Abstract
The anti-inflammatory activity of cannabinoids has been widely demonstrated in experimental animal models and in humans. CD40-CD40-ligand (L) interactions are among the most crucial initiators of inflammation. This study investigated the effects of Δ9-THC on CD40L expression in mouse splenic T cells after activation with various stimuli. Time course studies demonstrated that peak surface expression of CD40L by CD4+ T cells after anti-CD3/CD28 or phorbol ester plus calcium ionophore (PMA/Io) occurred 8 h post activation. Peak CD40L mRNA levels were observed at 2 h post PMA/Io treatment and at 4 h post anti-CD3/CD28 treatment. Pretreatment with Δ9-THC significantly impaired the upregulation of CD40L induced by anti-CD3/CD28 at both the protein and mRNA level. By contrast, Δ9-THC did not affect PMA/Io-induced surface CD40L expression on CD4+ T cells. Additionally, Δ9-THC also attenuated anti-CD3/CD28-induced CD40L expression on CD4+ T cells derived from CB1−/−/CB2−/− mice. We investigated whether the mechanism by which Δ9-THC suppressed CD40L expression involved putative cannabinoid activation of the glucocorticoid receptor (GR). Although activation of GR resulted in suppression of CD40L induction by anti-CD3/CD28, no interaction between Δ9-THC and GR was observed by a glucocorticoid response element (GRE) luciferase reporter assay in HEK293T cells. Collectively, these results suggest that Δ9-THC targets proximal T cell receptor-associated signaling in a cannabinoid receptor- and glucocorticoid receptor-independent manner. These findings identify suppression of CD40L expression as a novel part of the mechanism by which Δ9-THC exerts anti-inflammatory activity.
Keywords: Δ9-THC, CD40L, Anti-CD3/CD28, PMA/Io, CB1, CB2, Inflammatory diseases
Introduction
Δ9-THC, the major psychoactive component of Cannabis sativa, possesses immunomodulatory properties [reviewed in (Croxford and Yamamura 2005)]. Previous studies by this laboratory have identified a number of T cell-mediated responses modulated by Δ9-THC including T cell-dependent humoral immune responses, T cell proliferation, production of cytokines, and expression of inducible co-stimulator (ICOS) molecules (Lu et al. 2009; Springs et al. 2008). Nuclear factor of activated T cells (NFAT), a transcription factor, controls many aspects of T cell regulation including activation, differentiation, thymocyte development, and self-tolerance [reviewed in (Macian 2005)]. Suppressive effects of Δ9-THC on T cell functions were mediated, at least in part, through impairment of NFAT signaling as evidenced by decreased NFAT reporter gene activity after treatment with Δ9-THC (Lu et al. 2009).
Two cannabinoid receptors, cannabinoid type 1 receptor (CB1) and cannabinoid type 2 receptor (CB2), have been identified and extensively characterized [reviewed in (Mackie 2006)]. Δ9-THC binds both receptors, but with greater affinity to CB1 [reviewed in (Pertwee 1999)]. CB1 and CB2 are members of the G protein-coupled receptor superfamily and couple to inhibitory (Gαi/o) heterotrimeric G proteins. Binding of ligands to these receptors inhibits adenylyl cyclase activity resulting in decreased level of cAMP [reviewed in (Demuth and Molleman 2006)]. Studies from our laboratory showed that cannabinoid-mediated suppression in cAMP signaling is one of many putative contributing mechanisms responsible for the suppressive effects on T cell function [reviewed in (Kaminski 1998)]. However, the precise role of CB1 and/or CB2 in cannabinoid-mediated immune modulation remains elusive. Both receptor-dependent and receptor-independent mechanisms are involved depending on cell type and response being measured. Specifically, CB1 and/or CB2 were found to be involved in Δ9-THC-mediated suppression of the T cell-dependent IgM response induced by sRBC in vivo or by CD40 in vitro (Springs et al. 2008). Conversely, CB1 and CB2 involvement were ruled out in Δ9-THC-mediated suppression of interleukin 2 and interferon-gamma production in mouse splenocytes (Newton et al. 2009; Kaplan et al. 2003; Springs et al. 2008). This suggested the existence of additional targets other than CB1/CB2 receptors [reviewed in (Brown 2007)].
The CD40-CD40L interaction is initially thought to play a predominant role in the T cell-dependent humoral immune response. However, emerging evidence suggests that CD40-CD40L interactions are also involved in allograft rejection, oxidative stress, and vascular disease [reviewed in (Elgueta et al. 2009; Law and Grewal 2009; Rizvi et al. 2008)]. The expression of CD40, a member of the tumor necrosis factor receptor superfamily, is found on a variety of cell types including endothelial, epithelial, and immune cells. In immune cells, CD40 is constitutively expressed on the surface of antigen-presenting cells, such as B cells, macrophages, and dendritic cells (Schonbeck and Libby 2001; van Kooten and Banchereau 2000). CD40L (CD154) is the endogenous ligand for CD40. Similar to its receptor, the expression of CD40L is also found in both immune and non-immune cells. However, the highest level of CD40L expression is on activated CD4+ T cells (Schonbeck et al. 2000). Aberrant CD40L expression on activated T cells was demonstrated in the pathogenesis of many autoimmune and inflammatory diseases. Mutations in the gene encoding CD40L were found in patients suffering from X-linked hyper-IgM syndrome resulting in antibody class switching deficiencies. In addition, T cells derived from patients with systemic lupus erythematosus, rheumatoid arthritis, psoriatic arthritis, or inflammatory bowel disease showed higher induction of surface CD40L compared to T cells derived from healthy volunteers after in vitro stimulation. Thus, modulating signaling initiated by CD40-CD40L interactions serves as a potential therapeutic target in uncontrolled immune reactions or chronic inflammatory diseases [reviewed in (Law and Grewal 2009)]. With the potential therapeutic use of cannabinoids in treating inflammatory disorders (Croxford and Yamamura 2005), the objective of the present study was to investigate the effect of Δ9-THC on the expression of CD40L on activated CD4+ T cells.
Materials and methods
Reagents
Δ9-THC was provided by the National Institute on Drug Abuse (Bethesda, MD) dissolved in 100 % ethanol (EtOH). Unless otherwise noted, all other chemicals were obtained from Sigma-Aldrich (St Louis, MO).
Animals
Female C57BL/6 mice (6 weeks of age) were purchased from Charles River Laboratories (Portage, MI). CB1/CB2 null mice (CB1−/−/CB2−/−) on C57BL/6 background were a generous gift from Dr. Andreas Zimmer (University of Bonn, Germany) and were bred at Michigan State University (MSU). Mice were maintained under specific pathogen-free conditions as previously described (Kaplan et al. 2003). All experimental mouse protocols were reviewed and approved by the Institutional Animal Care and Use Committee at MSU.
Lymphocyte activation
Spleens were aseptically isolated and made into single-cell suspensions in RPMI media (Gibco Invitrogen, Carlsbad, CA) supplemented with either 2 % bovine calf serum or 5 % Charcoal/Dextran Treated Fetal Bovine Serum (HyClone, Logan, UT), 50 μM 2-mercaptoethanol, and the antibiotics Penicillin (100 units/mL)/Streptomycin (100 μg/mL) (Gibco Invitrogen). Splenocytes, 5×106 cells/mL, were cultured in 48-well plates at 37 °C in 5 % CO2. Cells were stimulated with 40 nM PMA plus 0.5 μM ionomycin (PMA/Io) or with 1 μg/mL immobilized anti-mouse CD3 (clone 145-2C11; BD Pharmingen, San Diego, CA) plus 1 μg/mL soluble anti-mouse CD28 (clone 37.51; BD Pharmingen) antibodies (anti-CD3/CD28) in the presence or absence of Δ9-THC. Stock solutions of Δ9-THC, 0.1 μM cyclosporin A (CsA) and 0.5 μM dexamethasone (DEX) were used at a final EtOH concentration of 0.1 % and were added 30 min before T cell stimulation. 0.1 % EtOH was used as a vehicle control (VH).
Cell culture
HEK293T were purchased from Open Biosystems and maintained in DMEM media (Gibco Invitrogen) supplemented with 5 % Charcoal/Dextran Treated Fetal Bovine Serum (CD-FBS, Hyclone), 1X HT supplement (Gibco Invitrogen), and the antibiotics Penicillin (100 units/mL)/Streptomycin (100 μg/mL). Jurkat T cells (clone E6-1) were obtained from American Type Culture Collection (Manassas, VA) and maintained in RPMI media supplemented with 5 % CD-FBS, 1X non-essential amino acids (Gibco Invitrogen), 1X sodium pyruvate (Gibco Invitrogen), and the antibiotics Penicillin (100 units/mL)/Streptomycin (100 μg/mL). In all cases, cells were cultured at 37 °C in 5 % CO2.
Surface staining for flow cytometry
At the indicated time points, 1×106 cells were harvested and washed once with FACS buffer [1X Hank’s Balanced Salt Solution containing 1 % bovine serum albumin (Calbiochem, San Diego, CA) and 0.1 % sodium azide, pH 7.4–7.6]. Fcγ III/II receptors were blocked using purified anti-mouse CD16/CD32 (BD Biosciences, San Jose, CA) for 10 min at 4 °C. Cell surface expression of CD40L on mouse CD4+ T cells was assessed by simultaneously staining with allophycocyanin (APC)-conjugated anti-mouse CD154 (CD40L) antibodies (eBiosciences, San Diego, CA) and fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD4 antibodies (BD Biosciences) for 30 min at 4 °C in the dark. After washing with FACS buffer, cells were fixed with BD CytoFix™ Buffer (BD Biosciences) for 10 min at 4 °C in the dark. Following washing, cells were resuspended in FACS buffer, and analyzed on a FACSCanto II cell analyzer (BD Biosciences). Data were analyzed using FlowJo (TreeStar, Ashland, OR) and presented as percentage of CD4+CD40L+ within the CD4+ population.
Real time polymerase chain reaction (Real-time PCR)
Total RNA was isolated from activated mouse splenocytes using TRI Reagent. RNA was quantified using a Nanodrop 1000 (Thermo Scientific, Wilmington, DE). Total RNA was reverse-transcribed into cDNA using random primers with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). TaqMan® Gene Expression Assay primers for mouse CD40L (Mm00441911_m1) were purchased from Applied Biosystems. The relative steady-state levels of CD40L mRNA was determined using ABI PRISM® 7900HT Sequence Detection System (Applied Biosystems). The fold change value of relative steady-state CD40L mRNA levels were calculated using the delta-delta-Ct method as described previously (Livak and Schmittgen 2001) and normalized to the endogenous reference, 18s rRNA.
Plasmids
GRE-luciferase reporter gene plasmid (pGRE-luc) and the vector control plasmid (pTAL-luc) were purchased from Clontech (Mountain View, CA).
Transient transfection assays
HEK293T, 1×106 cells, were cultured in 6-well plates at 37 °C in 5 % CO2 overnight before transfection. Cells were incubated with transfection reagents [1 μg of plasmid and 10 μL of Lipofectamine 2000 (Invitrogen)] for 3 h. The transfected HEK293T cells were treated with either Δ9-THC or DEX alone and the combination of Δ9-THC and DEX. 24 h after treatment, luciferase activity was assayed using the Promega luciferase assay system according to the manufacturer’s protocol (Promega). Fluorescence was measured using the BioTek Synergy™ HT autoreader. Data were analyzed using KC4 software (Bio-Tek Instruments, Highland Park, VT) and presented as count per second (CPS) normalized to total protein. Protein determinations were performed using a Bicinchoninic Acid Assay.
Statistical analyses
GraphPad Prism 4.00 (Graphpad Software, San Diego, CA) was used for all statistical analysis. The percentage data from flow cytometry were transformed into log scale before performing statistical analysis. The delta-Ct values representing the steady-state mRNA level of CD40L and the normalized CPS data representing luciferace activity were used in statistical analysis. For comparisons among treatment groups, one-way ANOVA was used. Dunnett’s post-hoc test was used to test for significance between treatment groups and control. A value of p<0.05 was considered significant.
Results
Kinetics of CD40L expression by splenic T cells activated with different stimuli
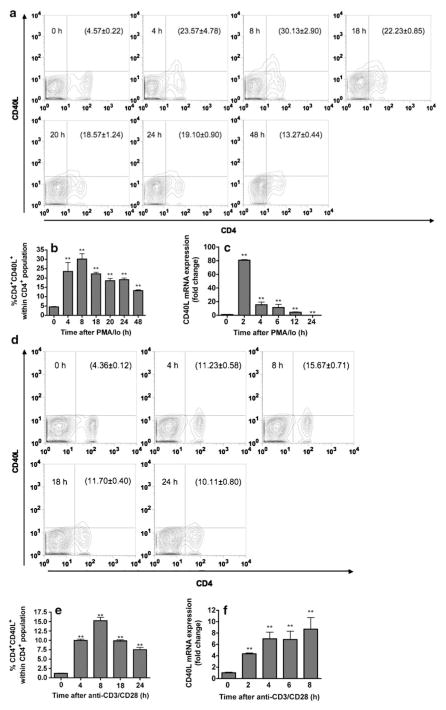
Expression of CD40L is readily induced on T cells upon activation both in vivo and in vitro (Roy et al. 1993; Fuleihan et al. 1994; Ford et al. 1999; Nusslein et al. 1996; Van den Eertwegh et al. 1993; Klaus et al. 1994). Here the peak time of CD40L expression in response to two different T cell activation stimuli, PMA/Io and anti-CD3/CD28, was defined at both the protein (cell surface expression) and mRNA level in splenic T cells. As shown in Fig. 1a and b, upregulation of surface CD40L by PMA/Io-activated CD4+ T cells was detectable as early as 4 h (23.04±4.75 %), maximal at 8 h (29.90±2.93 %), and was declining by 18 h (19.29±1.26 %). In concordance with surface expression, induction of CD40L mRNA levels by PMA/Io was rapid (as early as 2 h post activation), but transient, as evidenced by a decrease as early as 4 h after activation (Fig. 1c). We observed similar kinetic profiles of CD40L surface expression induced by anti-CD3/CD28 and PMA/Io. However, the proportion of CD4+CD40L+ cells induced by PMA/Io was greater than with anti-CD3/CD28. As shown in Fig. 1d and e, the upregulation of cell surface CD40L on activated CD4+ T cells by anti-CD3/CD28 was detectable at 4 h (9.97±0.34 %), maximal at 8 h (15.23±0.91 %), and decreased by 18 h (9.83±0.31 %) after activation. By contrast, the induction of CD40L mRNA by anti-CD3/CD28 had a different profile in which it peaked at 4 h post stimulation and then reached a plateau (Fig. 1f). Although splenocytes were used as a source of T cells, we found enrichment of T cells did not further increase the magnitude of anti-CD3/CD28-induced CD40L surface expression (data not shown).
Fig. 1.
Kinetics of CD40L expression in mouse splenocytes after stimulation with various T cell stimuli. Splenocytes (5×106 cells) were stimulated with either 40 nM PMA plus 0.5 μM ionomycin (a, b, and c) or immobilized anti-CD3 plus soluble anti-CD28 antibodies, 1 μg/ml each (d, e, and f). a and d Cell surface CD40L on activated CD4+ T cells was determined at indicated time points by flow cytometry. Numbers in parentheses represent the percentage of CD4+CD40L+ cell population ± SEM from triplicates. b and e Bar graphs represent data in a and d, respectively. c and f Steady-state expression of CD40L mRNA was determined at indicated time points by real-time PCR. The fold difference of CD40L mRNA molecules relative to unstimulated cells (cells at 0 h) was normalized using the endogenous reference, 18s rRNA. Results are the mean of triplicates per time point. The **=p≤0.01 compared to unstimulated cells. Results are representative of two separate experiments
Differential effects by Δ9-THC on CD40L upregulation in response to different T cell activation stimuli
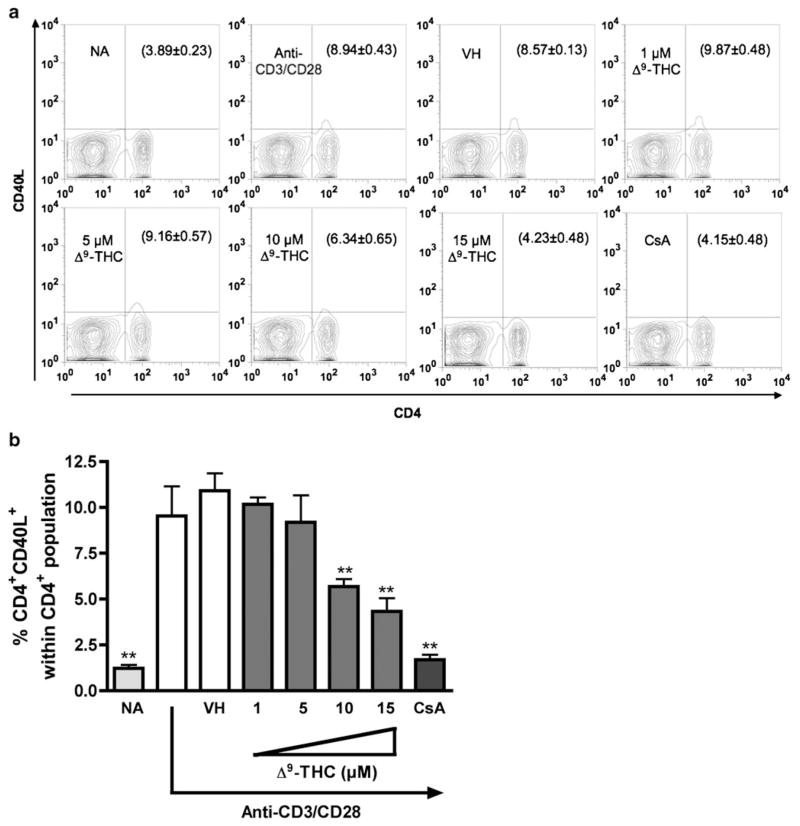
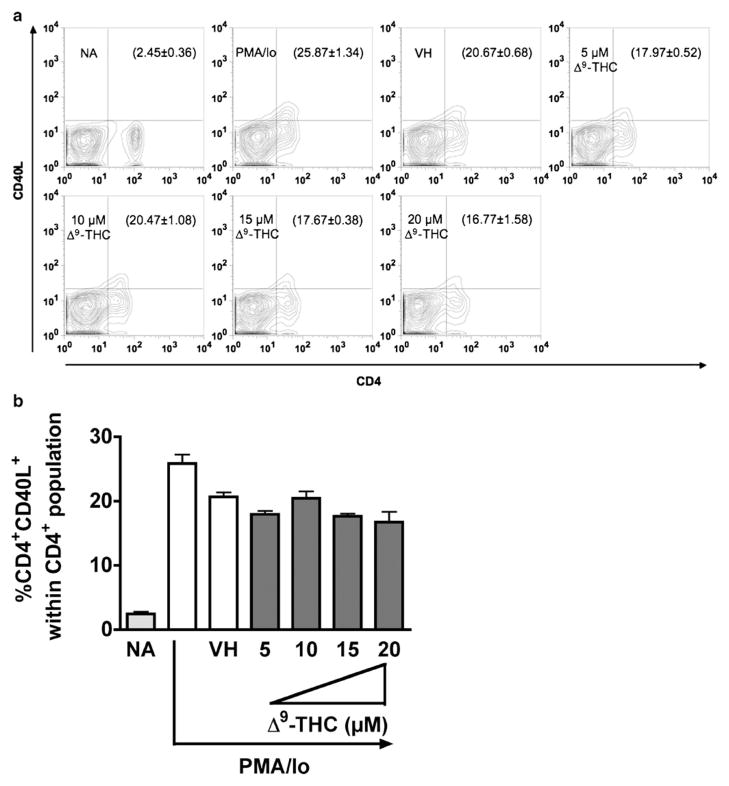
Based on the above kinetic studies, the effects of Δ9-THC on CD40L expression levels by CD4+ T cells activated with either anti-CD3/CD28 or PMA/Io was investigated at 8 h (i.e., the peak time of CD40L surface expression). CsA was included as a positive control for suppression of T cell activation. As shown in Fig. 2, Δ9-THC suppressed anti-CD3/CD28-induced CD40L surface expression by reducing the number of CD4+ T cells expressing CD40L, but not the expression level of CD40L on each CD4+ T cell (no differences in mean fluorescence intensity between treatment groups, data not shown). Significant suppression by Δ9-THC of CD4+CD40L+ double positive cells occurred in a concentration-dependent manner and was most pronounced at 10 and 15 μM Δ9-THC. Conversely, Δ9-THC treatment did not suppress PMA/Io-induced upregulation of CD40L surface expression on CD4+ T cells (Fig. 3).
Fig. 2.
Δ9-THC suppresses anti-CD3/CD28-induced surface CD40L expression on CD4+ T cells. Splenocytes (5×106 cells) were treated with various concentrations of Δ9-THC (1, 5, 10, and 15 μM) for 30 min and then stimulated with immobilized anti-CD3 plus soluble anti-CD28 antibodies (1 μg/ml each). a Cell surface CD40L expression on activated CD4+ was determined by flow cytometry 8 h after stimulation. Numbers in parentheses represent the percentage of CD4+CD40L+ cell population ± SEM from triplicates. b Bar graph is a representation of data in a. The * or ** indicates significant effect compared to vehicle control (VH=0.1 % EtOH) at p≤0.05 or p≤0.01, respectively. 0.1 μM CsA was used as the positive control. Results are representative of two separate experiments with three replicates per treatment group
Fig. 3.
Δ9-THC does not suppress PMA/Io-induced surface CD40L expression on CD4+ T cells. Splenocytes (5×106 cells) were treated with various concentrations of Δ9-THC (5, 10, 15, and 20 μM) for 30 min and then stimulated with 40 nM PMA plus 0.5 μM ionomycin for 8 h. a Cell surface CD40L expression on activated CD4+ was determined by flow cytometry. Number in parentheses represents the percentage of CD4+CD40L+ cell population±SEM. b Bar graph is a representation of data in a. The * or ** indicates significant effect compared to vehicle control (VH=0.1 % EtOH) at p≤0.05 or p≤0.01, respectively. Results are representative of two separate experiments with three replicates per treatment group
Δ9-THC decreases steady-state mRNA levels of CD40L induced by anti-CD3/CD28
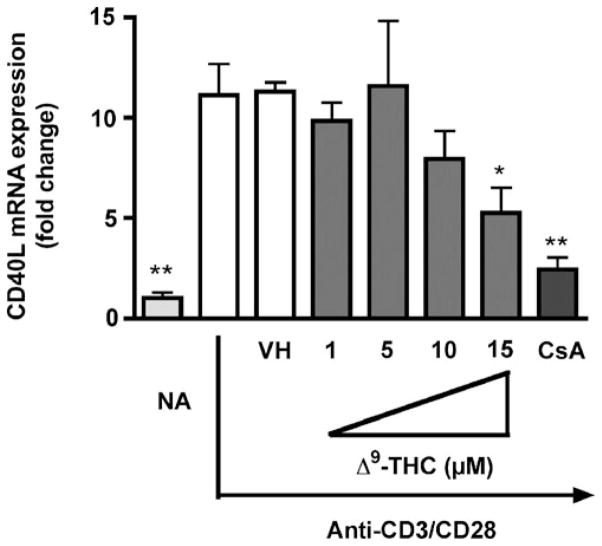
The expression of surface CD40L on activated T cells is tightly controlled and occurs at the transcriptional, post-transcriptional, and/or post-translational level. However, it appears to be primarily controlled at the level of transcription and post-transcription [reviewed in (Cron 2003; van Kooten and Banchereau 2000; Vavassori and Covey 2009)]. Therefore the effect of Δ9-THC was investigated on the steady-state mRNA levels of CD40L induced by anti-CD3/CD28. Splenocytes were treated with increasing concentrations of Δ9-THC followed by anti-CD3/CD28 activation for 4 h (i.e., the peak time of CD40L mRNA). Δ9-THC suppressed the induction of CD40L mRNA level by anti-CD3/CD28 in a concentration-dependent manner (Fig. 4).
Fig. 4.
Δ9-THC suppresses anti-CD3/CD28-induced CD40L mRNA expression in mouse splenocytes. Splenocytes (5×106 cells) were treated with various concentrations of Δ9-THC (1, 5, 10, and 15 μM) for 30 min and then stimulated with immobilized anti-CD3 plus soluble anti-CD28 antibodies (1 μg/ml ) each. Steady-state expression of CD40L mRNA was determined by real-time PCR at 4 h after stimulation. The fold difference of CD40L mRNA relative to unstimulated cells (naïve, NA) was normalized using the endogenous reference, 18s rRNA and results are the average of triplicates at various concentrations of Δ9-THC. The * or ** indicates significant effect compared to vehicle control (VH=0.1 % EtOH) at p≤0.05 or p≤0.01, respectively. 0.1 μM CsA was used as the positive control. Results are representative of two separate experiments with three replicates per treatment group
CB1 and/or CB2 are not involved in suppression by Δ9-THC of anti-CD3/CD28-induced CD40L expression on mouse splenic CD4+ T cells
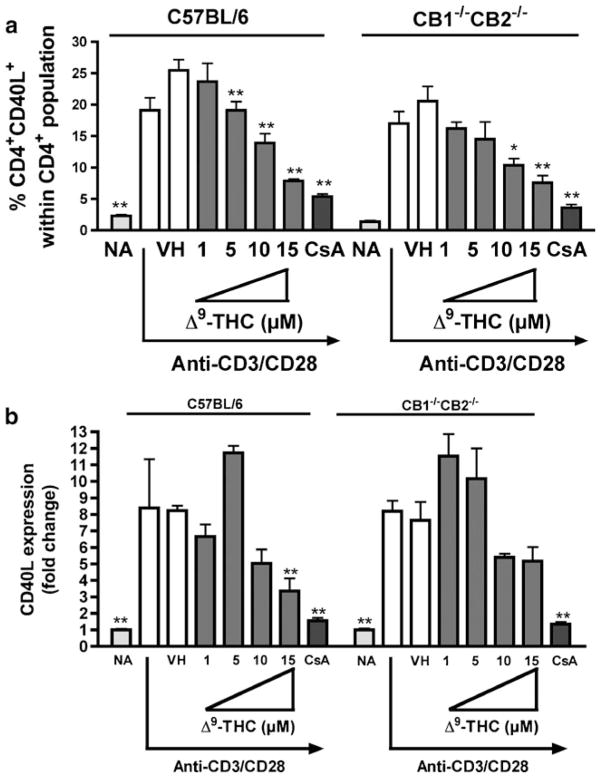
CB1 and/or CB2-dependent and -independent mechanisms have been suggested to account for cannabinoid-mediated effects on immune function (Kaplan et al. 2003; Springs et al. 2008). To investigate the involvement of CB1 and/or CB2 on Δ9-THC-mediated suppression of anti-CD3/CD28-induced CD40L expression, comparative studies were performed using splenocytes from C57BL/6 wild type and CB1−/−CB2−/− mice. As shown in Fig. 6, the absence of CB1 and CB2 did not affect Δ9-THC-mediated suppression of either anti-CD3/CD28-induced surface CD40L expression in CD4+ T cells (Fig. 5a) or CD40L mRNA level (Fig. 5b). Although we observed an increase of CD40L mRNA expression in CD4+ T cells derived from C57BL/6 at 5 μM Δ9-THC (Fig. 5b), it was not consistent across the experiments.
Fig. 6.
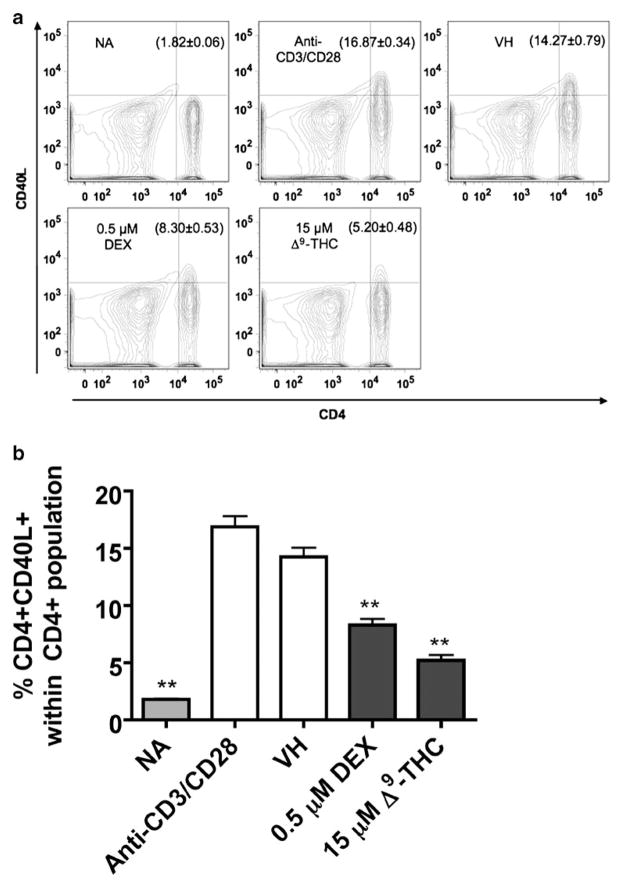
DEX suppresses anti-CD3/CD28-induced surface CD40L expression on CD4+ T cells. Splenocytes (5×106 cells) from wildtype or CB1−/−CB2−/− mice were treated with 0.5 μM DEX or 15 μM Δ9-THC for 30 min and then stimulated with immobilized anti-CD3 plus soluble anti-CD28 antibodies (1 μg/ml each). a Cell surface CD40L expression on activated CD4+ was determined by flow cytometry at 8 h after stimulation. Number in parentheses represents the percentage of CD4+CD40L+ cell population ± SEM. b Bar graph is a representation of data in a. The ** indicates significant effect compared to vehicle control (VH=0.1 % EtOH), at p≤0.01. Results are representative of two separate experiments with three replicates per treatment group
Fig. 5.
Comparison of the effect of Δ9-THC on anti-CD3/CD28-induced CD40L expression in splenic T cells derived from wildtype or CB1−/−CB2−/− mice. Splenocytes (5×106 cells) from wildtype or CB1−/−CB2−/− mice were treated with various concentrations of Δ9-THC (1, 5, 10, and 15 μM) for 30 min and then stimulated with immobilized anti-CD3 plus soluble anti-CD28 antibodies (1 μg/ml each). a Cell surface CD40L expression on activated CD4+ was determined by flow cytometry at 8 h after stimulation. The bar graph represents the percentage of CD4+CD40L+ cell population ± SEM. b Steady-state expression of CD40L mRNA was determined by real-time PCR at 4 h after stimulation. The fold difference of CD40L mRNA molecules relative to unstimulated cells (naïve, NA) was normalized using the endogenous reference, 18s rRNA and results are the average of triplicates at various concentrations of Δ9-THC. The * or ** indicates significant effect compared to vehicle control (VH=0.1 % EtOH), at p≤0.05 or p≤0.01, respectively. 0.1 μM CsA was used as the positive control. Results are representative of two separate experiments with three replicates per treatment group
The effects of Δ9-THC are not mediated via GR
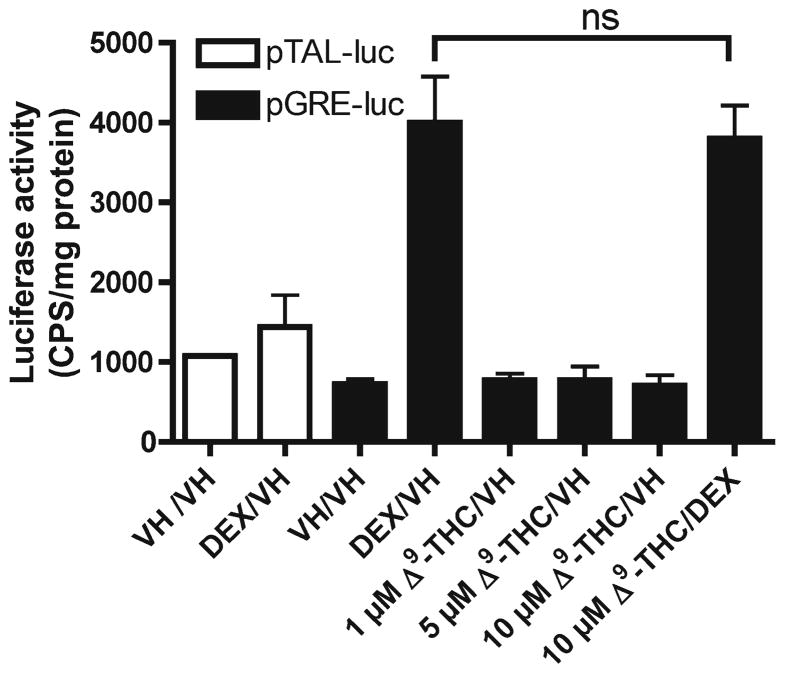
Since we have demonstrated that Δ9-THC-mediated suppression of CD40L protein and mRNA expression was not mediated through CB1 or CB2, we attempted to identify an alternative receptor. One possibility is the GR since it was demonstrated that Δ9-THC specifically bound rat hippocampal GR in vivo and in vitro (Eldridge and Landfield 1990) and activation of GR is well known to be immune suppressive [reviewed in (Baschant and Tuckermann 2010)]. First, we demonstrated that activation of GR using DEX, a GR agonist, suppressed CD40L expression-induced by anti-CD3/CD28 (Fig. 6). We next investigated the interaction between Δ9-THC and GR using a GRE-luc reporter assay in HEK293T cells. However, treatment with Δ9-THC did not affect basal or DEX-induced luciferase activity (Fig. 7). In addition, Δ9-THC did not affect the expression of glucocorticoid-induced leucine zipper or IkB mRNA, which are GRE-dependent genes, in both HEK293T and Jurkat T cells (data not shown).
Fig. 7.
GRE luciferase reporter activity in HEK293T cells treated with Δ9-THC and/or DEX. HEK293T cells (5×106 cells) were preseeded in a 6 well plate in growth medium overnight. The cells were then transiently transfected with pGRE-luc or pTAL-luc (vector control plasmid). After transfection, cells were treated with 0.1 % EtOH (VH), 0.5 μM DEX, different concentration of Δ9-THC (1, 5, and 10 μM), or the combination of 0.5 μM DEX plus 10 μM Δ9-THC. 24 h after transfection, luciferase activity was quantified in CPS and normalized to total protein (μg). The results are the mean±SEM. The “ns” indicates no significant difference. Results are representative of two separate experiments with three replicates per treatment group
Discussion
The strength and duration of CD40 signaling is crucial in initiating inflammatory responses and is controlled by the presence of its cognate ligand, CD40L, which is mainly expressed on the surface of activated CD4+ T cells. Δ9-THC impairs multiple aspects of T cell action, including activation, accessory, and effector functions (Lu et al. 2009; Springs et al. 2008). Toward this end, this study investigated the influence of Δ9-THC on the upregulation of CD40L on activated CD4+ T cells. Using mouse splenic T cells, we observed that the effect of Δ9-THC on CD40L induction depends on the mode of T cell activation, and its suppressive effect occurs, at least in part, at the transcriptional level, independently of CB1 and CB2. Additionally, we excluded the possibility that GR might be a cellular target of Δ9-THC.
In concordance with prior reports, similar CD40L expression kinetics were observed with CD4+ T cells activated by either PMA/Io or anti-CD3/CD28 [reviewed in (Cron 2003)]. It is noteworthy that while we observed a similar kinetic profile, the magnitude of surface CD40L expression induced by both stimuli under our experimental condition was modest compared to published reports and could be due to several factors including differences in culture conditions, mouse strain, and species (human versus mouse). Another possibility that might contribute to the modest CD40L induction is the internalization of CD40L after binding to its receptor, CD40, on B cells (Yellin et al. 1994). Although it seems possible since we utilized splenocytes, which are heterogeneous and contain B cells, we excluded this possibility, as T cell enrichment did not increase the magnitude of CD40L expression. We also found that the kinetics of CD40L mRNA upregulation induced by these two stimuli were quite different. PMA/Io induced CD40L mRNA levels rapidly, but transiently, while steady-state CD40L mRNA levels induced by anti-CD3/CD28 were slow, but sustained. These differences might be explained by the observation that the CD28 costimulatory signal promoted mRNA stability for many T cell-derived cytokines (Lindstein et al. 1989). However, CD40L mRNA is still very unstable, with a t1/2 of approximately 23 min in mouse (Vavassori et al. 2009).
We show here that Δ9-THC distinctly modulated the upregulation of surface CD40L induced by different T cell activation stimuli. Δ9-THC suppressed the upregulation of both surface CD40L expression and CD40L mRNA levels induced by anti-CD3/CD28 in a concentration-dependent manner. Δ9-THC did not affect the upregulation of surface CD40L induced by PMA/Io. This is in contrast to our previous reports showing thatΔ9-THC suppressed PMA/Io-induced cytokines (such as interleukin 2 and interferon gamma), and co-stimulatory molecules like ICOS (Lu et al. 2009; Springs et al. 2008). Unlike other proteins that are induced by T cell activation, the expression of CD40L on activated T cells largely depends on an increase in intracellular calcium through the activation of calcineurin and Ca2+/Calmodulin kinase Type IV (Lobo et al. 1999; Nusslein et al. 1996). This correlates well with numerous reports showing that NFAT, the activation of which requires calcineurin, is a critical transcription factor in regulating CD40L expression (Crist et al. 2008; Lindgren et al. 2001; Lobo et al. 2000). The importance of NFAT is also evidenced by a marked decrease in CD40L expression in the presence of CsA, an immunosuppressive drug that blocks NFAT activation and nuclear translocation (Fuleihan et al. 1994). As stated previously, our laboratory has demonstrated marked suppression by Δ9-THC of NFAT activation (Lu et al. 2009) and leads us to speculate that impaired NFAT likely also contributes to suppression of CD40L by Δ9-THC. It is also noteworthy that the downregulation of surface CD4, a co-receptor on helper T cells, after PMA/Io treatment as observed here is a common phenomenon (Kaldjian et al. 1988; Martinez-Valdez et al. 2002; Pelchen-Matthews et al. 1993; Weyand et al. 1987), but did not affect the upregulation of CD40L.
Based on the fact that PMA/Io activates T cells by bypassing the proximal TCR signaling, it is possible that Δ9-THC might target one or more signaling molecules between the T cell receptor and the activation of PKC-θ and/or induction of intracellular calcium. This is in accordance with a study demonstrating that Δ9-THC suppressed the activation of proximal TCR signaling components, partly due to the stabilization of Lck, the kinase responsible for initiating TCR signaling, when present in its inactive form (Borner et al. 2009). Borner and coworkers also demonstrated the involvement of CB1 and CB2 in Δ9-THC-mediated impairment of proximal TCR signaling, as evidenced by reversion of the suppressive effect of Δ9-THC, in the presence of CB1 and CB2 antagonists. This is in contrast to the results reported here, in which the suppressive effect of Δ9-THC on anti-CD3/CD28-induced CD40L expression occurred in the presence and absence of CB1 and CB2. It is noteworthy that Borner and coworkers studied the involvement of CB1 and CB2 by using IL-4 stimulated Jurkat or primary human T cells to induce CB1 expression (prestimulated cells) together with CB1 and CB2 selective antagonists (AM281 and AM630, respectively). In contrast, in our experiments, splenocytes from CB1/CB2 null mice (CB1−/−CB2−/− mice) were used eliminating the need for cannabinoid receptor antagonists, which are known to have inverse agonist activity as well as off-target effects, particularly in case of AM281 (Pertwee 2010). These differences in experimental conditions might account for the discrepancy between our findings and that of Borner and coworkers. At this time, we cannot exclude the possibility that cannabinoids target various orphan G protein-coupled receptors (e.g. GPR55 and GPR119), nuclear hormone receptors (e.g. the peroxisome proliferator-activated receptors α and γ), or ion channels (e.g. the transient receptor potential vanilloid type 1 and 2 receptors, and the transient receptor potential cation channel, subfamily A, member 1) [reviewed in (Brown 2007; Pertwee 2010)]. However, we have ruled out the possible involvement of GR in suppression of CD40L by Δ9-THC. Finally, although less likely, the CB1/CB2-independent mechanism may also be explained by the fact that Δ9-THC, which is very lipophilic, preferentially aligns its hydrophobic portion parallel with the membrane phospholipid chain [reviewed in (Makriyannis et al. 2005)]. This perpendicular orientation results in alteration of plasma membrane’s thermodynamic properties (Makriyannis et al. 1990) and may disrupt the migration and interaction of TCR/co-receptors in the plasma membrane during activation. Thus, it is possible with that Δ9-THC may exert its suppressive effect by interfering with the formation of TCR microcluster, which has been shown to be important in T cell activation [reviewed in (Yukosuka and Saito 2010)].
In conclusion, the present study shows the suppressive effects of Δ9-THC on CD40L upregulation on mouse CD4+ T cells are dependent on the mode of T cell activation. We also show that suppression of anti-CD3/CD28-induced CD40L expression in mouse splenic CD4+ T cells by Δ9-THC likely occurs at the transcriptional level and is mechanistically independent of CB1, CB2 and GR. Our findings provide further insights toward understanding the effect of Δ9-THC on CD40L regulation by activated T cells and suggests a role for immunomodulatory cannabinoids in the treatment of certain inflammatory-diseases.
Acknowledgments
This work was supported in part by National Institute of Health grant RO1 DA07908 to N.E.K., and Royal Thai Government Scholarship to T.N. We thank Dr. Andreas Zimmer (University of Bonn) for providing the CB1−/−CB2−/− mice. We also thank Dr. Schuyler Pike for providing editorial assistance and critical comments, and Mrs. Kimberly Hambleton for assistance in submitting the manuscript.
Footnotes
Conflicts of interest The authors declare that they have no conflicts of interest.
References
- Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 2010;120(2–3):69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- Borner C, Smida M, Hollt V, Schraven B, Kraus J. Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J Biol Chem. 2009;284(51):35450–35460. doi: 10.1074/jbc.M109.006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. 2007;152 (5):567–575. doi: 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist SA, Sprague DL, Ratliff TL. Nuclear factor of activated T cells (NFAT) mediates CD154 expression in megakaryocytes. Blood. 2008;111(7):3553–3561. doi: 10.1182/blood-2007-05-088161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cron RQ. CD154 transcriptional regulation in primary human CD4 T cells. Immunol Res. 2003;27(2–3):185–202. doi: 10.1385/IR:27:2-3:185. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005;166(1–2):3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78 (6):549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Eldridge JC, Landfield PW. Cannabinoid interactions with glucocorticoid receptors in rat hippocampus. Brain Res. 1990;534 (1–2):135–141. doi: 10.1016/0006-8993(90)90123-s. [DOI] [PubMed] [Google Scholar]
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford GS, Barnhart B, Shone S, Covey LR. Regulation of CD154 (CD40 ligand) mRNA stability during T cell activation. J Immunol. 1999;162(7):4037–4044. [PubMed] [Google Scholar]
- Fuleihan R, Ramesh N, Horner A, Ahern D, Belshaw PJ, Alberg DG, Stamenkovic I, Harmon W, Geha RS. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J Clin Invest. 1994;93(3):1315–1320. doi: 10.1172/JCI117089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldjian E, McCarthy SA, Sharrow SO, Littman DR, Klausner RD, Singer A. Nonequivalent effects of PKC activation by PMA on murine CD4 and CD8 cell-surface expression. FASEB J. 1988;2(12):2801–2806. doi: 10.1096/fasebj.2.12.3261700. [DOI] [PubMed] [Google Scholar]
- Kaminski NE. Inhibition of the cAMP signaling cascade via cannabinoid receptors: a putative mechanism of immune modulation by cannabinoid compounds. Toxicol Lett. 1998:102–103. 59–63. doi: 10.1016/s0378-4274(98)00284-7. [DOI] [PubMed] [Google Scholar]
- Kaplan BL, Rockwell CE, Kaminski NE. Evidence for cannabinoid receptor-dependent and -independent mechanisms of action in leukocytes. J Pharmacol Exp Ther. 2003;306(3):1077–1085. doi: 10.1124/jpet.103.051961. [DOI] [PubMed] [Google Scholar]
- Klaus SJ, Pinchuk LM, Ochs HD, Law CL, Fanslow WC, Armitage RJ, Clark EA. Costimulation through CD28 enhances T cell-dependent B cell activation via CD40-CD40L interaction. J Immunol. 1994;152(12):5643–5652. [PubMed] [Google Scholar]
- Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Adv Exp Med Biol. 2009;647:8–36. doi: 10.1007/978-0-387-89520-8_2. [DOI] [PubMed] [Google Scholar]
- Lindgren H, Axcrona K, Leanderson T. Regulation of transcriptional activity of the murine CD40 ligand promoter in response to signals through TCR and the costimulatory molecules CD28 and CD2. J Immunol. 2001;166(7):4578–4585. doi: 10.4049/jimmunol.166.7.4578. [DOI] [PubMed] [Google Scholar]
- Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lobo FM, Zanjani R, Ho N, Chatila TA, Fuleihan RL. Calcium-dependent activation of TNF family gene expression by Ca2+/calmodulin kinase type IV/Gr and calcineurin. J Immunol. 1999;162 (4):2057–2063. [PubMed] [Google Scholar]
- Lobo FM, Xu S, Lee C, Fuleihan RL. Transcriptional activity of the distal CD40 ligand promoter. Biochem Biophys Res Commun. 2000;279(1):245–250. doi: 10.1006/bbrc.2000.3914. [DOI] [PubMed] [Google Scholar]
- Lu H, Kaplan BL, Ngaotepprutaram T, Kaminski NE. Suppression of T cell costimulator ICOS by Delta9-tetrahydrocannabinol. J Leukoc Biol. 2009;85(2):322–329. doi: 10.1189/jlb.0608390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Makriyannis A, Yang DP, Griffin RG, Das Gupta SK. The perturbation of model membranes by (−)-delta 9-tetrahydrocannabinol. Studies using solid-state 2H- and 13C-NMR. Biochim Biophys Acta. 1990;1028(1):31–42. doi: 10.1016/0005-2736(90)90262-m. [DOI] [PubMed] [Google Scholar]
- Makriyannis A, Tian X, Guo J. How lipophilic cannabinergic ligands reach their receptor sites. Prostaglandins Other Lipid Mediat. 2005;77(1–4):210–218. doi: 10.1016/j.prostaglandins.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Martinez-Valdez H, Madrid-Marina V, Cohen A. Phorbol esters and cAMP differentially regulate the expression of CD4 and CD8 in human thymocytes. BMC Immunol. 2002;3:1. doi: 10.1186/1471-2172-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton CA, Chou PJ, Perkins I, Klein TW. CB(1) and CB(2) cannabinoid receptors mediate different aspects of delta-9-tetrahydrocannabinol (THC)-induced T helper cell shift following immune activation by Legionella pneumophila infection. J Neuro-immune Pharmacol. 2009;4(1):92–102. doi: 10.1007/s11481-008-9126-2. [DOI] [PubMed] [Google Scholar]
- Nusslein HG, Frosch KH, Woith W, Lane P, Kalden JR, Manger B. Increase of intracellular calcium is the essential signal for the expression of CD40 ligand. Eur J Immunol. 1996;26(4):846–850. doi: 10.1002/eji.1830260418. [DOI] [PubMed] [Google Scholar]
- Pelchen-Matthews A, Parsons IJ, Marsh M. Phorbol ester-induced downregulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J Exp Med. 1993;178(4):1209–1222. doi: 10.1084/jem.178.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6(8):635–664. [PubMed] [Google Scholar]
- Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem. 2010;17(14):1360–1381. doi: 10.2174/092986710790980050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi M, Pathak D, Freedman JE, Chakrabarti S. CD40-CD40 ligand interactions in oxidative stress, inflammation and vascular disease. Trends Mol Med. 2008;14(12):530–538. doi: 10.1016/j.molmed.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151(5):2497–2510. [PubMed] [Google Scholar]
- Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58(1):4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbeck U, Mach F, Libby P. CD154 (CD40 ligand) Int J Biochem Cell Biol. 2000;32(7):687–693. doi: 10.1016/s1357-2725(00)00016-9. [DOI] [PubMed] [Google Scholar]
- Springs AE, Karmaus PW, Crawford RB, Kaplan BL, Kaminski NE. Effects of targeted deletion of cannabinoid receptors CB1 and CB2 on immune competence and sensitivity to immune modulation by Delta9-tetrahydrocannabinol. J Leukoc Biol. 2008;84(6):1574–1584. doi: 10.1189/jlb.0508282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eertwegh AJ, Noelle RJ, Roy M, Shepherd DM, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T-B cell interactions. J Exp Med. 1993;178(5):1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- Vavassori S, Covey LR. Post-transcriptional regulation in lymphocytes: the case of CD154. RNA Biol. 2009;6(3):259–265. doi: 10.4161/rna.6.3.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavassori S, Shi Y, Chen CC, Ron Y, Covey LR. In vivo post-transcriptional regulation of CD154 in mouse CD4+ T cells. Eur J Immunol. 2009;39(8):2224–2232. doi: 10.1002/eji.200839163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand CM, Goronzy J, Fathman CG. Modulation of CD4 by antigenic activation. J Immunol. 1987;138(5):1351–1354. [PubMed] [Google Scholar]
- Yellin MJ, Sippel K, Inghirami G, Covey LR, Lee JJ, Sinning J, Clark EA, Chess L, Lederman S. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J Immunol. 1994;152(2):598–608. [PubMed] [Google Scholar]
- Yokosuka T, Saito T. The immunological synapse, TCR micro-clusters, and T cell activation. Curr Top Microbiol Immunol. 2010;340:81–107. doi: 10.1007/978-3-642-03858-7_5. [DOI] [PubMed] [Google Scholar]