Abstract
To address the role of D1 receptors in the medial prefrontal cortex, we combined pharmacological and genetic manipulations to examine long-term synaptic potentiation (LTP)/long-term synaptic depression (LTD) in brain slices of rats and mice. We found that the D1 antagonist SCH23390 selectively blocked the maintenance but not the induction of LTP in the prefrontal cortex. Conversely, activation of D1 receptors facilitated the maintenance of LTP, and this effect is impaired in heterozygous D1 receptor knockout mice. Low-frequency stimulation induced a transient depression in the medial prefrontal cortex. This depression could be transformed into LTD by coapplication of dopamine. Coapplication of dopamine, however, shows no facilitating effect on LTD in heterozygous D1 receptor knockout mice. These results provide pharmacological and genetic evidence for a role of D1 receptors in the bidirectional modulation of synaptic plasticity in the medial prefrontal cortex. The absence of this modulation in heterozygous knockout mice shows that a dysregulation of dopamine receptor expression levels can have dramatic effects on synaptic plasticity in the prefrontal cortex.
The prefrontal cortex is thought to be the highest association area in the mammalian cortex and is required for proper executive control. In primates the dorsolateral prefrontal cortex, alone, has been implicated in several different, partly overlapping, cognitive processes, including inhibitory control, working memory, selective attention, attentional-set shifting, rule learning, and strategy switching. In rodents, where the architecture of the cortex is simpler than that of primates, the medial prefrontal cortex (mPFC), the cortex that encompasses the infralimbic and prelimbic areas, is considered to be homologous to the primate dorsolateral prefrontal cortex (1, 2).
Consistent with this homology, a variety of studies have shown that dopamine modulates neuronal activity in the prefrontal cortex and affects working memory, attentional performance, attentional-set shifting, and strategy switching (3-9). Some functions of mPFC, such as strategy shifting or rule learning, may be affected by dopaminergic modulation of long-term synaptic plasticity. Dopamine indeed affects long-term plasticity in the prefrontal cortex. It facilitates long-term synaptic depression (LTD) via D1 and D2 receptors (10, 11), and D1 activation has been shown to be required for N-methyl-d-aspartate (NMDA) receptor-dependent long-term synaptic potentiation (LTP) at hippocampal-prefrontal cortex synapses (12).
To further our understanding of how dopamine modulates long-term synaptic plasticity in opposite directions, and to develop a model for exploring the underlying molecular signaling pathways, we examined LTP and LTD in slices of the mPFC from rats, wild-type mice, and heterozygous dopamine D1 receptor knockout mice. Heterozygous D1 receptor knockout mice show a 50% reduction in receptor number. They allow us to address specifically the function of the D1 receptor as opposed to both D1 and D5 receptors, which cannot be distinguished pharmacologically. We analyzed only heterozygous knockout mice because complete loss of the D1 receptor has developmental effects (13) and heterozygous loss allows us to address the issue of chronic D1 receptor dysregulation.
We find that in heterozygous D1 receptor knockout mice the dopaminergic modulation of both LTP and LTD is disrupted, providing genetic evidence for a D1-mediated bidirectional modulation of synaptic plasticity. Moreover, these findings emphasize the importance of tightly regulated receptor expression levels.
Materials and Methods
Animals. The initial pharmacological LTP studies were conducted in rats. Sprague-Dawley rats (4-5 weeks old) were purchased from Hilltop Lab Animals, Scottdale, PA. To investigate the effects of D1 receptor down-regulation, genetically modified mice were used. The D1 receptor knockout mice (13) were purchased from The Jackson Laboratory on a C57BL/6 background. D1 heterozygous mice were bred to D1 heterozygous mice to generate heterozygote and wild-type littermate mice (4-8 weeks old).
Recordings. Coronal slices (400-500 μm) including the prefrontal area (1.5-2.5 mm anterior to bregma) were cut and transferred to an interface chamber. The slices were perfused constantly with artificial cerebrospinal fluid (ACSF) at a rate of 2 ml/min and bubbled with 95% O2/5% CO2. The composition of ACSF was as follows: 124 mM NaCl, 1.3 mM MgSO4, 4 mM KCl, 1.0 mM Na2HPO4, 2.0 mM CaCl2, 26 mM NaHCO3, and 10 mM d-glucose. In mouse slice experiments, 10 μM picrotoxin was added to reduce the γ-aminobutyric acid (GABA)ergic interneuronal inhibition. Experiments were started at least 2.5 hr after the slice dissection, and the temperature of the slices was maintained at 28°C. Extracellular recording was obtained by a glass electrode filled with 2 M NaCl (3-5 MΩ) placed on layer V of the mPFC, where the basal dendrites and cell body of pyramidal neuron are located (Fig. 1A1). Stimuli (10-40 V) were delivered by a concentric bipolar electrode with a 25-μm diameter (FHC, Bowdoinham, ME), placed on layer II-III of the prefrontal cortex, where the input fibers are located (Fig. 1 A1). The testing stimuli for the basal synaptic response was 0.017 Hz (0.05 ms pulse duration). To induce LTP, 1-5 trains of 300-Hz (0.5-s, 0.05-ms pulse duration) tetanus in 3-min intervals were used in prefrontal cortex slices of rats and 10 trains of 300-Hz tetanus were used in mPFC of mice. To induce LTD, 3-Hz (15-min) stimulation was applied in the presence of dopamine (200 μM).
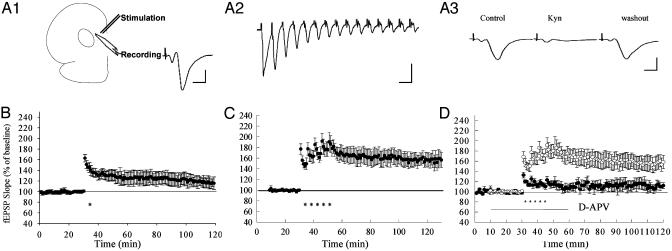
Fig. 1.
LTP in the mPFC of rat. (A1) Schematic illustration of the prefrontal cortex slice preparation and the placement of electrodes. The stimulation electrode was placed on layer II, and the recording electrode was placed on layer V. A sample field excitatory postsynaptic potential (fEPSP) from a rat slice is shown in the Inset. Calibration: 10 ms, 1 mV. (A2) The fEPSPs recorded during 50-Hz stimulation. Calibration: 20 ms, 1 mV. (A3) fEPSP in mPFC is blocked reversibly by kynurenic acid (Kyn, 10 mM). Left, control; center, during kynurenic acid; right, 90 min after washout of drug. Calibration: 5 ms, 1 mV. (B) LTP induced by a single train of tetanus (300 Hz, 0.5 s). (C) LTP induced by five trains of tetanus (300 Hz, 0.5 s) in a 3-min interval. (D) LTP in mPFC is dependent on NMDA receptor. ○, Control; •, in the presence of d-2-amino-5-phosphonovaleric acid (D-APV; 50 μM).
Results
Early- and Late-Phase LTP in the Prefrontal Cortex of Rats. Orthodromic stimuli applied to the layer II of rat prefrontal cortex slices elicited a typical field excitatory postsynaptic potential (fEPSP) in layer V (Fig. 1 A1), similar to that of the Schaffer collateral pathway of hippocampus. This fEPSP is blocked reversibly by the glutamate antagonist kynurenic acid (Fig. 1 A3), consistent with glutamate being the transmitter at this synapse. Moreover, this EPSP could follow high-frequency stimulation (50-Hz tetanization) reliably, without failure, indicating that it presumably reflects a monosynaptic response (Fig. 1 A2). The clear and stable fEPSP recorded in our experiments provides a reliable basis for the long-lasting analysis of synaptic plasticity for periods of up to 3 hr.
We first tested the early and late phases of LTP in slices of rat prefrontal cortex. It has been shown that high-frequency stimulation applied to layer II can elicit a synaptic potentiation in layer V. In in vivo experiments with rats, 10 trains of 250-Hz tetanus have been used (12), whereas in in vitro slice preparations 50- to 300-Hz tetanus or theta burst stimulation has been used (15-17). We found that in vitro a single train of tetanus (300 Hz, 0.5 S) induced only a transient LTP, that is 130 ± 9% of control at 20 min and decayed to 112 ± 10% in about 90 min (Fig. 1B, n = 5). In contrast, five repeated trains of such tetanization induced a stable LTP (156 ± 13% at 90 min, n = 5) that lasted more than 3 hr (Fig. 1C).
As previously reported (18), we found that the LTP in the mPFC depends on NMDA receptor function. In the presence of D-APV, LTP was blocked at initiation (Fig. 1D). Ninety minutes after the first tetanus, the potentiation is 112 ± 6%, which is significantly different from 158 ± 12% (n = 6) in the untreated slices (P < 0.01, Student t test).
D1, but Not D2, Receptors Are Involved in the Maintenance of LTP in the Rat mPFC. We next asked, how is LTP modulated by the dopaminergic input that reaches the prefrontal cortex? Toward that goal we tested the effect of the D1/D5 receptor antagonist SCH23390 on LTP induced by a single or repeated tetanus. We found that SCH23390 had no effect on LTP induced by a single tetanus (Fig. 2A) but it significantly reduced the maintenance phase of late-phase LTP induced by five trains (Fig. 2B). Although LTP could be induced in the presence of the antagonist it was decreased 30 min after the first tetanic stimulation. At 100 min, LTP was 121 ± 6% (n = 7) in SCH23390-treated slices and 162 ± 5% in control slices (n = 7, P < 0.01). Considered together, these results suggest that the activation of D1 receptor is mainly involved in the late maintenance of LTP, which is consistent with the effects of D1 receptor inhibition of Schaffer-collateral LTP in the hippocampal slices (19, 20). The D2-type receptor antagonist sulpiride (50 μM) had no affect on LTP (Fig. 2C).
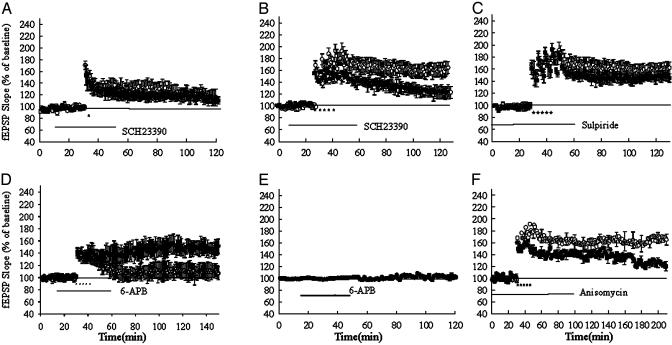
Fig. 2.
The D1 receptor is involved in the maintenance but not the induction of LTP (rat). The D1 receptor inhibitor SCH23390 had no significant effect on the LTP induced by a single train of tetanus. ○, Control; •, SCH23390 (2 μM). (B) SCH23390 (2 μM) blocked the late phase of LTP induced by repeated tetanus. SCH23390 was applied 20 min before the first tetanus and perfused for 50 min. ○, Control; •, SCH23390. (C) The D2 inhibitor sulpiride had no significant effect on the LTP induced by repeated train of tetanus. ○, Control; •, sulpiride (50 μM). (D) D1 receptor agonist facilitated the maintenance of LTP. Subthreshold tetanus (100 Hz, 0.5 s) induced a short synaptic potentiation (○). In the presence of 6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide (6-APB; 2 μM), a stable long-lasting LTP was induced (•). (E) The application of 6-APB alone (2 μM) had no effect on the baseline fEPSP. (F) Anisomycin blocked the LTP induced by five trains of tetanus. ○, Control; •, anisomycin (20 μM) applied 30 min before the tetanus and perfused for 90-100 min.
We next examined the effect of D1 receptor agonist. As shown in Fig. 2D, five-train subthreshold tetanization (100 Hz, 0.5 s) leads to a lasting potentiation in the mPFC that decays to baseline after 30 min (Fig. 2D). Coapplication of D1 receptor agonist 6-APB (2 μM) and subthreshold tetanus resulted in the induction of a long-lasting potentiation (Fig. 2D). At 150 min, the LTP was 150 ± 11% in the presence of 6-APB, which significantly differs from 113 ± 10% in the control solution (n = 6, P < 0.05). The application of 6-APB (2 μM in 0.1% DMSO) alone did not induce any synaptic potentiation (Fig. 2E). This result is consistent with our D1 inhibition experiments and supports the hypothesis that D1 receptors are mainly involved in the maintenance of LTP.
D1 receptors are coupled to stimulatory G proteins; activation therefore results in increased cAMP levels and a resultant increase in protein kinase A (PKA) activity. Indeed, LTP in the mPFC is associated with an enhancement of PKA activity (21). We examined the effect of the PKA inhibitor adenosine RP-3′,5′-cyclic monophosphothioate (Rp-cAMPS) on LTP. In the presence of Rp-cAMPS (50 μM), LTP induced by five trains of tetanus (300 Hz, 0.5 s) was reduced to 120 ± 5% at 90 min, which is significantly different from 166 ± 11% (n = 6, P < 0.01, data not shown).
We also examined the effect of the protein synthesis inhibitor anisomycin. LTP induced by repeated tetanization was decayed to 114 ± 7% 3 hr after tetanization in the presence of anisomycin (20 μM), which is significantly different from 169 ± 4% (n = 6) in the untreated control slices (Fig. 2F, P < 0.01). The application of anisomycin alone had no effect on the baseline fEPSP (data not shown). Late-Phase LTP in D1 Receptor Heterozygous Knockout Mice Is Impaired. D1 receptor knockout mice were generated by homologous recombination in embryonic stem cells (13). Experiments were performed using heterozygous (D1+/-) mice and their wild-type (D1+/+) littermates. We first performed ligand-binding studies to show that in both the striatum and frontal cortex D1 receptor density in +/- mice is ≈50% that of the density in +/+ mice (ref. 13 and data not shown).
We examined basal synaptic plasticity and found no significant difference between +/+ and +/- mice for the input-output curve, paired-pulse facilitation, or synaptic response during a 20-Hz train of 15 pulses, indicating that basal synaptic transmission and short-term synaptic plasticity were normal (Fig. 3). We then examined LTP in mutant and wild-type mice. In contrast to the induction of LTP in the mPFC of rat slices, the induction of LTP in the mouse mPFC slice preparation required a stronger tetanus, and the LTP was weaker and decayed with time. LTP induced by repeated tetanus (10 trains of 300 Hz, 0.5 s) was 130 ± 9% at 30 min and decayed to 116 ± 7% at 120 min after tetanus in wild-type mice. This is comparable with the published LTP study in mouse mPFC in vivo, in which only 110% LTP was elicited (14). We found, however, in the presence of the D1 receptor agonist 6-APB a stable long-lasting LTP could be induced in the mPFC of wild-type mice (146 ± 3% at 2 hr, n = 6, significantly different from 116 ± 7%, n = 6, in the control, P < 0.05; Fig. 4A). This facilitating effect of 6-APB could be reversed by the protein synthesis inhibitor anisomycin (121 ± 2%, n = 5, P < 0.01; Fig. 4B). These results are consistent with our pharmacological study in rat slices. Because our results showed that pharmacological D1 inhibition affected mainly the maintenance of LTP, we compared the LTP between D1+/+ and D1+/- mice in the presence of 6-APB. Although there is no significant difference between the LTP of D1+/+ and D1+/- mice in the absence of D1 agonist (Fig. 4C), a clear difference could be obtained when the tetanus was applied in the presence of 6-APB (2 μM). As shown in Fig. 4D, 10 repeated trains induced a long-lasting and stable LTP in wild-type mice, with the potentiation being 145 ± 7% (n = 6) at 120 min after tetanus, which is significantly different from 115 ± 9% (n = 6) in control slices (P < 0.05). However, the same tetanus induced only a decaying LTP in the D1+/- mice. The LTP in D1 heterozygous mice was 112 ± 6% (n = 6) at 120 min after tetanus, which is significantly different from that of the wild-type mice (Fig. 4D, P < 0.01) but not significantly different from non-6-APB-treated heterozygous D1 receptor knockout mice (Fig. 4C, 117 ± 6%, n = 6, P > 0.5).
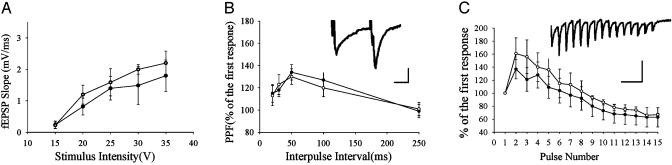
Fig. 3.
Basic synaptic plasticity in heterozygous D1 receptor knockout mice. (A) Input-output synaptic relation is normal in D1+/- mice. ○, Wild-type mice (n = 6); •,D1+/- mice (n = 6). (B) Paired-pulse facilitation is normal in the D1+/- mice. ○, Wild-type mice (n = 5); •,D1+/- mice (n = 5). A sample recording of paired-pulse facilitation is shown in the Inset. Calibration: 12 ms, 1 mV. (C) Synaptic response during a 20-Hz (15-pulse) stimulation. There is no significant difference in response curve as percentage change of amplitude to the first response between wild-type (○) and D1+/- mice (•) (n = 5 each). A sample recording of 20 Hz (15 pulses) is shown in the Inset. Calibration: 50 ms, 1.5 mV.
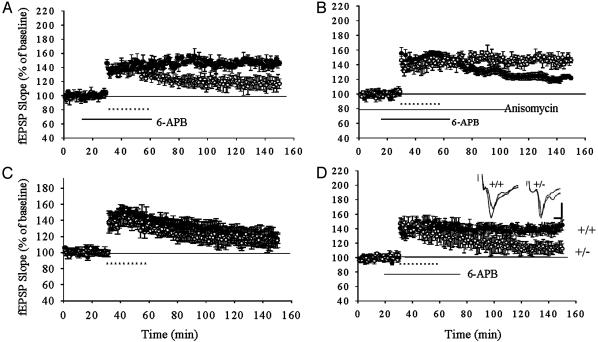
Fig. 4.
LTP in the heterozygous D1 receptor knockout mice. (A) Application of D1 receptor agonist facilitated the maintenance of LTP in wild-type mice. Ten trains of tetanus (300 Hz, 0.5 s) in a 3-min interval induced a decaying LTP (○); in the presence of 6-APB (2 μM), a stable long-lasting synaptic potential was induced (•). (B) Anisomycin blocked the facilitation of D1 agonist on LTP. ○, LTP in the presence of 6-APB without anisomycin; • LTP in the presence of 6-APB with anisomycin (20 μM). (C) LTP is unaffected in the heterozygous D1 receptor knockout mice. There is no significant difference between the LTP in the D1+/+ mice (○, n = 6) and D1+/- mice (•, n = 6). (D) The effect of D1 agonist on LTP in wild-type and heterozygous D1 knockout mice. In the presence of 6-APB, a stable long-lasting synaptic potentiation could be induced in D1+/+ mice (•) but not in D1+/- mice (○). Representative fEPSPs before and 2 hr after tetanus in the D1+/+ (right) and D1+/- mice (left) are shown in the Insets. Calibration: 5 ms, 1 mV.
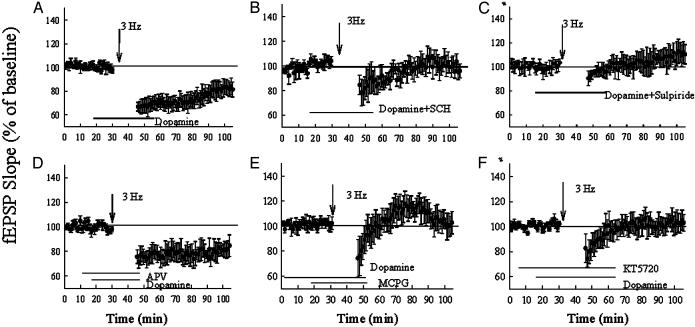
Facilitation of LTD in the mPFC by Dopamine Is Impaired in Heterozygous D1 Receptor Mice. To investigate the specific role of the D1 receptor in LTD, we performed experiments using both wild-type and heterozygous D1 knockout mice. In contrast to the study of Otani et al. (11), in our experimental conditions the application of dopamine (200 μM) alone to wild-type slices had no significant effect on basal synaptic transmission (Fig. 5A). Three-hertz tetanization (15 min) alone elicited only a weak and transient depression that lasted about 15 min (Fig. 5B). However, in the presence of dopamine (200 μM, dissolved in 20 μM ascorbic acid), 3 Hz (15 min) elicited a deep and long-lasting depression in the mPFC of wild-type mice. Twenty minutes after the 3-Hz stimulation, the fEPSP was 74 ± 6% (n = 9) of baseline level and 60 min after it was 79.3 ± 7% (Fig. 5C). This form of LTD could be blocked by the D1 antagonist SCH23390 (2 μM) (Fig. 6B), the PKA inhibitor KT5720 (2 μM) (Fig. 6F), or the D2 antagonist sulpiride (50 μM) (Fig. 6C). In contrast to prefrontal LTP, LTD is independent of NMDA receptor function, because in the presence of D-APV (50 μM) a substantial LTD (83 ± 9% at 60 min) could still be elicited (Fig. 6D). However, the application of the metabotropic glutamate receptor (mGluR) antagonist (R,S)-α-methyl-4-carboxyphenylglycine (MCPG; 500 μM) abolished LTD, suggesting that this form of LTD is dependent on the mGluR (Fig. 6E).
Fig. 5.
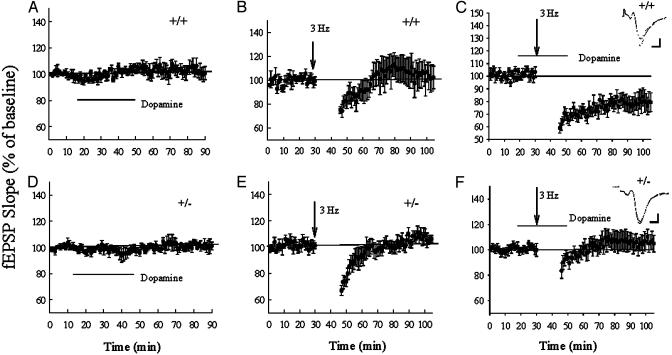
LTD in heterozygous D1 knockout mice. (A and D) The application of dopamine (200 μM) alone not induce significant depression in D1+/+ mice (A) or D1+/- mice (D). (B and E) Three-hertz stimulation (15 min) induced a transient depression in D1+/+ mice (B) and D1+/- mice (E). (C and F) Coapplication of 3-Hz stimulation and dopamine induced a LTD in the D1+/+ mice (C), whereas application of 3-Hz stimulation and dopamine failed to induce any LTD in the D1+/- mice (F). Representative fEPSPs before and 1 hr after the 3-Hz stimulation in the D1+/+ and D1+/- mice are shown in the Insets. Calibration: 5 ms, 0.5 mV.
Fig. 6.
Pharmacological analysis of the LTD in wild-type mice. (A) Coapplication of 3-Hz stimulation and dopamine induced LTD in wild-type mice (n = 8). (B) D1 antagonist SCH23390 (2 μM) blocked the LTD induced by coapplication of dopamine (200 μM) and 3-Hz stimulation. (C) D2 receptor antagonist sulpiride (50μM) blocked the LTD induced by coapplication of dopamine and 3-Hz stimulation. (D) NMDA inhibitor D-APV (50 μM) did not block the LTD induced by coapplication of dopamine and 3-Hz stimulation. (E) Metabotropic glutamate receptor antagonist (R,S)-α-methyl-4-carboxyphenylglycine (MCPG; 500 μM) blocked the LTD induced by coapplication of dopamine and 3-Hz stimulation. (F) PKA inhibitor KT5720 (2 μM) blocked the LTD induced by coapplication of dopamine and 3-Hz stimulation.
We next compared LTD in wild-type and D1+/- mice. We found that brief application of dopamine did not induce significant depression in either genotype (Fig. 5 A and D). Three-hertz (15 min) stimulation induced a transient depression lasting about 15 min in both D1+/+ and D1+/- mice (Fig. 5 B and E) and there is no significant difference between genotypes. When 3-Hz stimulation was coapplied with the perfusion of dopamine, a long-lasting depression could be obtained in the D1+/+ mice (79.3 ± 7% at 60 min, n = 9, Fig. 5C). However, the coapplication of 3-Hz stimulation and dopamine failed to induce deep and long-lasting LTD in D1+/- mice. The synaptic potential was 105 ± 9% of control baseline at 60 min in the D1+/- mice (n = 11), which is significantly different from the change in the wild-type mice (Fig. 5 F and C; P < 0.025).
Discussion
The mPFC of the rat receives rich dopaminergic innervation from the ventral tegmentum (22-24). Anterograde labeling techniques have revealed dopaminergic terminals in layers 1, 2, 3, and 5, which are distributed to both the basal and apical dendrites of the pyramidal neurons. Dopamine receptors are found in all layers of the cortex, although the expression of the most prominent dopamine receptor gene, the D1 receptor gene is highest in the deep layers 5 and 6 of the cortex.
Previous studies addressing the role of dopamine for synaptic plasticity in the mPFC focused mainly on LTD (10, 11, 15) and less on LTP (12, 14, 16, 25). Here we show with pharmacological and genetic tools that the action of dopamine is bidirectional and that dopamine modulates long-term synaptic changes in rats and in mice by facilitating both LTP and LTD.
We find that the D1/D5 receptor plays a critical role in the intracortical prefrontal LTP, as it does in the hippocampal-prefrontal pathway in the intact animal (12). The facilitation is quite selective. A D1 receptor agonist selectively facilitates not the induction but the maintenance of LTP; similarly, a D1 receptor antagonist selectively depressed the maintenance, but not the induction, of LTP. D1 receptors are known to activate adenylate cyclase by Gs proteins, leading to an increase of cAMP that in turn activates PKA. We found that a PKA inhibitor and a protein synthesis inhibitor attenuate LTP in a similar time course as PKA and D1 receptor activation. These findings lead us to suggest that activation of D1 receptors results in activation of PKA, which then phosphorylates substrates, including transcription factors and translational regulators required for long-term synaptic changes. Thus, our data indicate that the molecular pathways regulating LTP are largely shared between the hippocampus and the cortex.
To separate out the function of the D1 receptor from the D1/D5 receptors in long-term synaptic plasticity we analyzed heterozygous D1 receptor knockout mice. Surprisingly, the facilitating effect of dopamine on LTP seems to be absent in D1 heterozygous mice. This finding means that a 50% reduction has a strong influence on synaptic plasticity in the cortex, and it may indicate that altered D1 receptor levels observed in schizophrenia may have significant electro- and pathophysiological consequences (26, 27).
Not only LTP but also LTD is facilitated by dopamine, but in different ways. To induce LTD in the prelimbic area of the prefrontal cortex we stimulated with 3 Hz for 15 min, leading to a weak and transient depression that lasts about 10 min. Dopamine facilitated this form of LTD, leading to a depression that can be measured after 90 min. The facilitating effect of dopamine depends on both D1 and D2 receptors, since blockage of either of them impairs LTD. This is somewhat similar to what has been observed in rats, although in rats inactivation of one receptor alone is not sufficient, both receptors have to be activated in parallel (11) to elicit this facilitation. Like prefrontal LTP, LTD is dependent on PKA, indicating that the two forms of synaptic plasticity share similar signal transduction pathways. Direct activation of a D1/PKA pathway by Gs protein seems to play an important role in the bidirectional modulation of synaptic plasticity in the prefrontal cortex, and dopaminergic modulation of synaptic plasticity is at least partly independent of the NMDA receptor. Facilitation of LTD by dopamine is absent in heterozygous knockout mice, again emphasizing the importance of well regulated receptor expression level.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute and by grants from the Lieber Center for Schizophrenia Research (to E.R.K.), the National Institute of Mental Health (to E.R.K.), and the Mather Foundation (to E.R.K.), a Fellowship of the Deutsche Forschungsgemeinschaft (to C.K.), and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to C.K.).
Abbreviations: LTP, long-term synaptic potentiation; LTD, long-term synaptic depression; NMDA, N-methyl-D-aspartate; mPFC, medial prefrontal cortex; fEPSP, field excitatory postsynaptic potential; D-APV, D-2-amino-5-phosphonovaleric acid; 6-APB, 6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide; PKA, protein kinase A.
References
- 1.Kolb, B. (1990) in The Cerebral Cortex of the Rat, eds. Kolb, B. & Tees, R. (MIT Press, Cambridge MA), pp. 437-58.
- 2.Guldin, W. O., Pritzel, M. & Markowitsch, H. J. (1981) Brain Behav. Evol. 19, 93-107. [DOI] [PubMed] [Google Scholar]
- 3.Brozoski, T. J., Brown, R. M., Rosvold, H. E. & Goldman, P. S. (1979) Science 205, 929-932. [DOI] [PubMed] [Google Scholar]
- 4.Sawaguchi, T. & Goldman-Rakic, P. S. (1991) Science 251, 947-950. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten, A. F., Cai, J. X., Murphy, B. L. & Goldman-Rakic, P. S. (1994) Psychopharmacology 116, 143-151. [DOI] [PubMed] [Google Scholar]
- 6.Seamans, J. K., Floresco, S. B. & Phillips, A. G. (1998) J. Neurosci. 18, 1613-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zahrt, J., Taylor, J. R., Mathew, R. G. & Arnsten, A. F. (1997) J. Neurosci. 17, 8528-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granon, S., Passetti, F., Thomas, K. L., Dalley, J. W., Everitt, B. J. & Robbins, T. W. (2000) J. Neurosci. 20, 1208-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birrell, J. M. & Brown, V. J. (2000) J. Neurosci. 20, 4320-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law-Tho, D., Desce, J. M. & Crepel, F. (1995) Neurosci. Lett. 188, 125-128. [DOI] [PubMed] [Google Scholar]
- 11.Otani, S., Blond, O., Desce, J. M. & Crepel, F. (1998) Neuroscience 85, 669-676. [DOI] [PubMed] [Google Scholar]
- 12.Gurden, H., Takita, M. & Jay, T. M. (2000) J. Neurosci. 20, RC106 (1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drago, J., Gerfen, C. R., Lachowicz, J. E., Steiner, H., Hollon, T. R., Love, P. E., Ooi, G. T., Grinberg, A., Lee, E. J. & Huang, S. P. (1994) Proc. Natl. Acad. Sci. USA 91, 12564-12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herry, C. & Garcia, R. (2002) J. Neurosci. 22, 577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch, J. C. & Crepel, F. (1990) J. Physiol. 427, 31-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vickery, R. M., Morris, S. H. & Bindman, L. J. (1997) J. Neurophysiol. 78, 3039-3046. [DOI] [PubMed] [Google Scholar]
- 17.Morris, S. H., Knevett, S., Lerner, E. G. & Bindman, L. J. (1999) J. Neurophysiol. 82, 1927-1933. [DOI] [PubMed] [Google Scholar]
- 18.Jay, T. M., Burette, F. & Laroche, S. (1995) Eur. J. Neurosci. 7, 247-250. [DOI] [PubMed] [Google Scholar]
- 19.Huang, Y. Y. & Kandel, E. R. (1995) Proc. Natl. Acad. Sci. USA 92, 2446-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthies, H., Becker, A., Schroeder, H., Kraus, J., Hollt, V. & Krug, M. (1997) NeuroReport 8, 3533-3535. [DOI] [PubMed] [Google Scholar]
- 21.Jay, T. M., Gurden, H. & Yamaguchi, T. (1998) Eur. J. Neurosci. 10, 3302-3306. [DOI] [PubMed] [Google Scholar]
- 22.Thierry, A. M., Blanc, G., Sobel, A., Stinus, L. & Glowinski, J. (1973) Science 182, 499-501. [DOI] [PubMed] [Google Scholar]
- 23.Hökfelt, T., Ljungdahl, A., Fuxe, K. & Johansson, O. (1974) Science 184, 177-179. [DOI] [PubMed] [Google Scholar]
- 24.Conde, F., Maire-Lepoivre, E., Audinat, E. & Crepel, F. (1995) J. Comp. Neurol. 352, 567-593. [DOI] [PubMed] [Google Scholar]
- 25.Blond, O., Crepel, F. & Otani, S. (2002) Eur. J. Pharmacol. 438, 115-116. [DOI] [PubMed] [Google Scholar]
- 26.Abi-Dargham, A., Mawlawi, O., Lombardo, I., Gil, R., Martinez, D., Huang, Y., Hwang, D. R., Keilp, J., Kochan, L., Van Heertum, R., et al. (2002) J. Neurosci. 22, 3708-3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okubo, Y., Suhara, T., Suzuki, K., Kobayashi, K., Inoue, O., Terasaki, O., Someya, Y., Sassa, T., Sudo, Y., Matsushima, E., et al. (1997) Nature 385, 634-636. [DOI] [PubMed] [Google Scholar]