, such as calcium, for prolonged periods (2, 3). Activation of purinergic receptors on microglial cells changes their phenotype; they retract their processes and increase their rate of migration (1, 3-5). They also release immune-related mediators, such as cytokines, chemokines, cytotoxins, and growth factors, all of which orchestrate neuroinflammatory responses (6, 7). Because many neuropathological conditions are associated with exacerbated neuroinflammation (8), the molecular machinery underlying the production of immune-related mediators released by microglial cells constitutes an appealing target for therapies aimed at reducing exacerbated neuroinflammation.
Traumatic brain injury and experimental autoimmune encephalomyelitis, both of which are associated with exacerbated neuroinflammation, lead to increased production of endocannabinoid (eCBs) in brain parenchyma (9, 10). This increased eCB production is thought to prevent cell damage in multiple ways. Sustained activation of cannabinoid receptors (i) on neurons inhibits excitotoxicity (10-13), (ii) on endothelial cells induces hypotension and decreases edema (14), and (iii) on microglial cells and other invading immune cells regulates their immune response (15, 16). The cellular source of eCBs produced during neuroinflammation is unknown, although it likely involves microglial cells, for they produce ≈20-fold more eCBs than neurons and astrocytes (16).
Several eCBs have been identified: arachidonoylethanolamide (anandamide), 2-arachidonoylglycerol (2-AG), homo-γ-linolenoylethanolamide, and docosatetraenoylethanolamide (17-19). Evidence suggests that the phospholipase D pathway produces anandamide, homo-γ-linolenoylethanolamide, and docosatetraenoylethanolamide, whereas the PI-PLC-diacylglycerol (DG) lipase pathway produces 2-AG (17, 18). eCBs are inactivated by different molecular mechanisms. Anandamide is hydrolyzed into arachidonic acid and ethanolamine by fatty acid amide hydrolase (20), and 2-AG is hydrolyzed into arachidonic acid and glycerol by monoacylglycerol (MG) lipase (21). The rate of eCB production and inactivation determines the extent and duration of eCB production. These rates are important, considering that the extent and duration of cannabinoid receptor activation likely determines the cellular function that is regulated. For example, activation of cannabinoid receptors for seconds induces short-term and long-term plasticity (22-24), whereas activation of cannabinoid receptors for minutes or even hours regulates the extent of excitotoxicity and neuroinflammation (9, 13, 25). Because the molecular mechanism that controls sustained eCB production is unknown, we sought to identify the receptors and enzymatic steps that control ATP-induced 2-AG production in microglial cells (16).
Materials and Methods
Materials. ADP, ATP, oxidized ATP, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate-acetoxymethyl ester (BAPTA-AM), benzoylbenzoyl (Bz)-ATP, DG kinase inhibitor 1, EGTA, and UTP were from Sigma. U73122, RHC-80267, and propranolol were from Biomol (Plymouth Meeting, PA). Thirteen-millimeter coverslips (Thermanox) were from Nalge Nunc. Primeria tissue culture dishes and well plates were from VWR Scientific. CellGro (complete serum-free cell culture media) was from Mediatech (Washington, DC).
Cell Culture. Mouse microglial cells in primary cultures were prepared as described (26), according to the guidelines of the Institutional Animal Care and Use Committee of the University of Washington (Seattle). Microglial cells were plated in serum-free MEM (i.e., MEM supplemented with 1 mM l-glutamine/10 mM Hepes/5 mM NaHCO3/100 units/ml penicillin/100 μg/ml streptomycin/10% CellGro) onto 60-mm culture dishes (5 × 105 cells per dish), 35-mm culture dishes (3 × 105 cells per dish), and 24-well plates (1 × 105 cells per well).
Incubation. Dishes or plates containing microglial cells were rinsed once with HB buffer (20 mM Hepes/5 mM NaHCO3/120 mM NaCl/5 mM KCl/2 mM CaCl2/1 mM MgSO4/1 mM NaH2PO4/10 mM glucose) and placed on a shaking water bath at 37°C for a 30-min preincubation period. Cells were then stimulated by directly adding agents prepared in 10× HB buffer and incubated for an additional 10 min.
Lipid Extraction and eCB Analysis. Amounts of eCBs in cells plated in 60-mm dishes were determined as described (26, 27). In brief, placing dishes on ice and adding ice-cold methanol stopped incubations. Lipids were extracted with chloroform containing internal standards (200 pmol of [2H4]eCB). Organic phases were purified by open-bed silica gel chromatography followed by HPLC, and eCB amounts were determined by chemical ionization-GC/MS by using isotope dilution as a quantification method.
PLC Activity. PLC activity was determined as described (28). In brief, cells plated in 24-well plates were incubated for 18 h in myo-[3H]inositol (4 μCi/ml, American Radiolabeled Chemicals, St. Louis) and then rinsed, preincubated, and incubated as described above, except that lithium chloride (10 mM) was added to the HB buffer during preincubation and incubation. Incubation was stopped with Triton X-100. Radioactive lipids were isolated from lysate with methanol/chloroform (1:2, vol/vol), and an aliquot of the upper aqueous phase was loaded onto Dowex AG 1 X-8 columns (formate form, 200-400 mesh, Bio-Rad). myo-[2-3H]Inositol was eluted with myo-inositol, and radioactivity in this elute was determined. Columns were then washed with formic acid, and total [3H]inositol phosphates ([3H]IP) were eluted with ammonium formate/formic acid. The radioactivity in the latter elute was determined. Results were expressed as [3H]IP divided by myo-[2-3H]inositol.
DG Lipase and MG Lipase Activity. Cells grown in 60-mm dishes were rinsed with calcium-free HB buffer, lysed, and homogenized in 50 mM Tris (pH 7.5). For each condition, 0.5 ml of homogenates (35 μg of protein final) were incubated with [14C]DG (9 nCi/ml, Amersham Pharmacia) or 2-[3H]AG (15 nCi/ml, American Radiolabeled Chemicals) for the indicated period, which was stopped by adding methanol (1 ml). Lipids were extracted with chloroform containing the following carriers: 15 μM of DG, 2-AG (Cayman Chemical, Ann Arbor, MI), and arachidonic acid. Samples were separated by TLC by using silica gel G plates (Analtech) and chloroform/methanol/ammonium hydroxide (80:20:1) as solvent system (29). Lipids were visualized with phosphomolybdic acid (10%), bands were scraped off, and radioactivity therein was determined.
Western Blot Analysis. Western blot analysis was performed as described (16). In brief, cells grown in 60-mm dishes were lysed, and membrane protein fractions were collected by ultracentrifugation. Samples were electrophoresed (SDS/PAGE), transferred to nitrocellulose, and immunoblotted by using antibody directed against the P2X7 receptor (1:200, Alomone, Jerusalem).
Calcium Imaging. Calcium imaging was performed as described (30). In brief, cells grown on coverslips placed in 24-well plates were incubated in HB buffer containing 5 μM fura-2-acetoxymethyl ester (Molecular Probes) for 30 min at room temperature. Cells were placed in a perfusion chamber on the stage of an inverted microscope (Diaphot 200, Nikon) equipped with a 40×/1.3-numerical-aperture oil immersion objective. Fura-2 was excited by using a Lambda DG-4 filter system (Sutter Instruments, Novato, CA) at 340 and 380 nm and fluorescence emission collected at 510 ± 20 nm by a bandpass filter. Acquisition of fluorescence and image analysis were performed by using a digital imaging system (R3, Inovision, Durham, NC). Ratios were collected at 2-s intervals. Drugs were added to the chamber under nonperfused conditions so that these conditions were similar to the ones used for analyzing eCB production and PI-PLC activation.
Results
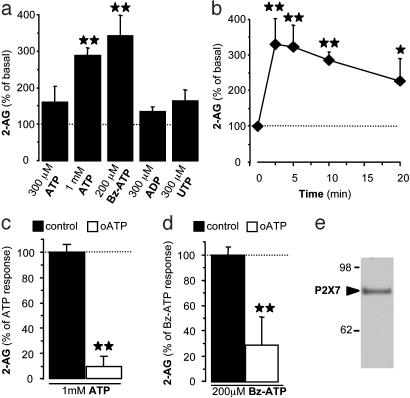
P2X7 Receptors Control 2-AG Production by Microglia. Under basal conditions, mouse microglial cells in primary culture produced low, yet clearly detectable amounts of 2-AG (7.0 ± 0.8 pmol/mg protein, n = 112 individual culture dishes). The levels of anandamide, homo-γ-linolenoylethanolamide, or docosatetraenoylethanolamide were below detection limit. As shown (16), millimolar concentrations of ATP significantly increased 2-AG production in microglial cells (Fig. 1a), whereas anandamide, homo-γ-linolenoylethanolamide, and docosatetraenoylethanolamide amounts remained below detection limits. ATP increased 2-AG production in a time-dependent manner (Fig. 1b). At 10 min, the EC50 of this response was 1.2 mM (not shown), which is consistent with activation of P2X7 receptors (1). To determine whether activation of P2X7 receptors is sufficient to increase 2-AG production, we tested the effect of Bz-ATP, a selective P2X7 receptor agonist (2). Bz-ATP (200 μM) increased 2-AG production to the same extent as ATP (Fig. 1a). Pretreatment of microglial cells with oxidized-ATP, a P2X7 antagonist, abolished both the ATP- and Bz-ATP-increased 2-AG production (Fig. 1 c and d). In line with these results, we confirmed that microglial cells express P2X7 receptor proteins (Fig. 1e) (1, 31). These results show that activation of P2X7 receptors is both sufficient and necessary to increase 2-AG production in microglial cells.
Fig. 1.
P2X7 receptors control 2-AG production. (a-d) Microglial cells were incubated with vehicle (basal), ATP, Bz-ATP, ADP, or UTP for 10 min (a) or with ATP (1 mM) for increasing periods of time (b). Microglial cells were preincubated for 2 h with vehicle (control) or oxidized-ATP (o-ATP, 300 μM) and incubated for 10 min with ATP (c) or Bz-ATP (d). Lipids were extracted and HPLC-purified, and amounts of eCB were quantified by chemical ionization-GC/MS. N values are mean ± SEM of independent eCB quantifications, each performed on one 60-mm dish of cells. In a and b, n = 14-82 dishes (i.e., 7-41 separate experiments performed in duplicate); **, P < 0.01, significantly different from basal (dotted line) (ANOVA followed by Dunnett's post test). In c and d, n = 6 dishes (i.e., three separate experiments performed in duplicate). **, P < 0.01, significantly different from respective control (Student's t test). Dotted line represents respective control response. (e) Western blot performed on microglial cell homogenates by using a P2X7 receptor antibody.
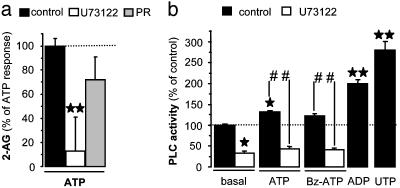
ATP-Induced 2-AG Production Requires PI-PLC Activity. Many studies have shown that PI-PLC activity is necessary for 2-AG production; yet, alternative pathways, such as the phospholipase D pathway, have been proposed (32). Fig. 2a shows that the PI-PLC inhibitor U73122 abolished ATP-induced 2-AG production by microglia, whereas the phospholipase D inhibitor propranolol had no effect, confirming the evidence that PI-PLC is necessary for 2-AG production.
Fig. 2.
ATP-induced 2-AG production involves PI-PLC. (a) Microglial cells were preincubated for 30 min with vehicle (control), U73122 (2 μM), or propranolol (200 μM) and incubated for 10 min with ATP (1 mM). Lipids were extracted and HPLC-purified, and amounts of eCB were quantified by chemical ionization-GC/MS. n = 6 independent eCB quantifications, each performed on one 60-mm dish (i.e., three separate experiments performed in duplicate). **, P < 0.01, significantly different from control (dotted line) (ANOVA followed by Dunnett's post test). (b) Microglial cells were preincubated for 30 min with vehicle (control) or U73122 (2 μM), incubated for 10 min with vehicle (basal), ATP (1 mM), Bz-ATP (200 μM), ADP (300 μM), or UTP (300 μM), and [3H]IP production was determined. Values are mean ± SEM of n = 9-54 determinations of [3H]IP production (i.e., 3-18 separate experiments performed in triplicate). **, P < 0.01, significantly different from basal (dotted line) (ANOVA followed by Dunnett's post test). ##, P < 0.01 significantly different from either the ATP or Bz-ATP response (Student's t test).
Considering this latter result, and the fact that activation of calcium-permeable ionotropic receptors, such as N-methyl-d-aspartate receptors, increase PI-PLC activity (33, 34), we tested the hypothesis that activation of P2X7 receptors on microglial cells increases PI-PLC activity. Thus, microglial cells were incubated with ATP and Bz-ATP, and the production of [3H]IP, a product of PI-PLC, was measured. We obtained two unexpected results. We found that ATP only induced a small increase in [3H]IP production and Bz-ATP had no significant effect (Fig. 2b), thus ruling out the possibility that activation of P2X7 receptors increases 2-AG production by merely increasing PI-PLC activity. We also found that ADP and UTP, two P2Y receptor agonists, significantly increased [3H]IP production (Fig. 2b), although they had no effect on 2-AG production (Fig. 1a).
Together, these results suggest that stimulation of PI-PLC activity is not sufficient to increase 2-AG production, and basal PI-PLC activity provides enough DG for ATP-induced 2-AG production to occur. If this second point holds true, U73122 should reduce PI-PLC activity below its basal activity. Fig. 2b shows that this was indeed the case.
DG Kinase Restrains 2-AG Production. Because an increase in PI-PLC activity does not lead to an increase in 2-AG production, microglial cells must express an enzyme that shunts the PI-PLC-derived DG toward a different metabolic pathway. Such an enzyme could be DG kinase, an enzyme that yields phosphatidic acid by phosphorylating DG (35). To test this hypothesis, we investigated the effect of a DG kinase inhibitor, DG kinase inhibitor I, on 2-AG production by microglial cells. When applied to microglial cells, DG kinase inhibitor I dramatically increased both basal 2-AG production (278 ± 122% of basal 2-AG production) and ATP-induced 2-AG production (11,178 ± 6,032% of basal) (both results are from n = 3 independent experiments performed in duplicate). These results suggest that microglial cells express a constitutively active DG kinase that shunts the majority of the PI-PLC-derived DG toward phosphatidic acid. These results also indicate that ATP does not increase 2-AG production by inhibiting DG kinase, because 2-AG production induced by the combination of ATP plus DG kinase inhibitor I is more than additive.
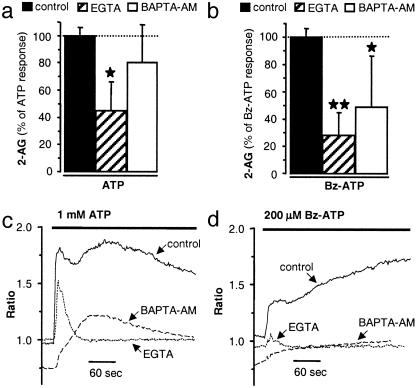
ATP-Induced 2-AG Production Requires Sustained Rise in Intracellular Calcium. Whereas many studies have shown that eCB production requires calcium (17, 18), at least one study suggested that increased eCB production may occur independently of a rise in [Ca2+]i (36). We tested the effect of EGTA, a chelator of extracellular calcium, and BAPTA-AM, a chelator of intracellular calcium, on ATP- and Bz-ATP-induced 2-AG production to determine whether these responses require a rise in [Ca2+]i and, if so, whether calcium was mobilized from extra- or intracellular compartments. EGTA inhibited both ATP- and Bz-ATP-induced 2-AG production, whereas BAPTA-AM only inhibited Bz-ATP-induced 2-AG production (Fig. 3 a and b). This result suggests that calcium from the extracellular milieu is clearly necessary for P2X7-induced 2-AG production, whereas the involvement of intracellular calcium is ambiguous.
Fig. 3.
ATP-induced 2-AG production requires sustained rise in [Ca2+]i. (a and b) Microglial cells were preincubated for 30 min with vehicle (control), EGTA (1 mM), or BAPTA-AM (10 μM) and incubated for 10 min with ATP (1 mM) or Bz-ATP (200 μM). Lipids were extracted and HPLC-purified, and amount of eCB was quantified by chemical ionization-GC/MS. Values are mean ± SEM of n = 6-62 independent eCB quantifications, each performed on one 60-mm dish (i.e., 3-31 separate experiments performed in duplicate). **, P < 0.01, significantly different from basal (dotted line) (ANOVA followed by Dunnett's post test). (c and d) [Ca2+]i in microglial cells was determined as described in Materials and Methods. Ratio plots in figures were averaged from ≈25 cells in the field of view and are representative of three experiments.
To determine why ATP- and Bz-ATP-induced 2-AG production had different sensitivities toward BAPTA-AM, we used fura-2 imaging to monitor changes in [Ca2+]i. It is known that activation of P2X7 receptors leads to a sustained rise in [Ca2+]i, a result of their high permeability to calcium, whereas activation of P2Y receptors induces a transient rise in [Ca2+]i, due to the release of calcium from intracellular stores (2). As expected, ATP induced a rise in [Ca2+]i that was both rapid and sustained (Fig. 3c). EGTA prevented the sustained elevation in [Ca2+]i without affecting the rapid rise in [Ca2+]i, whereas BAPTA-AM prevented the rapid rise in [Ca2+]i and merely reduced the sustained elevation in [Ca2+]i (Fig. 3c). On the other hand, Bz-ATP induced only a sustained increase in [Ca2+]i that was abolished by EGTA and clearly reduced by BAPTA-AM (Fig. 3d).
Together, these results suggest that increased 2-AG production induced by ATP and Bz-ATP requires sustained rise in [Ca2+]i, likely the result of P2X7 receptor activation, whereas transient elevations in [Ca2+]i, likely mediated through P2Y receptors, are not sufficient to increase 2-AG production. BAPTA-AM did not prevent ATP-induced 2-AG production because this chelator does not efficiently prevent the ATP-induced sustained rise in [Ca2+]i.
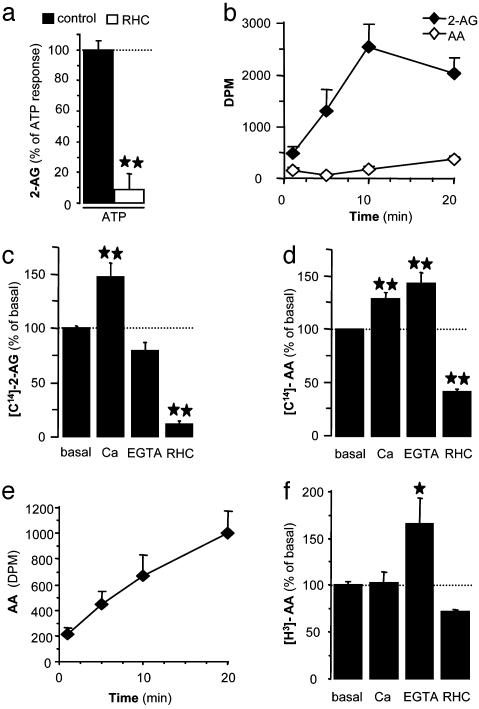
2-AG Production Is Controlled by Calcium-Dependent DG Lipase and MG Lipase Activity. DG lipase is known to produce 2-AG by hydrolyzing DG (17, 18). Indeed, the DG lipase inhibitor RHC-80267 prevented the ATP-increased 2-AG production in microglial cells (Fig. 4a). To determine whether DG lipase activity constitutes an enzymatic step that controls 2-AG production, we measured DG lipase activity in microglial cell homogenates by quantifying the production of 2-[14C]AG when adding DG that contains [14C]arachidonic acid in its sn-2 position. In the same experiments, we indirectly measured MG lipase activity by quantifying the production of [14C]arachidonic acid. Fig. 4b shows that, within minutes, large amounts of 2-[14C]AG were generated under basal conditions. On the contrary, under these conditions, little [14C]arachidonic acid was produced, suggesting that MG lipase is less active than DG lipase in microglial cell homogenates. 2-[14C]AG production was significantly increased by calcium, slightly reduced by EGTA and significantly inhibited by RHC-80267 (Fig. 4c), suggesting that calcium increases DG lipase activity. Remarkably, [14C]arachidonic acid production was increased by both calcium and EGTA and inhibited by RHC-80267 (Fig. 4d). The effect of calcium and RHC-80267 on [14C]arachidonic acid production likely reflects their effect on DG lipase. These results suggest that calcium inhibits MG lipase activity. We addressed this latter possibility by incubating microglial cell homogenates with 2-AG labeled with [3H]arachidonic acid and measured the production of free [3H]arachidonic acid as direct measure of MG lipase activity. Indeed, microglial cell homogenates express MG lipase activity (Fig. 4e), which was not significantly affected by calcium and RHC-80267 but was increased by EGTA (Fig. 4f), confirming that MG lipase activity is inhibited by calcium.
Fig. 4.
ATP-induced 2-AG production requires modulation of DG lipase and MG lipase activity. (a) Microglial cells were preincubated for 30 min with vehicle (control) or RHC-80267 (30 μM) and incubated for 10 min with ATP (1 mM). Lipids were extracted and HPLC-purified, and amounts of eCB were quantified by chemical ionization-GC/MS. Values are mean ± SEM of n = 6 independent eCB quantifications, each performed on one 60-mm dish (i.e., three separate experiments performed in duplicate). **, P < 0.01, significantly different from control response (dotted line) (Student's t test). (b-f) Microglial cell homogenates were incubated with [14C]DG (b-d) or 2-[3H]AG (e and f). After increasing times, lipids were chloroform-extracted and analyzed by TLC. Bands with the same retention time as 2-AG and arachidonic acid were scraped off, and the radioactivity therein was determined. (b-f) Microglial cell homogenates were incubated in the presence of vehicle (basal), calcium (3 mM), EGTA (1 mM), or RHC-80267 (30 μM) for 5 min in c and d and for 10 min in f. Values are mean ± SEM of 6-12 independent determinations of radioactivity (three to six separate experiments performed in duplicate). **, P < 0.01, significantly different from basal (dotted line) (ANOVA followed by Dunnett's post test).
Discussion
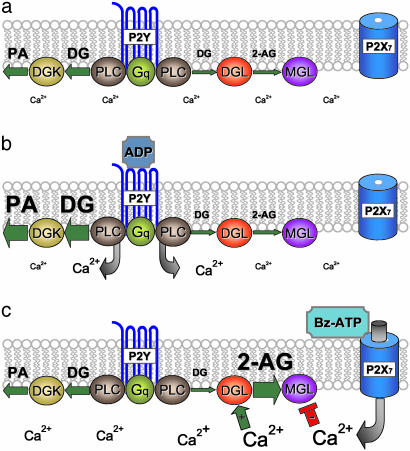
Our study identifies the purinergic receptor and enzymatic steps controlling 2-AG production in microglial cells. Fig. 5 depicts the model that we propose. Under basal conditions, microglial cells express constitutive PI-PLC activity, which continuously produces DG; yet, 2-AG production is low. Two mechanisms are likely to prevent 2-AG accumulation. DG kinase shunts the majority of the PI-PLC-produced DG toward phosphatidic acid, thus reducing substrate availability for DG lipase (Fig. 5a). Although some DG may reach DG lipase and produce 2-AG, the latter is promptly hydrolyzed by MG lipase, which should be active at the low [Ca2+]i present in intact cells (Fig. 5a). Under conditions whereby PI-PLC activity is greatly increased, for example, when ADP or UTP activates P2Y receptors, DG kinase activity is still sufficient to shunt all the PI-PLC-produced DG (Fig. 5b). Under conditions whereby P2X7 receptors are activated, for example, by millimolar concentrations of ATP, the sustained rise in [Ca2+]i that ensues increases DG lipase activity and decreases MG lipase activity, thus allowing 2-AG to accumulate (Fig. 5c). This inverse sensitivity of two lipases to calcium constitutes an original and efficient modality for sustained accumulation of a signaling lipid.
Fig. 5.
Model depicting the receptors and enzymatic steps that control 2-AG production by microglial cells. Levels of 2-AG, DG, and phosphatidic acid under basal (unstimulated) conditions (a) and in the presence of ADP (b) or Bz-ATP (c). The size of abbreviations represents amounts of lipids and calcium. The following abbreviations were also used: DGL, DG lipase; MGL, monoacylglycerol lipase; and DGK, diacylglycerol kinase. The size of arrows represents levels of activities.
RHC-80267 inhibits both ATP-induced 2-AG production by microglial cells and the calcium-sensitive DG lipase activity in microglial cell homogenates, confirming the evidence that DG lipase is absolutely necessary for 2-AG production (29, 32). Although no DG lipase has been cloned to date, various studies have shown that more than one DG lipase entity exists and that these enzymes have different sensitivities toward calcium (37). Recently, MG lipase has been cloned (21). Here, we show that the activity of this enzyme is inhibited by calcium.
Here, DG kinase is implicated in regulating eCB production. Nine DG kinase isoforms have been identified, each having a unique tissue and subcellular distribution (35). We show that, in microglial cells, DG kinase actively shunts the majority of PI-PLC-produced DG from the DG lipase pathway. Indeed, DG kinase inhibitor I increases basal 2-AG production by 3-fold and the ATP-increased 2-AG production by 30-fold. Selective inhibition of the specific DG kinase isoform involved in this response would provide an appealing pharmacological tool to increase 2-AG production.
Activation of P2X7 receptors results in a prolonged opening of its pore, which allows large quantities of extracellular calcium to enter the cell (2, 3). In line with this evidence, we show that chelation of extracellular calcium by EGTA prevents ATP- and Bz-ATP-induced sustained rise in [Ca2+]i. In the presence of EGTA, the rapid rise in [Ca2+]i induced by ATP, which is likely due to activation of P2Y receptors, is not affected. In the presence of EGTA, both ATP- and Bz-ATP-induced 2-AG production are prevented, suggesting that 2-AG production is induced by a sustained rise in [Ca2+]i coming from the extracellular space. Do sustained rises in [Ca2+]i systematically increase 2-AG production? This seems to be the case. In macrophage-like cells, both platelet-activating factor and lipopolysaccharide induce sustained increases in [Ca2+]i (38, 39) and increase 2-AG production (40, 41). In neurons, activation of N-methyl-d-aspartate receptors enhance [Ca2+]i and increase 2-AG production (29). In support of the notion that sustained rises in [Ca2+]i is required to increase 2-AG production, ADP and UTP, which transiently elevate [Ca2+]i in microglial cells in a manner that is not affected by EGTA (data not shown), do not increase 2-AG production (Fig. 1a).
Why did BAPTA-AM significantly affect Bz-ATP-induced 2-AG production, but it did not affect ATP-induced 2-AG production? When monitoring [Ca2+]i we saw that BAPTA-AM prevented the Bz-ATP-induced rise in [Ca2+]i, whereas it only reduced the ATP-induced rise in [Ca2+]i. One likely explanation for this difference is that ATP also induces a release of calcium from intracellular stores (i.e., the initial rise in [Ca2+]i), which are replenished through calcium-operated calcium channels (38). This strong influx of calcium is unlikely to be chelated by BAPTA-AM, because this chelator is likely to already be saturated by calcium. Thus, ATP still induces 2-AG production in the presence of BAPTA-AM because of the rise in [Ca2+]i that remains.
Both ATP and 2-AG concurrently increase in brain parenchyma under neuropathological conditions (9, 10). Our data provide a functional link between these two phenomena. Immunohistochemical studies show that microglial cells and astrocytes express P2X7 receptors (1). Here, we show that activation of P2X7 receptors on microglial cells increases 2-AG production, but recent experiments from our laboratory show that P2X7 receptor activation on astrocytes in primary culture also increases 2-AG production (unpublished data). Note that microglial cells in primary culture produce ≈20-fold higher amounts of eCB (expressed in pmol/mg of protein) compared with astrocytes in primary culture, but astrocytes in brain parenchyma are more abundant than microglial cells. Therefore, we propose that, under neuropathological conditions, microglial cells and astrocytes are responsible for producing a significant amount of the eCB that accumulates in brain parenchyma.
Can 2-AG control the extent of neuroinflammatory responses? As extracellular ATP concentrations increase to millimolar levels and activate P2X7 receptors expressed by neural cells, local amounts of 2-AG will increase. 2-AG then activates presynaptic CB1 receptors, which reduces glutamate releases and tempers excitotoxicity (11-13). 2-AG will also activate CB1 receptors expressed by blood vessels, which will decrease cerebral blood flow and reduce edema expansion (14). Finally, 2-AG will activate CB2 receptors expressed by microglial cells and invading immune cells, which inhibits their ability to release cytotoxic agents (42). Therefore, compounds that affect eCB production from microglial cells might constitute promising therapeutics to temper exacerbated neuroinflammatory responses and allied cell damage.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grant WI 1965/1-1 (to A.W.), Public Health Service National Research Service Award T32 from National Institute of General Medical Sciences Grant GM07270 (to L.W.), and National Institutes of Health Grants DA14486 (to N.S.) and NS044337 (to T.M.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: 2-AG, 2-arachidonoylglycerol; DG, diacylglycerol; PI-PLC, phosphatidylinositol-specific phospholipase C; eCB, endocannabinoid; MG, monoacylglycerol; BAPTA-AM, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate-acetoxymethyl ester; IP, inositol phosphate; Bz, benzoylbenzoyl.
References
- 1.James, G. & Butt, A. M. (2002) Eur. J. Pharmacol. 447, 247-260. [DOI] [PubMed] [Google Scholar]
- 2.Ralevic, V. & Burnstock, G. (1998) Pharmacol. Rev. 50, 413-492. [PubMed] [Google Scholar]
- 3.Le Feuvre, R., Brough, D. & Rothwell, N. (2002) Eur. J. Pharmacol. 447, 261-269. [DOI] [PubMed] [Google Scholar]
- 4.Honda, S., Sasaki, Y., Ohsawa, K., Imai, Y., Nakamura, Y., Inoue, K. & Kohsaka, S. (2001) J. Neurosci. 21, 1975-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreutzberg, G. W. (1996) Trends Neurosci. 19, 312-318. [DOI] [PubMed] [Google Scholar]
- 6.Aloisi, F. (2001) Glia 36, 165-179. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima, K. & Kohsaka, S. (2001) J. Biochem. (Tokyo) 130, 169-175. [DOI] [PubMed] [Google Scholar]
- 8.Wyss-Coray, T. & Mucke, L. (2002) Neuron 35, 419-432. [DOI] [PubMed] [Google Scholar]
- 9.Panikashvili, D., Simeonidou, C., Ben Shabat, S., Hanus, L., Breuer, A., Mechoulam, R. & Shohami, E. (2001) Nature 413, 527-531. [DOI] [PubMed] [Google Scholar]
- 10.Baker, D., Pryce, G., Croxford, J. L., Brown, P., Pertwee, R. G., Makriyannis, A., Khanolkar, A., Layward, L., Fezza, F., Bisogno, T. & Di Marzo, V. (2001) FASEB J. 15, 300-302. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan, J. M. (1999) J. Neurophysiol. 82, 1286-1294. [DOI] [PubMed] [Google Scholar]
- 12.Shen, M. & Thayer, S. A. (1998) Mol. Pharmacol. 54, 459-462. [DOI] [PubMed] [Google Scholar]
- 13.Marsicano, G., Goodenough, S., Monory, K., Hermann, H., Eder, M., Cannich, A., Azad, S. C., Cascio, M. G., Gutierrez, S. O., Van Der Stelt, M., et al. (2003) Science 302, 84-88. [DOI] [PubMed] [Google Scholar]
- 14.Parmentier-Batteur, S., Jin, K., Mao, X. O., Xie, L. & Greenberg, D. A. (2002) J. Neurosci. 22, 9771-9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang, Y. H., Lee, S. T. & Lin, W. W. (2001) J. Cell Biochem. 81, 715-723. [DOI] [PubMed] [Google Scholar]
- 16.Walter, L., Franklin, A., Witting, A., Wade, C., Xie, Y., Kunos, G., Mackie, K. & Stella, N. (2003) J. Neurosci. 23, 1398-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Marzo, V., Melck, D., Bisogno, T. & De Petrocellis, L. (1998) Trends Neurosci. 21, 521-528. [DOI] [PubMed] [Google Scholar]
- 18.Piomelli, D., Beltramo, M., Giuffrida, A. & Stella, N. (1998) Neurobiol. Dis. 5, 462-473. [DOI] [PubMed] [Google Scholar]
- 19.Howlett, A. C., Barth, F., Bonner, T. I., Cabral, G., Casellas, P., Devane, W. A., Felder, C. C., Herkenham, M., Mackie, K., Martin, B. R., et al. (2002) Pharmacol. Rev. 54, 161-202. [DOI] [PubMed] [Google Scholar]
- 20.Cravatt, B. F. & Lichtman, A. H. (2002) Chem. Phys. Lipids 121, 135-148. [DOI] [PubMed] [Google Scholar]
- 21.Dinh, T. P., Carpenter, D., Leslie, F. M., Freund, T. F., Katona, I., Sensi, S. L., Kathuria, S. & Piomelli, D. (2002) Proc. Natl. Acad. Sci. USA 99, 10819-10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson, R. I. & Nicoll, R. A. (2002) Science 296, 678-682. [DOI] [PubMed] [Google Scholar]
- 23.Robbe, D., Kopf, M., Remaury, A., Bockaert, J. & Manzoni, O. J. (2002) Proc. Natl. Acad. Sci. USA 99, 8384-8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chevaleyre, V. & Castillo, P. E. (2003) Neuron 38, 461-472. [DOI] [PubMed] [Google Scholar]
- 25.Franklin, A., Parmentier-Batteur, S., Walter, L., Greenberg, D. A. & Stella, N. (2003) J. Neurosci. 23, 7767-7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter, L., Franklin, A., Witting, A., Moller, T. & Stella, N. (2002) J. Biol. Chem. 277, 20869-20876. [DOI] [PubMed] [Google Scholar]
- 27.Walter, L. & Stella, N. (2003) Glia 44, 85-90. [DOI] [PubMed] [Google Scholar]
- 28.Stella, N., Pellerin, L. & Magistretti, P. J. (1995) J. Neurosci. 15, 3307-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stella, N., Schweitzer, P. & Piomelli, D. (1997) Nature 388, 773-778. [DOI] [PubMed] [Google Scholar]
- 30.Moller, T., Nolte, C., Burger, R., Verkhratsky, A. & Kettenmann, H. (1997) J. Neurosci. 17, 615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, M., Spelta, V., Sim, J., North, R. A. & Surprenant, A. (2001) J. Biol. Chem. 276, 23262-23267. [DOI] [PubMed] [Google Scholar]
- 32.Bisogno, T., Melck, D., De Petrocellis, L. & Di Marzo, V. (1999) J. Neurochem. 72, 2113-2119. [DOI] [PubMed] [Google Scholar]
- 33.Nicoletti, F., Wroblewski, J. T., Novelli, A., Alho, H., Guidotti, A. & Costa, E. (1986) J. Neurosci. 6, 1905-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurd, J. W. & Bissoon, N. (1997) J. Neurochem. 69, 623-630. [DOI] [PubMed] [Google Scholar]
- 35.Topham, M. K. & Prescott, S. M. (2002) Thromb. Haemostasis 88, 912-918. [PubMed] [Google Scholar]
- 36.Kondo, S., Kondo, H., Nakane, S., Kodaka, T., Tokumura, A., Waku, K. & Sugiura, T. (1998) FEBS Lett. 429, 152-156. [DOI] [PubMed] [Google Scholar]
- 37.Moriyama, T., Urade, R. & Kito, M. (1999) J. Biochem. (Tokyo) 125, 1077-1085. [DOI] [PubMed] [Google Scholar]
- 38.Khoo, C., Helm, J., Choi, H. B., Kim, S. U. & McLarnon, J. G. (2001) Glia 36, 22-30. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann, A., Kann, O., Ohlemeyer, C., Hanisch, U. K. & Kettenmann, H. (2003) J. Neurosci. 23, 4410-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berdyshev, E. V., Schmid, P. C., Krebsbach, R. J. & Schmid, H. H. (2001) FASEB J. 15, 2171-2178. [DOI] [PubMed] [Google Scholar]
- 41.Matias, I., Pochard, P., Orlando, P., Salzet, M., Pestel, J. & Di Marzo, V. (2002) Eur. J. Biochem. 269, 3771-3778. [DOI] [PubMed] [Google Scholar]
- 42.Klegeris, A., Bissonnette, C. J. & McGeer, P. L. (2003) Br. J. Pharmacol. 139, 775-786. [DOI] [PMC free article] [PubMed] [Google Scholar]