Abstract
Purpose
Abiraterone is an oral inhibitor of CYP17, essential for androgen biosynthesis. This multicenter study assessed its efficacy in patients with CRPC without prior exposure to chemotherapy or CYP17 targeted therapy, and assessed the frequency of interpretation of bone scans discordant with PSA and clinical response.
Patients and Methods
33 patients received abiraterone acetate 1000 mg daily with prednisone 5 mg twice daily in continuous 28-day cycles. Patients were evaluated monthly for efficacy and safety. Bone scan flare was defined as the combination, after 3 months of therapy, of an interpreting radiologist's report indicating “disease progression” in the context of a ≥50% decline in PSA, with scan improvement 3 months later.
Results
A ≥ 50% PSA decline at week 12 was confirmed in 22/33 (67%) patients. PSA declines of ≥ 50% were seen in 26 (79%) patients. Undetectable PSA levels (≤ 0.1 ng/mL) occurred in 2 patients. Median time on therapy and time to PSA progression are 63 and 71 weeks, respectively. Twenty three patients were evaluable for bone scan flare. Progression was indicated in the radiologist's report in 12/23 (52 %), and 10/12 subsequently showed improvement. As prospectively defined, bone scan flare was observed in 10/23 (43.5%) evaluable patients or 10/33 (30%) enrolled patients. Adverse events were typically grade 1/2 and consistent with prior published abiraterone reports.
Conclusion
Clinical response to abiraterone acetate plus prednisone was frequent and durable in men with metastatic CRPC progressing on hormonal therapy with over half of patients on therapy > 1 year. Further investigation is needed to clarify the potential confounding effect of the frequently occurring bone scan flare phenomena on patient management and interpretation of clinical trial results.
Keywords: abiraterone acetate, castration-resistant prostate cancer, CRPC, hormone-resistant prostate cancer, therapy, efficacy
INTRODUCTION
As data emerge suggesting that the progression of prostate cancer in an androgen-deprived milieu is, in part, mediated through the selective growth of tumor cells with a heightened sensitivity to androgens[1–4], progress and interest in the development of therapies that target extra-gonadal androgen synthesis has accelerated[5, 6]. Abiraterone acetate is an orally available selective androgen biosynthesis inhibitor that specifically inhibits CYP17. Abirateroned has shown activity as a monotherapy in castration-resistant prostate cancer (CRPC) in both chemotherapy-naïve and chemotherapy-exposed patients receiving an luteinizing hormone releasing hormone (LHRH) analogue.[7–12]
Based on the phase I experience with abiraterone acetate monotherapy, it was determined that the safety profile of this therapy could be improved through concomitant corticosteroid administration - an approach that reduces the compensatory elevations in adrenocorticotropic hormone (ACTH) and mineralocorticoid excess induced by CYP17 blockade. Because corticosteroids have shown modest anti-tumor efficacy when given as monotherapy, the current phase II study was designed to determine the efficacy and safety of the combination of abiraterone acetate and prednisone in patients with metastatic CRPC. In particular, the present trial is the first trial with abiraterone to focus on patients who had not been treated with either docetaxel or with an androgen synthesis inhibitor, such as ketoconazole.
A well-recognized barrier to therapy development in CRPC is the lack of reliable surrogate markers of response to treatment coupled with a potentially exaggerated reliance on changes in serum prostate-specific antigen (PSA) as an indicator of treatment efficacy. This is particularly true with agents that have the potential for PSA modulation, such as abiraterone acetate or ketoconazole. Consequently, changes in bone scan images have been advocated as important markers of disease response and progression. However, the utility of bone scans has been called into question because of a transient “flare” of bone lesion intensity in the context of treatment response resulting in a false determination of disease progression[13, 14]. Although the bone scan flare has been described in breast cancer and in hormone sensitive prostate cancer, no systemic evaluation of the flare phenomenon has been undertaken in CRPC[15, 16]. Therefore, changes in objective imaging, particularly bone scans, were incorporated into this study in addition to PSA decline and time to PSA progression. As noted, these imaging changes are particularly important in the context of a novel agent that can potentially modulate PSA, potentially rendering it a less useful biomarker.
PATIENTS AND METHODS
Patients
Eligibility required histologically confirmed adenocarcinoma of the prostate progressing on androgen deprivation therapy (either a LHRH agonist or orchiectomy, and following antiandrogen withdrawal, as appropriate). PSA progression was defined according to Prostate-Specific Antigen Working Group (PSAWG) criteria, [17]and all patients had to have baseline lesions identified by bone scan, CT, or MRI. Prior ketoconazole therapy or chemotherapy was not permitted, with the exception of neoadjuvant or adjuvant chemotherapy completed at least 1 year before study entry. Additional eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, adequate renal (serum creatinine ≤ 1.5 × ULN), hepatic (bilirubin ≤ 1.5 × ULN, AST and ALT ≤ 2.5 × ULN), and bone marrow (hemoglobin > 9 gm/dL, absolute neutrophil count > 1.5 × 109/L, platelets > 100 × 109/L) function, serum potassium level ≥ 3.5 mmol/L, and a castrate level of testosterone (< 50 ng/dL). Patients with clinically significant electrocardiogram abnormalities were ineligible, as were patients with uncontrolled hypertension, New York Heart Association Class III or IV congestive heart failure, those with active autoimmune disease requiring corticosteroid therapy, or any other serious medical or psychiatric illness. Radiation therapy or initiation of bisphosphonate therapy within 4 weeks of study entry was not permitted, although maintenance of a stable bisphosphonate dose was allowed. Use of hormonal therapies, systemic corticosteroids, or any other agent known to decrease PSA levels within 4 weeks prior to study initiation was not permitted. Written informed consent was obtained from all patients.
Treatment and Evaluations
Treatment consisted of abiraterone acetate 1000 mg daily with prednisone 5 mg twice daily. Abiraterone was administered without food in 28-day cycles. Treatment was given continuously until evidence of disease progression in patients not experiencing unacceptable toxicity.
Screening evaluations included a history and physical examination, performance status evaluation, and 12-lead electrocardiogram (ECG). Laboratory assessments included a CBC, serum chemistries and electrolytes, blood clotting evaluation (PT, PTT, INR), and serum PSA and testosterone levels. Baseline tumor imaging was performed by bone scan, CT, MRI, or other imaging procedure. Selected physical and laboratory assessments were repeated on days 1 and 8 of cycle 1, on day 1 of each subsequent cycle, and at the end of study. PSA values were obtained monthly. Adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3. Bone scans and tumor imaging studies were repeated every three cycles.
Study Design and Statistical Considerations
This was a single-arm, open-label, multicenter phase II study conducted under the auspices of the Department of Defense Prostate Cancer Clinical Trials consortium and sponsored by Cougar Biotechnology. The primary study end point was the proportion of patients achieving a ≥ 50% decline in PSA by week 12 of therapy. Secondary end points included durability of response as determined by time to PSA progression, objective radiographic response rate according to RECIST guidelines,[18]radiographic progression-free survival time, overall survival time, clinical benefit as determined by disease stabilization, change in ECOG performance status, and overall treatment safety.
Bone scan flare was prospectively defined as discordant results after 3 months of therapy based on the combination of an interpreting radiologist's report indicating “disease progression”, typically based on increased lesion intensity or number that occurred in the context of a ≥ 50% decline in PSA, which on subsequent reevaluation 3 months later showed improvement in the scan. Thus patients evaluable for bone scan flare included all patients who had bone scans available at baseline, after 3 months of therapy, and after 6 months of therapy.
A sample size of 32 evaluable patients was determined as necessary to detect a response rate of at least 50%, with response defined as ≥ 50% decline in PSA at week 12 from baseline measurement, at a significance level of 0.04 for a directional test and 81% power. Distributions of time-to-event variables were estimated using the Kaplan-Meier product limit method. All patients who received a minimum of three cycles of therapy were considered evaluable for response. All patients who received at least one dose of abiraterone acetate were evaluable for safety.
Adverse events were summarized by worst grade of severity per patient. The study protocol was approved by the institutional review boards at all participating sites and was conducted in accordance with the ethical principles of the World Medical Association Declaration of Helsinki.
RESULTS
Patient Characteristics and Treatment
Between October 2007 and May 2008, 33 men with metastatic prostate cancer progressing despite a castrate level of testosterone were enrolled across five study centers within the U.S. Table 1 lists the baseline patient demographics and clinical characteristics. The median baseline PSA level was 23 ng/mL and ranged from 5.9 to 1110 ng/mL. All patients had radiographic evidence of metastatic disease and 26/33 (79%) had bony metastases. All patients had received androgen deprivation therapy with an LHRH antagonist (n = 31) or orchiectomy (n = 2), and 32 (97%) had also received an antiandrogen and undergone antiandrogen withdrawal. All patients had castrate serum testosterone levels (median 8.5 ng/dL; range 0.9 to 24.1 ng/dL). The majority of patients (88%) had received two prior hormonal therapies, with three patients (9%) having received up to four hormonal therapies including estrogens or glucocorticoids. No patient had undergone prior treatment with abiraterone, ketoconazole, or chemotherapy. At the time of analysis (January 2010), the study population had received a median of 63 weeks (range 8 to 104 weeks) of treatment with abiraterone acetate plus prednisone, with 15 patients (46%) continuing to receive therapy. Treatment had been discontinued due to disease progression in 14 patients (42%) and adverse events in three patients (9%); two patients discontinued due to grade 3 adverse events (1 each for back pain and pathological fracture). Twenty-six patients were evaluable for the bone flare phenomenon. All 33 patients were evaluable for response and safety.
Table 1.
Baseline Demographic and Clinical Characteristics of 33 Patients Enrolled in a Phase II Trial of Abiraterone Acetate Plus Prednisone.
| Baseline Value | |
|---|---|
|
| |
| PSA (Median) ng/mL | 23.0 (range 5.9 – 1110.0) |
|
| |
| Metastases | n (%) |
| Visceral only | 1 (3) |
| Viscera plus bone/soft tissue | 2(6) |
| Bone only | 10 (30.3) |
| Soft tissue only | 6 (18.2) |
| Bone and soft tissue only | 14 (42.4) |
|
| |
| Gleason Score (Median) | 8 (range 5 – 9) |
|
| |
| Hemoglobin (Median) g/dL | 12.8 (range 10.6 – 15.3) |
|
| |
| Testosterone (Median) ng/dL | 25.5 (range 4.0 – 49.0) |
|
| |
| Alkaline Phosphatase (Median) units/L | 82.0 (range 39.0 – 1078.0) |
|
| |
| Number of Hormonal Therapies (Median) | 2 (range 2 – 4) |
PSA Response and Durability
Changes in PSA, both after 3 months of therapy and maximal, for each patient are depicted in Figure 1. A decline in PSA of ≥ 50% after 3 months, the primary study end point, was confirmed in 22 (67%) of 33 patients. Confirmed maximal declines in PSA of ≥ 50% and ≥ 90% were seen in 26 (79%) and 15 (46%) patients, respectively. In two patients, PSA levels became undetectable (≤ 0.1 ng/mL), declining from baseline values of 204 ng/mL and 9 ng/mL, respectively. These patients continue to receive study therapy after 20 and 21 months, both with continued stable bone scans and resolution of adenopathy in one patient.
Figure 1.
Changes in prostate specific antigen (PSA) levels in castration-resistant prostate cancer (CRPC) patients treated with abiraterone acetate plus prednisone. Waterfall plots of maximal PSA change (top panel) and PSA change at week 12 (bottom panel).
Median follow-up time for this analysis was 19.3 months. The median time to PSA progression was 16.3 months (95% CI; 9.2 months, not estimable, Fig 2). Nineteen (58%) patients of have received study treatment for at least 12 months.
Figure 2.
Time to prostate specific antigen (PSA) progression in castration-resistant prostate cancer (CRPC) patients treated with abiraterone acetate and prednisone (n = 33). CI, confidence interval; NE=Not Estimable
Objective Tumor Response
Of 13 patients with measurable disease consisting of lymphadenopathy, nine (69%) had a partial response and three (23%) had stable disease.
Bone Scan results and Bone Scan Flare
At baseline, 26 patients had positive bone scans. One had a solitary bone metastasis, seven patients had between two and four metastases, and 18 patients had more than four discrete metastases. Twenty three patients had the combination of a positive baseline bone scan, ≥50% decline in PSA after three months and bone scans at 3 and 6 months and thus are available for evaluation of bone scan flare. Reports are available on 92 total bone scans from 41 unique radiologists dispersed geographically amongst the study sites. Of the 23 eligible patients, bone scan progression was indicated in the radiologist's report in 12 (52 %) of the scans taken after 3 months of therapy. Four of the 12 patients had a report indicating “progression of disease” without new lesions whereas for 8/12 patients, progression of disease due to new lesions was noted. Imaging following 6 months of therapy, the radiologists report in 10/12 indicated subsequent improvement, thereby meeting the definition of bone flare. Thus, overall, bone scan flare was observed in 10 of 23 (43.5%) evaluable patients or 10 of 33 (30%) enrolled patients.
Two patients were not evaluable: one had persistent PSA declines (from baseline to the end of month 3 and from month 3 through month 6 of therapy) with a negative bone scan at baseline that was not repeated; the second discontinued study therapy after 4 months on therapy due to a pathological femoral neck fracture despite a PSA decline of 91.7%.
In the 10 patients with bone flare, median age was 72 (range 54 to 85) years, median PSA at baseline was 32.4 (range 6.8 to 204.3) ng/dL, and median alkaline phosphatase at baseline was 88.5 (range 49.0 to 372.0) units/L, not significantly different from the study population as a whole. Alkaline phosphatase did not change in patients experiencing flare: median baseline value was 88.5 units/L. After 3 months of therapy, 88.5 units/L and after 6 months, 83.5 units/L. Three patients had between two and four metastases and seven patients had multifocal disease. Patient disposition is summarized in Figure 3.
Figure 3.
Disposition of patients who did and did not experience a bone scan flare.
*Of the 2 patients: 1 patient had a negative bone scan at baseline that was not repeated given the prostate specific antigen (PSA) decline; 1 patient came off study at month 4 due to a pathologic femoral neck fracture.
†Of the 2 patients: 1 came off study after month 9 (and thus never underwent another bone scan) while the other patient came off study after month 16.
BL= baseline; m4, month 4; m7, month 7
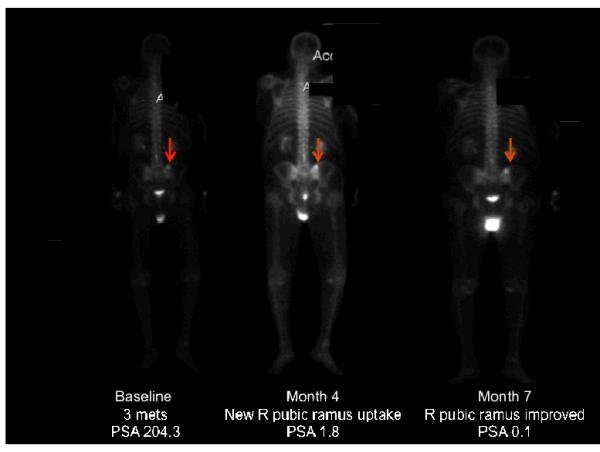
Radiologist interpretation of bone scan in these 10 patients was as follows, five patients had month 4 bone scans read as having increased intensity of existing lesions; the other five patients had bone scans that were read as having new lesions. Images in Figure 4 demonstrate this. Of these 10 patients with flare, six had continued declines in PSA, and four had modest increases in PSA (range 0.14–5.1 ng/dL). Of the two remaining patients with discordant results after 3 months of therapy, both continued to have PSA declines past 3 months but both had bone scans after 6 months of therapy that were interpreted as progressive disease. The first of these two patients came off study after 8 months of therapy (and thus never underwent another bone scan), while the other patient discontinued study therapy after 15 months.
Figure 4.
Examples of bone scan flare in patients receiving abiraterone acetate. (A) Example of a patient with a declining prostate specific antigen (PSA) but a month 4 bone scan being read as having a new metastasis in the right pubic ramus, as indicated by the arrow (although in retrospect one can see the lesion in the baseline bone scan). By month 7, this lesion shows improvement, indicating that this lesion seen at month 4 was present at baseline and thus was secondary to bone flare. (B) Example of a patient with a declining PSA but a month 4 bone scan being read as progression in existing lesions. By month 7, this progression has improved, indicating that the progression seen at month 4 was due to bone flare.
BL= baseline,; m4, month 4; m7, month 7
Safety
Adverse events were most often grade 1 or 2 (see Table 2) and clinically manageable. The most common treatment-related adverse events were fatigue, hot flush, bone pain, peripheral edema, arthralgia, dizziness, and hypokalemia. In addition to those listed in Table 2, there was a single occurrence each of grade 3 supraventricular arrhythmia and atrial flutter. One incident each of grade 3 hypokalemia and hypertension each was observed.
Table 2.
Incidence of Most Frequent (≥ 10%) Treatment-related Adverse Events (n = 33)*
| Total | Grade (% of patients) | ||||
|---|---|---|---|---|---|
| Toxicity by Preferred Term | Grade 1–4 | 1 | 2 | 3 | 4 |
| Fatigue | 15 (46) | 8 (24) | 6 (18) | 1 (3) | 0 |
| Hot flush | 10 (30) | 7 (21) | 3 (9) | 0 | 0 |
| Bone pain | 8 (24) | 6 (18) | 1 (3) | 1 (3) | 0 |
| Peripheral edema | 8 (24) | 7 (21) | 0 | 1 (3) | 0 |
| Arthralgia | 7 (21) | 6 (18) | 1 (3) | 0 | 0 |
| Dizziness | 7 (21) | 6 (18) | 0 | 1 (3) | 0 |
| Hypokalemia | 7 (21) | 6 (18) | 0 | 1 (3) | 0 |
| Back pain | 6 (18) | 3 (9) | 2 (6) | 1 (3) | 0 |
| Hypertension | 6 (18) | 3 (9) | 2 (6) | 1 (3) | 0 |
| Muscle spasms | 6 (18) | 6 (18) | 0 | 0 | 0 |
| Constipation | 5 (15) | 5 (15) | 0 | 0 | 0 |
| Ecchymosis | 5 (15) | 4 (12) | 1 (3) | 0 | 0 |
| Hyperbilirubinemia | 5 (15) | 4 (12) | 1 (3) | 0 | 0 |
| Hyperglycemia | 5 (15) | 2 (6) | 2 (6) | 1 (3) | 0 |
| Contusion | 4 (12) | 4 (12) | 0 | 0 | 0 |
| Nausea | 4 (12) | 3 (9) | 1 (3) | 0 | 0 |
| Musculoskeletal pain | 4 (12) | 2 (6) | 2 (6) | 0 | 0 |
| Pain in extremity | 4 (12) | 3 (6) | 1 (3) | 0 | 0 |
| Upper respiratory tract infection | 4 (12) | 2 (6) | 2 (6) | 0 | 0 |
| Vomiting | 4 (12) | 3 (9) | 1 (3) | 0 | 0 |
Most frequent according to the total percentage observed
DISCUSSION
In the current study a large proportion of patients (67%) with CRPC experienced a ≥ 50% decline in serum PSA while on abiraterone acetate, an effect that persisted for greater than 1 year in more than half of patients. An important and surprising observation in this study was that bone scan flare as prospectively defined was observed in a large proportion of patients (30% of the total and 44% of those who experienced a ≥50% decline in PSA).
In this study, the potential clinical utility of abiraterone acetate in CRPC used determinants of efficacy that included PSA decline, time to PSA progression, and changes in the objective imaging with bone scan and CT scans. Evaluation of bone scans resulted in an observation of a high incidence of bone flare during the first 6 months of the study. The high incidence of the flare phenomenon highlights several important issues. First, this phenomenon is not systematically assessed in the setting of highly active, hormonal therapies that modulate PSA expression in CRPC. As a result, incorporation of this data into subsequent phase II and phase III designs may further clarify the clinical meaning of this phenomenon (eg, whether it predicts a favorable long-term outcome). Second, it suggests that there may be an initial discordance between PSA and bone scan that will change with time, in many cases leading to the stable presence of bone lesions following an initial increase in tracer uptake. Of note, consensus criteria such as the PCWG2 formally addresses bone flare and includes the recommendation to perform a first follow-up bone scan 12 or more weeks after the start of therapy; and furthermore defines progression in bone when a minimum of two new lesions are observed and confirmed on a second scan performed 6 or more weeks later[19]. These bone flare findings further highlight a need for closer communication between clinicians and interpreting radiologists. This phase II study did not mandate central review of scans, nor did it require that scans be performed at the main clinical site of the study. Indeed, there were 41 individual radiologists from 4 different states (MA, TX, NM, and CA) who interpreted 92 bone scans in this study – which suggests that these results are not the result of an institutional or regional bias. It is thus necessary that this phenomenon be recognized to avoid prematurely discontinuing efficacious therapy on the basis of a potentially erroneous bone scan. Mandating central review of scans where possible is recommended. Lastly, bone flare can potentially be evaluated by the measurement of markers of bone turnover, as changes in bone-specific isoenzyme of alkaline phosphatase and osteocalcin have been reported to be sensitive predictors of subsequent radiologic response. [20] These parameters may provide useful clinical information to a physician after only 1 month of therapy, long before radiological evidence of response can be expected.[21] Imaging techniques such as Positron Emission Tomography (PET) utilizing novel tracers such as radiolabelled dihydrotestosterone (DHT) are also in development as a potential means to more specifically image androgen receptor and ligand interactions.
The PSA response proportion of 67% in this study is slightly higher than that observed in previous phase II studies with abiraterone acetate (36% to 55% [9, 12, 22]. Potential explanations for this observation are many and include the fact that this was a population with less extensive disease than in past studies (eg, median PSA was 23 in this study), no patients were previously treated with either chemotherapy or CYP17 inhibitors such as ketoconazole, and finally that prednisone may contribute to a modest increase in the likelihood of a PSA decline.
Grade 3 and 4 toxicities were infrequent in this study, and toxicities related to mineralocorticoid excess that were observed in earlier studies of abiraterone acetate without steroid replacement therapy were rare [9–12].Although hypokalemia remained a common event, it occurred with lower incidence and severity than was observed in patients previously treated with steroid replacement therapy. This decrease was most likely due to suppression of the compensatory rise in ACTH levels by prednisone, as well as a heightened awareness of this potential toxicity and early intervention with potassium supplementation.
Collectively, these results, as well as the favorable long-term safety profile of this combination suggest that this therapy is highly active in CRPC. Advancement of this dose and schedule of abiraterone to a pivotal phase III study in this patient population is warranted.
Acknowledgments
Supported by: Cougar Biotechnology and the Department of Defense Prostate Cancer Clinical Trials Consortium
Footnotes
Presented in part at the 2009 American Society of Clinical Oncology Annual Meeting, Orlando, FL, and the 2009 American Society of Clinical Oncology Genitourinary Cancers Symposium, Orlando, FL
REFERENCES
- 1.Chen CD, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–99. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 2.Holzbeierlein J, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164(1):217–27. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mostaghel EA, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67(10):5033–41. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RB, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Small E, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–33. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Ryan CJ, et al. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: results from a cancer and leukemia group B study. Clin Cancer Res. 2007;13(7):2030–7. doi: 10.1158/1078-0432.CCR-06-2344. [DOI] [PubMed] [Google Scholar]
- 7.Barrie SE, et al. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17–20 lyase) J Steroid Biochem Mol Biol. 1994;50(5–6):267–73. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell A, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90(12):2317–25. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan CJ, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28(9):1481–8. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attard G, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27(23):3742–8. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attard G, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 12.Reid AH, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28(9):1489–95. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johns WD, Garnick MB, Kaplan WD. Leuprolide therapy for prostate cancer. An association with scintigraphic “flare” on bone scan. Clin Nucl Med. 1990;15(7):485–7. doi: 10.1097/00003072-199007000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Sundkvist GM, et al. Dynamic quantitative bone scintigraphy in patients with prostatic carcinoma treated by orchiectomy. Eur J Nucl Med. 1990;16(8–10):671–6. doi: 10.1007/BF00998167. [DOI] [PubMed] [Google Scholar]
- 15.Janicek MJ, Hayes DF, Kaplan WD. Healing flare in skeletal metastases from breast cancer. Radiology. 1994;192(1):201–4. doi: 10.1148/radiology.192.1.8208938. [DOI] [PubMed] [Google Scholar]
- 16.Pollen JJ, Shlaer WJ. Osteoblastic response to successful treatment of metastatic cancer of the prostate. AJR Am J Roentgenol. 1979;132(6):927–31. doi: 10.2214/ajr.132.6.927. [DOI] [PubMed] [Google Scholar]
- 17.Bubley GJ, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17(11):3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Scher HI, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22(3):537–56. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 20.Coleman RE, et al. Osteocalcin: a potential marker of metastatic bone disease and response to treatment. Eur J Cancer Clin.Oncol. 1988;24:1211–1217. doi: 10.1016/0277-5379(88)90130-7. [DOI] [PubMed] [Google Scholar]
- 21.Sundkvist GM, et al. Quantitative bone scintigraphy in prostatic carcinoma--long-term response to treatment. Nuklearmedizin. 1993;32(5):231–5. [PubMed] [Google Scholar]
- 22.Danila DC, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28(9):1496–501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]