Abstract
Knockout (KO) mice missing the sweet taste receptor subunit T1r3 or the signaling protein Trpm5 have greatly attenuated sweetener preferences but learn to prefer sucrose in 24-h tests. Here, we examined 24-h preferences of T1r3 KO, Trpm5 KO, and C57BL/6J wild-type (WT) mice for glucose, fructose, galactose, and corn starch. Unlike glucose, fructose has little postoral reward effect in WT mice, whereas conflicting data have been obtained with galactose. Naïve KO mice were initially indifferent to dilute glucose solutions (0.5–4%) but exhibited strong preferences for 8–32% concentrations. In a second test, they strongly preferred (~90%) all glucose concentrations although they drank less sugar than WT mice. Naïve KO mice were indifferent to 0.5–8% fructose and avoided 16–32% fructose. However, the glucose-experienced KO mice displayed significant preferences for all fructose solutions. Naïve KO mice preferred only 8% galactose, whereas WT mice preferred 4–16% galactose, and all mice avoided 32% galactose. Galactose experience enhanced the preference for this sugar in KO and WT mice. Naïve T1r3 KO and WT mice displayed similar preferences for 0.5–32% corn starch, which were enhanced by starch experience. Naïve Trpm5 KO mice did not prefer starch but did so after 1-bottle starch experience. The results confirm the sweet taste deficits of T1r3 KO and Trpm5 KO mice but demonstrate their ability to develop strong glucose and milder galactose preferences attributed to the postoral actions of these sugars. The acquired preference for the non-sweet flavor properties of glucose generalized to those of fructose. The findings further demonstrate that although Trpm5 (but not T1r3) signaling is essential for starch preference, Trpm5 KO mice can learn to prefer starch based on its postoral effects.
Key words: experience, fructose, glucose, galactose, postoral conditioning, starch, T1r3 KO mice, Trpm5 KO mice
Introduction
The sweet taste of sugar is mediated in mammals by the T1r2 + T1r3 taste receptor and downstream signaling elements including the G-protein gustducin and the Ca2-activated cation channel Trpm5 (Chaudhari and Roper 2010). The importance of the T1r2 + T1r3 receptor to sweet taste processing is demonstrated by the greatly reduced gustatory nerve and behavioral responses to sugars and artificial sweeteners in knockout (KO) mice missing one or both of the genes Tas1r2 or Tas1r3 that code for the sweet receptor components (Damak et al. 2003; Zhao et al. 2003). In particular, the T1r3 KO mice are indifferent to sucrose solutions in brief access licking tests and also fail to prefer dilute sugar solutions in 24-h sugar versus water choice tests (Zhao et al. 2003; Treesukosol et al. 2009; Zukerman et al. 2009a). However, T1r3 KO mice develop significant preference and acceptance for concentrated sucrose solutions in 24-h tests (Damak et al. 2003; Zhao et al. 2003; Zukerman et al. 2009a, 2009b; Brasser et al. 2010). Furthermore, after experience with concentrated sucrose solutions, T1r3 KO mice significantly prefer dilute sugar solutions to which they were initially indifferent (Zukerman et al. 2009a, 2009b). The experience-induced sucrose preference of T1r3 KO mice has been attributed to a learned association between the T1r3-independent orosensory properties (e.g., odor, texture) and the postoral nutritive effects of the sugar (Zhao et al. 2003; Zukerman et al. 2009a). This interpretation is supported by 2 subsequent studies from our laboratory. First, we reported that anosmia induced by olfactory bulbectomy attenuated sucrose preference in sugar-experienced T1r3 KO mice (Zukerman et al. 2009b). Second, we observed that T1r3 KO mice, like normal C57BL/6 wild-type (B6 WT) mice, learned a strong preference (92%) for a flavored solution (the CS+; e.g., grape) paired with intragastric (IG) self-infusions of 16% sucrose over a different flavored solution (the CS−; e.g., cherry) paired with IG water infusions (Sclafani et al. 2010). It remains possible, however, that a small residual taste sensitivity to concentrated sugar solutions observed in T1r3 KO mice (Damak et al. 2003; Zhao et al. 2003) contributed to their sucrose preference in 24-h tests.
Trpm5 KO mice are also indifferent to sucrose in brief taste tests, but they display significant preferences for concentrated sugar solutions in 24-h tests (Zhang et al. 2003; Damak et al. 2006). As with T1r3 KO mice, the preference for concentrated sucrose solutions is likely due to the post-oral actions of the sugar, which is digested to glucose and fructose in the gut. Supporting this view, Trpm5 KO mice learned to prefer a CS+ solution paired with IG glucose infusions (Sclafani and Ackroff 2012b). In addition, Trpm5 KO mice learned to prefer a bottle side-position associated with the consumption of a sucrose solution, which was attributed to the nutritive conditioning effects of the sugar (de Araujo et al. 2008). It is not known, however, if Trpm5 KO mice, after developing a preference for concentrated sugar solutions in 24-h tests, also prefer dilute sugar solutions as do T1r3 KO mice (Zukerman et al. 2009a, 2009b). If so, then this would demonstrate that the Trpm5 KO mice can learn to identify and prefer dilute sugar solutions even though they are not inherently attracted to them.
This study further investigated the process by which sweet-ageusic KO mice develop preferences for carbohydrates in 24-h tests. Although early work suggested that postoral sugar reward was due to the carbohydrate’s energy value, subsequent studies implicate the activation of postoral sugar-specific sensors in sugar appetite (Sclafani and Ackroff 2012b). For example, although B6 WT mice acquire a significant preference for a CS+ solution paired with IG glucose infusions, they are indifferent to a CS+ solution paired with IG (and isocaloric) fructose infusions (Sclafani and Ackroff 2012a). Rat studies also demonstrate that IG glucose is much more effective than IG fructose in conditioning flavor preferences (Sclafani et al. 1993;Ackroff et al. 1997; Sclafani et al. 1999; Ackroff et al. 2001). Based on these results, we predicted that T1r3 and Trpm5 KO mice would express preferences for glucose but not for fructose in long-term tests. Experiment 1 tested this prediction by comparing the sugar versus water preferences of T1r3 KO, Trpm5 KO, and WT mice subjected to 24-h tests with 0.5–32% concentrations of glucose or fructose (Test 1). The ascending test series was repeated (Test 2) to determine if the sugar experience during the first test would enhance subsequent sugar preferences of the KO mice, as was observed with sucrose-experienced T1r3 KO mice (Zukerman et al. 2009a, 2009b). Finally, a third test series was conducted to determine if experience with 1 sugar in the first 2 tests would influence the preference of the KO mice for the other sugar in Test 3. In particular, it was predicted that after acquiring a significant glucose preference in Tests 1 and 2, the KO mice would also prefer fructose in Test 3 because the 2 monosaccharides may share sweet taste-independent orosensory properties (i.e., odor, texture) (Zukerman et al. 2009b). On the other hand, initial fructose experience was not expected to enhance the subsequent preferences for fructose or glucose.
Experiment 2 compared preferences of T1r3 KO, Trpm5 KO, and WT mice for the monosaccharide galactose. Although less sweet than other sugars (Noma et al. 1971; Jakinovich Jr and Goldstein 1976), galactose was of interest because, like glucose but unlike fructose, it is a ligand for the intestinal SGLT1 glucose transporter and sensor (Wright et al. 2011), which may be involved in postoral sugar conditioning (Sclafani and Ackroff 2012b). Furthermore, a recent study reported that IG intubation of galactose and glucose, but not fructose, conditioned place preferences in mice suggesting that galactose has a postoral reward effect similar to glucose (Matsumura et al. 2010). However, IG infusions of 8% or 16% galactose failed to condition CS+ flavor preferences in B6 mice (Sclafani and Ackroff 2012a). Furthermore, IG 16% galactose conditioned a flavor avoidance in rats (Sclafani et al. 1999). Adult mice and rats have a limited capacity to metabolize galactose (Berman et al. 1978; Solberg and Diamond 1987; Niewoehner and Neil 1992), and the postoral reward effect of this sugar may be critically related to the amount of galactose consumed or infused. The sweet-ageusic T1r3 and Trpm5 KO mice should not be attracted to the taste of galactose, but they may develop a preference for this sugar if it has glucose-like postoral reward effects at specific concentrations. There are no previous reports of galactose preferences in mice and only 1 published rat study (Richter and Campbell 1940). Thus, a comparison of the preference responses of WT and KO mice to galactose solutions of varying concentrations will provide further insight into the postoral conditioning actions of this monosaccharide.
Experiment 3 evaluated the preferences of T1r3 KO, Trpm5 KO, and WT mice for corn starch. Prior studies suggest that rodents have 3 different carbohydrate tastes, that is, tastes for sugars, maltodextrins, and starch (Ramirez 1991a, 1991b; Ramirez 1994; Sclafani 2004; Sclafani et al. 2007). In particular, KO studies revealed that although T1r3 KO mice are deficient in their taste response to sugars, they show near-normal ingestive responses to polycose, a soluble maltodextrin that is rapidly digested and absorbed as glucose (Treesukosol et al. 2009; Zukerman et al. 2009a; Treesukosol et al. 2011). In contrast, Trpm5 KO mice are indifferent to dilute solutions of sucrose and polycose as well as starch in 24-h 2-bottle tests, indicating that Trpm5 signaling is critical for all 3 carbohydrate tastes (Sclafani et al. 2007). Because starch is digested to glucose in the gut, we predicted that Trpm5 KO mice would develop a preference for concentrated starch solutions in 24-h tests based on their flavor conditioning response to IG glucose infusions (Sclafani and Ackroff 2012b). There are no published data on starch preference in T1r3 KO mice. Given that these mice prefer starch-derived maltodextrin (polycose), they might be expected to prefer pure corn starch as well (Treesukosol et al. 2009; Zukerman et al. 2009a; Treesukosol et al. 2011). This is not certain, however, because gustducin KO mice show an impaired maltodextrin preference but a normal starch preference, suggesting that there are distinct taste pathways for maltodextrin and starch taste (Sclafani et al. 2007). Experiment 3 addressed these questions by measuring the 24-h preference responses of KO and WT mice to 0.5–32% starch solutions.
Experiment 1: glucose and fructose preferences in T1r3 KO, Trpm5 KO, and WT mice
Long-term 2-bottle tests (24h/day, which are also referred to as 48-h tests when extended over 2 days as in the present experiment) are commonly used to compare genotype differences in taste preference and acceptance (Lush 1989; Bachmanov et al. 2001b). This method is most informative at low sugar concentrations. At high concentrations, on the other hand, 24-h sugar preferences may be influenced by postoral effects that override inherent (or missing) taste preferences. We previously reported that T1r3 KO mice develop strong preferences for concentrated sucrose solutions, which generalize to dilute sugar solutions in subsequent testing (Zukerman et al. 2009a, 2009b). Experiment 1 tested the prediction that T1r3 KO and Trpm5 KO mice will display preferences for glucose but not fructose, which has minimal postoral preference conditioning effects in B6 mice (Sclafani and Ackroff 2012a). However, it is possible that the weak residual taste responses of T1r3 KO mice to concentrated sugars (Damak et al. 2003; Zhao et al. 2003) may support some preference for fructose in 24-h tests.
Materials and methods
Animals
A total of 20 naïve T1r3 KO (Damak et al. 2003) and 17 naïve Trpm5 KO (Damak et al. 2006) mice were derived from mice produced by homologous recombination in C57BL/6J embryonic stem cells and maintained on this background. A total of 20 naïve B6 WT mice were derived from mice obtained from the Jackson Laboratories (Bar Harbor, ME). Studies were limited to female mice (10 weeks old); in a previous study, T1r3 KO male and female mice did not differ in their preference for dilute or concentrated sucrose solutions (Zukerman et al. 2009a). The animals were singly housed in plastic tub cages with ad libitum access to chow (5001, PMI Nutrition International, Brentwood, MO) and deionized water in a room maintained at 22 °C with a 12:12 h light:dark cycle. Experimental protocols were approved by the institutional animal care and use committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Taste solutions
Sugar solutions were prepared using food-grade glucose and fructose (Tate and Lyle, Honeyville Food Products, Rancho Cucamonga, CA) in deionized water. A sodium saccharin solution (Sigma-Aldrich, St Louis, MO) was also used for screening purposes. The solutions were formulated on a w/w basis because intakes were measured by weight. The solution tests were conducted in the animal’s home cages as previously described (Zukerman et al. 2009a).
Procedure
The T1r3 KO and Trpm5 KO were given a 2-day choice test with 0.2% saccharin versus water to confirm their sweet aguesia phenotype as in previous studies (Zukerman et al. 2009a, 2009b). The WT mice were not tested with saccharin so that they would remain naïve to sweet solutions prior to the sugar tests. The mice of each genotype were divided into Glucose and Fructose groups equated for saccharin intake (KO mice only), water intake, and body weight (n = 10 each, except Glucose Trpm5 KO n = 8, Fructose Trpm5 KO n = 9). Four days later, they were given a series of 2-bottle sugar versus water tests. In each test, the sugar solutions were presented in order of increasing concentration (0.5%, 1%, 2%, 4%, 8%, 16%, and 32%) with each concentration presented for 2 consecutive days. The solutions were available 23h/day, and the bottles were weighed, cleaned, and refilled during the remaining hour. In Tests 1 and 2, the Glucose and Fructose groups were given glucose and fructose solutions, respectively, versus water. In Test 3, the mice in the Glucose group were given choice tests with 0.5–32% fructose versus water, whereas the mice in the Fructose group were tested with 0.5–32% glucose versus water. Four days of water only separated each test series. The Fructose group was then given a Test 4 with 0.5–32% fructose versus water.
Data analysis
Daily solution and water intakes were averaged over the 2 days at each concentration. Sugar intakes were also expressed as kcal/day, and sugar preferences were expressed as percent intakes (sugar intake/[sugar + water intakes] × 100). Genotype differences in sugar intakes and preferences were evaluated using separate mixed-model analysis of variance (ANOVAs) with genotype and sugar concentration as between-group and within-group factors, respectively; separate ANOVAs analyzed the Glucose and Fructose groups. When a significant genotype effect was shown, separate analyses were conducted that compared each KO genotype with the B6 WT mice; significant interaction effects were evaluated by simple main effects tests at each concentration according to Winer (1962). Additional ANOVAs compared glucose versus fructose intake and preference within a genotype. The significance of the sugar preference at each concentration was evaluated for each group by comparing sugar intake versus water intake using paired t-tests. To control for the use of multiple t-test comparisons across the several concentrations of each carbohydrates, the α-level (0.05) was corrected with the Bonferroni procedure (i.e., P = 0.05/7 or 0.00714).
Overall, WT and Trpm5 KO mice weighed slightly more than T1r3 KO mice (22.3, 22.0 and 21.1g) based on body weights averaged at the start and end of the study. Preliminary analyses of the saccharide intakes expressed as intake/mouse or intake/30-g body weight, as in previous studies (Sclafani 2007), produced very similar results and, therefore, the data are reported as intake/mouse.
Results
Pretest
The KO mice failed to prefer the 0.2% saccharin solution. In fact, the T1r3 KO mice consumed less saccharin than water (2.3 versus 2.8g/day, t[19] = 3.6, P < 0.05), whereas the Trpm5 KO mice did not differ in their saccharin and water intakes (3.2 versus 2.7g/day). These results agree with previous reports (Blednov et al. 2008; Zukerman et al. 2009a, 2009b; Glendinning et al. 2012).
Glucose groups
Tests 1–3.
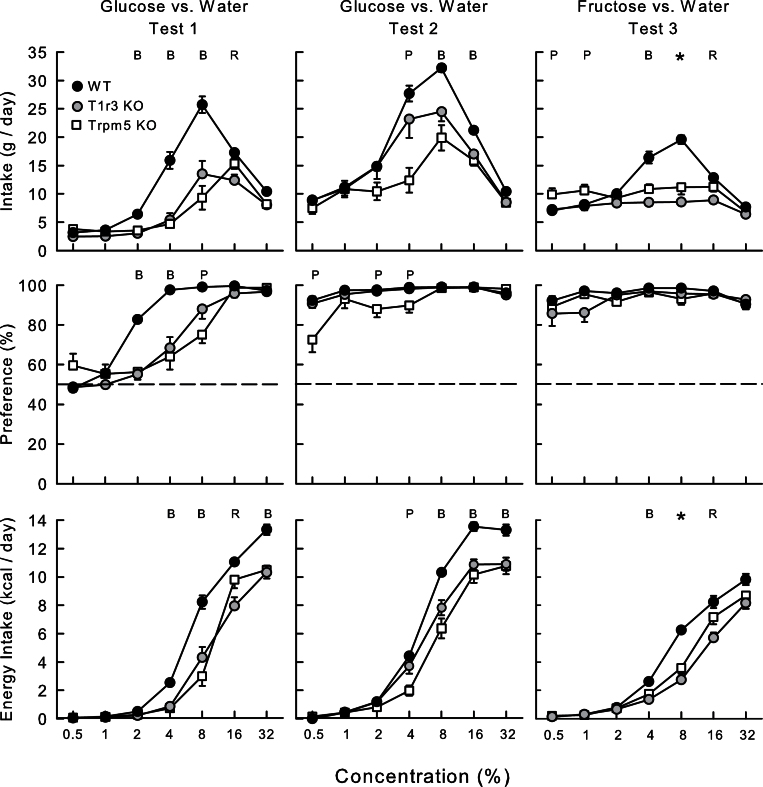
In Test 1 (Figure 1), overall the Trpm5 KO and the T1r3 KO mice consumed less glucose solution than did the WT mice (6.9, 6.8, and 11.8g/day, respectively; F[2,25] = 23.5, P < 0.001). All 3 groups increased and then decreased their solution intakes as concentration increased (F[6,150] = 132.8, P < 0.001); both KO groups consumed less than the WT group at 2–8% concentrations, and the T1r3 KO group also consumed less sugar (16%) than WT and Trpm5 KO groups (Group × Concentration interaction, F[12,150] = 16.8, P < 0.001). In addition, there was an overall effect of group on glucose energy intake (3.5, 3.2, and 4.9 kcal/day for Trpm5 KO, T1r3 KO, and WT mice, respectively; F[2,25] = 17.1, P < 0.001). Both KO groups consumed fewer calories at 4–8% and at 32% compared with the WT group, and the T1r3 KO group consumed fewer calories than the other groups at 16% (Group × Concentration interaction, F[12,150] = 12.2, P < 0.001). With respect to glucose preference, the WT mice consumed significantly more glucose than water at 2–32% concentrations, whereas the Trpm5 KO mice consumed significantly more glucose at 16–32% and the T1r3 KO mice at 8–32% concentrations. All 3 groups increased their percent glucose intakes with concentration (F[2,25] = 12.8, P < 0.01). However, there was a Group × Concentration interaction (F[12,150] = 12.4, P < 0.001), and both KO groups had reduced glucose preferences compared with WT mice at 2–4%, and the Trpm5 KO mice had reduced preference for 8% glucose compared with WT and T1r3 KO mice. All 3 groups displayed near-total glucose preferences at 16–32%.
Figure 1.
Mean (±standard error [SE]) glucose solution intake (top), percent glucose preference over water (middle), and glucose energy intake (bottom) in Trpm5 KO, T1r3 KO, and B6 WT mice during 24-h 2-bottle glucose versus water in Tests 1 and 2, and fructose versus water in Test 3. Water intakes are not shown. Significant (P < 0.05) group differences at individual concentrations are denoted by * where all the groups differ from one another, by B where B6 WT group differs from both KO groups, by P where the Trpm5 KO group is different from both other groups, and by R where the T1r3 KO group differs from the other groups. Note that the y axis range in the top graphs differs from those of Figures 2–5.
In glucose Test 2, overall the Trpm5 KO mice consumed less glucose than T1r3 KO mice, and both consumed less than the WT mice (12.2, 15.4, and 18.0g/day, respectively; F[2,25] = 7.4, P < 0.001). All 3 groups increased and then decreased their solution intakes as concentration increased (F[6,150] = 129.5, P < 0.001). As indicated in Figure 1, the Trpm5 KO group consumed less glucose than WT and T1r3 KO groups at 4%, and both KO groups consumed less than the WT group at 8–16% (Group × Concentration interaction, F[12,150] = 9.3, P < 0.001). Glucose solution intakes peaked at 8% for all groups although the intakes of the T1r3 KO and Trpm5 KO mice were 24% and 38% lower, respectively, than that of the WT mice. In addition, there was an overall group effect on glucose energy intake, and both KO groups consumed less than the WT mice (4.2, 4.8, and 5.9 kcal/day, respectively; F[2,25] = 14.6, P < 0.001). The Trpm5 KO group consumed less energy than T1r3 KO and WT groups at 4%, and both KO groups consumed less than WT group at 8–32% (Group × Concentration interaction, F[12,150] = 11.8, P < 0.001). With respect to glucose preference, the Trpm5 KO mice significantly preferred glucose to water at 1–32%, and the T1r3 KO and WT mice preferred the sugar at all concentrations. Analysis of the percent intake data revealed that Trpm5 KO mice had lower preferences at 0.5% and 2–4% compared with the other 2 groups (Group × Concentration interaction, F[12,150] = 4.4, P < 0.001).
Within-group test comparisons revealed that Trpm5 KO mice increased their absolute and percent glucose intakes from Test 1 to 2, with differences being most pronounced at 0.5–8% concentrations (Test × Concentration interactions, F[6,42] > 7.2, P < 0.001). The T1r3 KO mice also increased their absolute intake (0.5–16%) and percent intakes (0.5–8%) from Test 1 to 2 (Test × Concentration interactions, F[6,54] > 15.7, P < 0.001). Similarly, the WT mice increased their glucose intake at 0.5–16%, but their percent intake only at 0.5–2% from Test 1 to 2 (Test × Concentration interactions, F[6,54] > 18.3, P < 0.001).
In Test 3 (Figure 1), the Glucose groups were now tested with fructose versus water. Overall, the WT mice consumed more fructose than Trpm5 KO mice, which in turn consumed more than T1r3 KO mice (11.7, 10.0, and 8.0g/day, respectively; F[2,25] = 12.8, P < 0.001). In particular, the WT group consumed more than both KO groups at 4–8% and also more than the T1r3 KO group at 16%; the Trpm5 KO group consumed more fructose than the other groups at 0.5–1% and more than the T1r3 KO group at 8–16% (Group × Concentration interaction, F[12,150] = 23.3, P < 0.001). In terms of energy intake, the WT group consumed more calories than the KO groups at 4–8% and more than the T1r3 KO group at 16%; in turn, the Trpm5 KO group consumed more than T1r3 KO group at 8–16% (Group × Concentration interaction, F[12,150] = 8.8, P < 0.001). All 3 groups showed near-total fructose preferences for all concentrations and there were no group differences, which contrasts with the results obtained with the Fructose groups as described below. Yet, despite their strong preference, the KO mice showed no increase in fructose intake as concentration increased, although they decreased their sugar intake at the 32% concentration.
Fructose group
Tests 1–4.
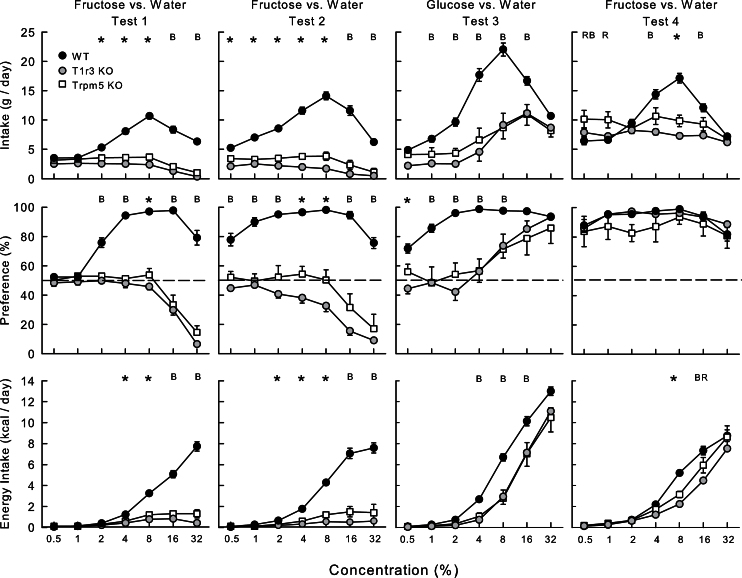
In Test 1 (Figure 2), the T1r3 KO mice consumed less fructose solution than Trpm5 KO mice, and both consumed substantially less than the WT mice (2.0, 2.9, and 6.6g/day, respectively; F[2,26] = 149.1, P < 0.001) (Figure 2). The KO mice displayed no change in fructose intake at low and intermediate concentrations but decreased intake at 16–32% concentrations, whereas the WT mice increased and then decreased their fructose intake as concentration increased (Group × Concentration interaction, F[12,156] = 47.8, P < 0.001). Analyses at individual concentrations revealed that the WT mice consumed more 2–32% fructose than both KO groups, and the Trpm5 KO group consumed more 2–8% sugar than T1r3 KO group. Likewise, in terms of energy intakes, the WT mice consumed more than both KO groups at 4–32%, and the Trpm5 KO mice consumed more than T1r3 KO mice at 4–8% (Group × Concentration interaction, F[12,156] = 93.5, P < 0.001). With respect to fructose preference, the WT mice consumed more fructose than water at 2–32% concentrations; the KO mice were indifferent to fructose at 0.5–8%, and the T1r3 KO mice consumed less (P < 0.05) sugar than water at 16–32% and the Trpm5 KO mice at 32% concentrations (F[2,26] = 187.7, P < 0.001). The percent fructose intakes of the WT mice exceeded those of both KO groups at 2–32% concentrations; in addition, the Trpm5 KO mice had higher percent intake of 8% fructose compared with T1r3 KO mice (Group × Concentration interaction, F[12,156] = 37.6, P < 0.001).
Figure 2.
Mean (±SE) fructose solution intake (top), percent fructose preference over water (middle), and fructose energy intake (bottom) in Trpm5 KO, T1r3 KO, and B6 WT mice during 24-h 2-bottle fructose versus water in Tests 1, 2, and 4, and glucose versus water in Test 3. Water intakes are not shown. Significant (P < 0.05) group differences at individual concentrations are denoted by * where all the groups differ from one another, by B where B6 WT group differ from both KO groups, by P where Trpm5 KO group differs from both other groups, and by R where the T1r3 KO group differs from the other groups.
In Test 2, group intake differences were more pronounced than in Test 1. The WT mice consumed more fructose solution than both KO groups at all concentrations, and the Trpm5 KO mice consumed more than T1r3 KO mice at 0.5–8% concentrations (Group × Concentration interaction, F[12,156] = 32.8, P < 0.001). In terms of energy intakes, the WT mice consumed more than KO mice at 2–32%, and Trpm5 KO mice consumed more than T1r3 KO mice at 2–8% (Group × Concentration interaction, F[12,156] = 53.8, P < 0.001). With respect to preference, the WT mice consumed more fructose than water at 0.5–32%, whereas the Trpm5 KO and T1r3 KO mice consumed significantly less fructose than water at 32% and 8–32% concentrations, respectively. The percent fructose intakes of the WT mice exceeded those of the KO mice at all concentrations, and the Trpm5 KO mice had a higher percent intake than T1r3 KO mice at the 8% concentration (Group × Concentration interaction, F[12,156] = 9.2, P < 0.001).
Within-group comparisons revealed that the WT mice had higher fructose solution intakes and percent intakes in Test 2 compared with Test 1 at 0.5–16% and 0.5–2% concentrations, respectively (Test × Concentration interactions, F[6,54] > 7.8, P < 0.001). In contrast, the Trpm5 KO mice did not change their fructose intakes or percent intakes from Test 1 to 2, whereas the T1r3 KO mice decreased their fructose intakes and percent intakes in Test 2 at 2–16% concentrations (Test × Concentration interactions, F[6,54] > 4.2, P < 0.001).
In Test 3, the Fructose groups were tested with glucose versus water (Figure 2). Overall, the WT mice consumed more glucose than the Trpm5 KO mice, which in turn consumed more than the T1r3 KO mice (12.6, 6.7, and 5.8g/day, respectively; F[2,26] = 22.1, P < 0.001). In particular, the WT mice consumed more glucose than the KO groups at 1–16% concentrations (Group × Concentration interaction, F[12,156] = 10.1, P < 0.001). In terms of energy intakes, the WT group consumed more than the KO groups at 4–32% concentrations (Group × Concentration interaction, F[12,156] = 4.1, P < 0.001). With respect to glucose preference, the WT mice consumed more glucose than water at all concentrations, whereas the T1r3 KO mice consumed more glucose only at 16–32% concentrations. The Trpm5 KO mice failed to drink significantly more glucose than water at any concentration, but this was due to 1 animal that avoided 16–32% glucose; the remaining animals preferred glucose to water at these concentrations. All 3 groups increased percent glucose intakes with concentration (F[2,26] = 15.7, P < 0.001). The percent intakes of the WT mice significantly exceeded that of the Trpm5 KO and T1r3 KO mice at 1–8%; in addition, percent intake of the Trpm5 KO mice was higher than that of the T1r3 KO mice at 0.5% (Group × Concentration interaction, F[12,156] = 4.9, P < 0.001).
In Test 4, the Fructose groups were tested again with fructose versus water. In this test, overall, the WT and Trpm5 KO mice consumed more fructose solution than did the T1r3 KO mice (F[2,26] = 6.5, P < 0.01). In addition, the WT mice consumed more fructose than did the KO groups at 4–16% concentrations (Group × Concentration interaction, F[12,156] = 18.5, P < 0.001). Other group differences in solution and energy intakes are indicated in Figure 2. The groups did not, however, differ in their percent fructose intakes, which were 80% or higher as a function of concentration (Figure 2).
Experiment 2: galactose preferences in T1r3 KO, Trpm5 KO, and WT mice
The galactose intakes and preferences of naïve T1r3 KO, Trpm5 KO, and WT mice were measured in two 24-h test series as in Experiment 1. In addition, the mice were given a third test with fructose to determine if previous galactose experience influenced their preference for this sugar.
Materials and methods
Naïve female T1r3 KO, Trpm5 KO, and WT mice (n =10 each, 10 weeks old) were tested as in Experiment 1, except that all mice were given 0.5–32 % galactose (Sigma-Aldrich) in Tests 1 and 2 and 0.5–32% fructose in Test 3. Two Trpm5 KO mice died during Test 1, and their data were eliminated from the study.
Results
Pretest
In the 0.2% saccharin versus water test, the T1r3 KO mice consumed less saccharin than water (1.4 g vs. 3.5g/day, t[9] = 4.3, P < 0.05), whereas the Trpm5 KO mice did not significantly differ in their saccharin and water intakes (3.3 g vs. 3.7g/day).
Test 1
Galactose versus water.
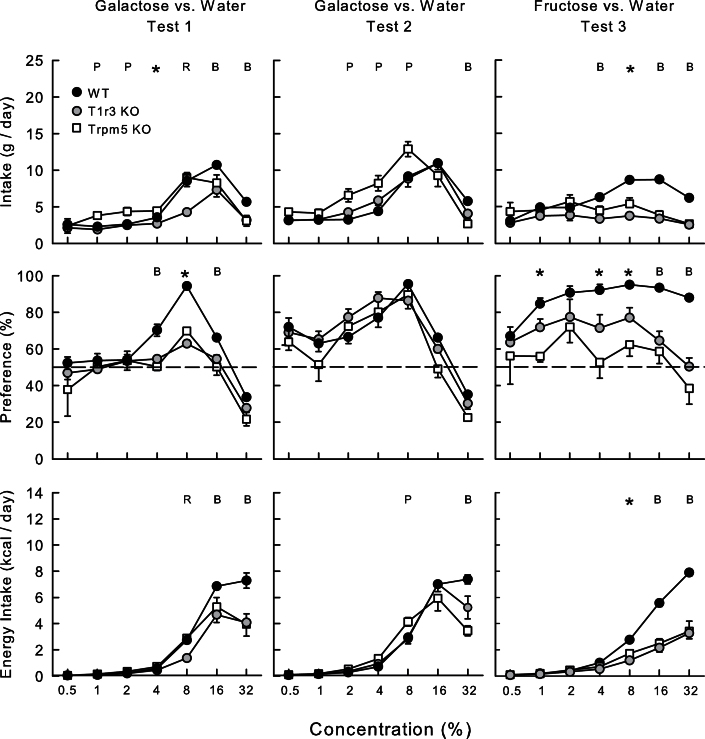
In Test 1 (Figure 3), the WT and the Trpm5 KO mice overall consumed more galactose than did the T1r3 KO mice (5.2, 5.1, and 3.4g/day, respectively; F[2,25] = 20.6, P < 0.001). All 3 groups increased and then decreased their solution intakes as concentration increased, although the WT mice increased their intakes more than the KO groups (Group × Concentration interaction, F[6,150] = 63.3, P < 0.001). Analyses of the individual concentrations indicated that the Trpm5 KO group consumed more (P < 0.05) galactose than the other groups at 1–4% concentrations. The WT mice consumed more (P < 0.05) galactose than T1r3 KO mice at 4–8% concentrations and more than both KO groups at 16–32% concentrations. Galactose energy intake increased with concentration (F[2,25] = 15.3, P < 0.001), and the WT mice consumed more than the KO groups at the 16–32% concentrations (Group × Concentration interaction, F[12,150] = 5.4, P < 0.001). With respect to galactose preference, WT mice consumed more sugar than water at 4–16% concentrations, whereas the KO groups preferred only 8% galactose to water; all 3 groups drank more water than 32% galactose. Percent galactose intakes of the WT mice exceed those of the KO mice at 4–16% concentrations (Group × Concentration interaction, F[12,150] = 2.6, P < 0.05).
Figure 3.
Mean (±SE) galactose solution intake (top), percent galactose preference over water (middle), and galactose energy intake (bottom) in Trpm5 KO, T1r3 KO, and B6 WT mice during 24-h 2-bottle galactose versus water in Tests 1 and 2, and fructose versus water in Test 3. Water intakes are not shown. Significant (P < 0.05) group differences at individual concentrations are denoted by * where all the groups differ from one another, by B where B6 WT group differ from both KO groups, by P where Trpm5 KO differs from both other groups, and by R where the T1r3 KO group differs from other groups.
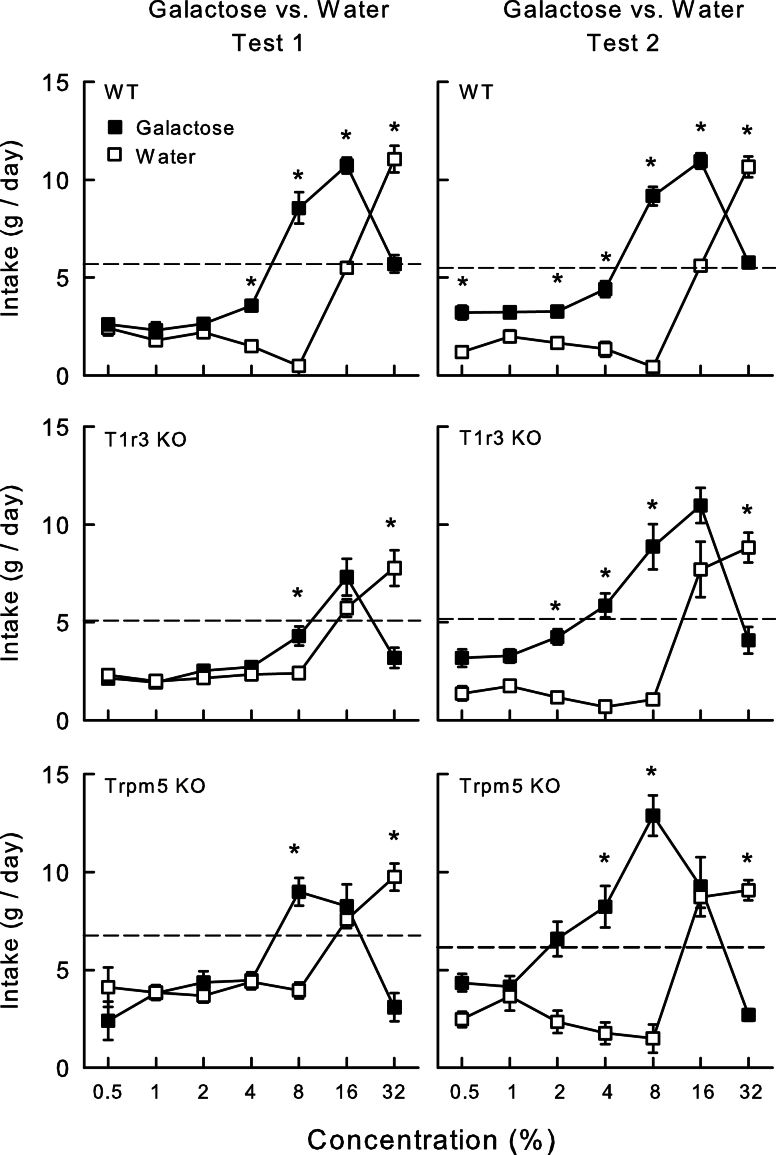
An unusual aspect of the galactose intake response is that the rapid decline in galactose preference at the 16% and 32% concentrations was accompanied by a rapid increase in water intake in all 3 groups (Figure 4). In particular, the apparent low preference for 32% galactose occurred not because the intake of the sugar solution was underconsumed relative to the other concentrations but because water intake was greatly elevated. Overall, the Trpm5 KO mice consumed more water than did the T1r3 KO and the WT mice (5.3g vs. 3.5g vs. 3.6g, respectively; F[2,25] = 22.5, P < 0.001). There was a Group × Concentration interaction, however, and the Trpm5 KO mice consumed more water than the WT mice at 0.5–16%, and T1r3 KO mice consumed more than the WT mice at 8%, but the WT mice consumed more water than the KO mice at 32% concentration (Group × Concentration interaction, F[12,150] = 5.1, P < 0.001).
Figure 4.
Mean (±SE) galactose solution and water intake in B6 WT mice (top), T1r3 KO mice (middle), and Trpm5 mice (bottom) during 24-h 2-bottle galactose versus water test in Tests 1 and 2. The dash line represents mean water intake prior to the 2-bottle test. Significant (P < 0.05) differences between galactose and water at individual concentrations are denoted by *.
Test 2
Galactose versus water.
In the second galactose test (Figure 3), there was no overall group difference in solution intake. However, the Trpm5 KO group consumed more galactose than the WT and T1r3 KO groups at 2–8%, whereas the WT group consumed more than both KO groups at 32% (Group × Concentration interaction, F[12,150] = 6.1, P < 0.001). In terms of energy intake, the Trpm5 KO group consumed more than the other groups at 8%, but less at 16–32% (Group × Concentration interaction, F[12,150] = 6.6, P < 0.001). Additionally, the T1r3 KO mice consumed less energy than the WT mice at 32%. With respect to preference, Trpm5 KO mice preferred galactose at 4–8%, T1r3 KO mice at 2–16%, and WT mice at 0.5% and 2–16%. All 3 groups avoided galactose at 32%. The groups did not differ in their percent intakes of 0.5–32% galactose solutions. As in Test 1, water intakes increased substantially with the 16% and 32% galactose solutions (Figure 4). The Trpm5 KO and T1r3 KO mice consumed more water than WT mice at 16%, but less water at 32% galactose concentration (Group × Concentration interaction, F[12,150] = 2.7, P < 0.05).
Within-group comparisons indicated that the WT mice overall consumed more galactose solution in Test 2 than 1 (F[1,9] = 6.4, P < 0.05) and more percent galactose in Test 2 at concentrations 0.5–2% (Test × Concentration interaction, F[6,54] = 3.4, P < 0.01). The Trpm5 KO mice increased their galactose solution and percent intakes from Tests 1 to 2 at 0.5–16% and 0.5–2%, respectively (Test × Concentration interactions, F[6,54] ≥ 18.3, P < 0.001). Similarly, the T1r3 KO mice increased their galactose solution and percent intakes from Tests 1 to 2 at 0.5–16% and 0.5–2%, respectively (Test × Concentration interactions, F[6,54] ≥ 7.8, P < 0.001).
Test 3
Fructose versus water.
When given fructose in Test 3 (Figure 3), overall the WT mice consumed more sugar solution and energy than did the KO mice (F[2,25] ≥ 26.4, P <0.001), and these differences varied as a function of concentration (Group × Concentration interaction, F[2,25] ≥ 7.1, P <0.001) (Figure 3). Overall, the percent fructose intakes of the WT mice exceeded that of the T1r3 KO mice which, in turn, exceeded that of the Trpm5 KO mice (87%, 68%, and 57%, respectively; F[2,25] = 36.3, P < 0.001). The WT mice preferred fructose to water at 1–32%, whereas the T1r3 KO mice significantly preferred only 1% and 8% fructose, and the Trpm5 KO mice failed to prefer fructose to water at any concentration. Importantly, both KO group avoided fructose at high concentrations.
Galactose versus glucose versus fructose.
Within-group analyses compared separately the response of the 3 groups to the galactose, glucose, and fructose solutions and is described in detail in Supplementary Figures 1–3. The WT mice consumed substantially more glucose than fructose and galactose in both Tests 1 and 2 and more fructose than galactose in both tests (Supplementary Figure 1). Glucose and fructose preferences were rather similar but greater than that for galactose with the notable exception at the 8% concentration. The T1r3 KO mice also consumed significantly more glucose than fructose and galactose but more galactose than fructose particularly in Test 2 (Supplementary Figure 2). Also, their glucose preferences exceeded that for the other 2 sugars, and their preference for galactose was greater than that for fructose. Similarly, the Trpm5 KO mice consumed more glucose than fructose and galactose and more galactose than fructose (Supplementary Figure 3). Their glucose preferences exceeded that for the other 2 sugars, and their galactose preferences were greater than those for fructose particularly in Test 2.
Experiment 3: starch preferences in T1r3 KO, Trpm5 KO, and WT mice
The first 2 experiments compared the preferences of T1r3 KO, Trpm5 KO, and WT mice to the monosaccharide sugars glucose, fructose, and galactose. This experiment compared their preferences for a complex polysaccharide, corn starch. As noted in the Introduction, behavioral studies indicate that rodents have a distinct “taste” for starch (Ramirez 1991a, 1991b; Ramirez 1994; Sclafani 2004), and we previously reported that Trpm5 KO mice, unlike WT mice, are indifferent to dilute (0.5–4%) starch (Sclafani et al. 2007). However, based on their response to glucose in Experiment 1, Trpm5 KO mice would be expected to develop a preference for concentrated starch solutions given that starch is digested to glucose in the gut. Whether T1r3 KO mice have a normal or impaired preference for starch is not known. This experiment, therefore, compared the preferences for 0.5–32% starch in T1r3 KO, Trpm5 KO, and WT mice.
Materials and methods
Naïve female Trpm5 KO, T1r3 KO, and WT mice (n =10 each, 17 weeks old) were used. As in the first 2 experiments, the KO mice were given a saccharin screening test, and all mice were then given 2 test series with ascending concentrations (0.5–32%) of corn starch (ACH Foods, Memphis, TN). Because starch is insoluble in water, the starch was presented as a suspension in 0.3% xanthan gum (Sigma-Aldrich); this same xanthan gum suspension, rather than water, was used as the control fluid as in previous studies (Bachmanov et al. 2001a; Sclafani et al. 2007). The starch suspension and vehicle were prepared with deionized water and were mixed in a kitchen blender for 5min. Following Test 2, additional tests were conducted with Trpm5 KO mice only in an attempt to induce all the mice in this group to prefer starch to vehicle as described in the Results. The drinking spouts in this experiment had a larger hole size (2.5mm) than used in the previous experiments (1.5mm) to accommodate the viscosity of the starch and gum suspensions. For simplicity, hereafter, the starch suspension will be referred to as a starch solution.
Statistical tests were performed as in Experiments 1 and 2.
Results
Pretests
In the 0.2% saccharin versus water test, the T1r3 KO mice drank more water than 0.2% saccharin (1.9 g vs. 2.6g/day, t[8] = 5.1, P < 0.05), whereas the Trpm5 KO mice were indifferent to saccharin and water (3.0 g vs. 3.3g/day).
Test 1
Starch versus gum.
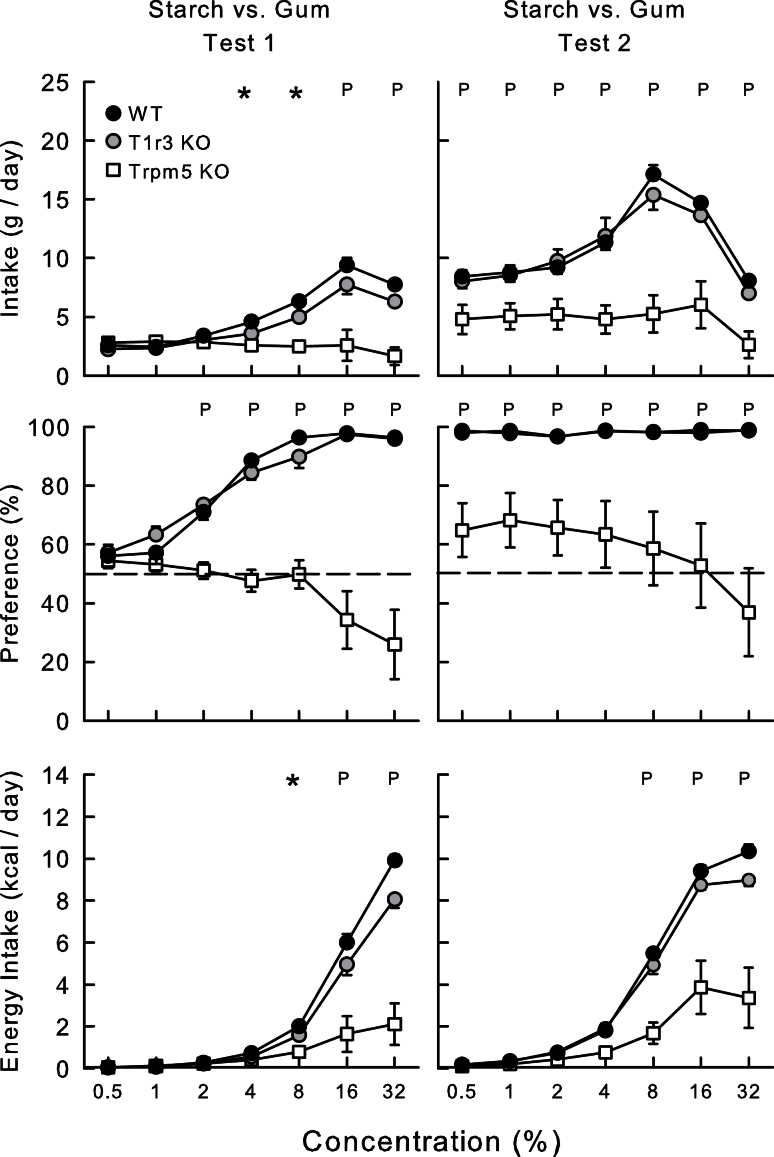
In Test 1 (Figure 5), overall the WT mice and the T1r3 KO mice consumed more starch than did the Trpm5 KO mice (5.2, 4.3, and 2.5g/day, respectively; F[2,27] = 28.1, P < 0.001). The WT and T1r3 KO groups increased their starch solution intake up to 16% and decreased it at 32% (Group × Concentration interaction, F[6,162] = 14.7, P < 0.001). The intakes of WT mice exceeded those of T1r3 KO mice, which exceeded those of Trpm5 KO mice at 4–8% concentrations (F[2,27] > 18.6, P < 0.001). The intakes of WT and T1r3 KO mice did not differ at 16–32% concentrations but exceeded (P < 0.05) those of the Trpm5 KO mice (F[2,27] > 13.6, P < 0.01). In terms of energy intake, the WT mice consumed more starch than T1r3 KO mice, which consumed more than Trpm5 KO mice at 4% and 8% concentrations (F[2,27] > 13.6, P < 0.001). Starch energy intake at 16–32% did not differ between WT and T1r3 KO groups, and both consumed more than Trpm5 KO group. In terms of preference, the T1r3 KO mice preferred starch over the vehicle at 1–32%, whereas the WT mice preferred it at 2–32%. In contrast, the Trpm5 KO mice failed to prefer starch at any concentration and tended to drink less starch than gum at 16% and 32% concentrations (Group × Concentration interaction, F[12,162] = 16.7, P < 0.001). The apparent avoidance of starch at high concentrations, however, was due in part to the starch + gum solution congealing at the tip of the sipper tube. This did not happen with the WT and T1r3 KO mice indicating that if the mice consistently consumed the concentrated starch solutions during the 24-h session, the starch did not congeal.
Figure 5.
Mean (±SE) starch + gum solution intake (top), percent starch preference over water (middle), and starch energy intake (bottom) in Trpm5 KO, T1r3 KO, and B6 WT mice during 24-h 2-bottle starch + gum versus gum solution in Tests 1 and 2. Gum solution intakes are not shown. Significant (P < 0.05) group differences at individual concentrations are denoted by * where all the groups differ from one another and by P where Trpm5 KO group differs both other groups.
Test 2
Starch versus gum.
In Test 2, the WT and T1r3 KO mice consumed similar amounts of starch and more than did the Trpm5 KO mice at all concentrations (11.1, 10.6, and. 4.8g/day, respectively; F[2,27] = 15.7, P < 0.001). Similarly, the WT and T1r3 KO mice consumed more starch energy than did the Trpm5 KO mice, and these differences were significant at 8–32% concentrations (Group × Concentration interaction, F[12,162] = 15.5, P < 0.001). In terms of preference, the WT and the T1r3 KO mice displayed near-total preferences for starch at all concentrations, whereas the Trpm5 KO mice failed to drink more starch than water (Group × Concentration interaction, F[12,162] = 6.1, P < 0.001).
A closer examination of the Trpm5 KO data revealed that 5 of 10 Trpm5 KO mice preferred starch during Test 2, whereas the remaining mice were indifferent or drank more water than starch. In an attempt to induce all the Trpm5 KO mice to prefer starch, the mice were given alternating 1-bottle access to 8% starch and gum on 4 consecutive days during which they consumed more starch than gum (8.4 g vs. 5.1g/day, t[9] = 2.4, P < 0.05). In a subsequent 2-day choice test, the Trpm5 KO group consumed significantly more 8% starch than gum (9.4g vs. 1.1g/day, t[9] = 3.2, P < 0.05) although 3 mice still failed to prefer the starch. All mice were then food restricted to 85–90% of their ad libitum body weight and given another four 1-bottle training days followed by a 2-bottle test with 8% starch and gum. The Trpm5 KO mice now consumed substantially more starch than gum in the 1-bottle training days (22.9 g vs. 5.2g/day, t[9] = 9.5, P < 0.001) and 2-day choice test (20.0g vs. 0.1g, t[9] = 26.7, P < 0.001). The mice were then returned to ad libitum chow and given another choice test during which all animals consumed substantially more 8% starch than gum (98% preference, 13.1 g vs. 0.2g/day, t[9] = 10.0, P < 0.001). Four days later, the Trpm5 KO mice were given a 2-day test with 0.5% starch versus gum, and all mice strongly preferred the 0.5% starch (97%, 9.5 g vs. 0.3g/day, t[9] = 11.4, P < 0.001].
Within-group comparisons showed that the WT mice increased (P < 0.05) their starch solution and percent intakes from Tests 1 to 2 at 0.5–16%, 0.5–4% concentrations, respectively (F[6,54] ≥ 38.0, P < 0.001). Similarly, the T1r3 KO mice increased (P < 0.05) their starch solution and percent intakes at 0.5–16%, 0.5–8%, respectively (F[6,54] ≥ 12.8, P < 0.001). In contrast, the Trpm5 KO mice did not increase their starch solution or percent intake from Tests 1 to 2 although, as noted above, they increased their 0.5% and 8% intakes after 1-bottle training.
General discussion
The T1r3 and Trpm5 signaling proteins are critical for the normal taste response to sugars and artificial sweeteners in mice (Damak et al. 2003; Zhang et al. 2003; Zhao et al. 2003; Damak et al. 2006; Treesukosol et al. 2011). We recently reported that T1r3 mice failed to display a normal preference response to sucrose solutions in 1-min choice tests, but in 24-h tests, they developed a significant preference and increased acceptance for concentrated sucrose solutions (Zukerman et al. 2009a). Furthermore, the T1r3 KO mice subsequently displayed significant preferences for dilute sucrose solutions to which they were initially indifferent. We attributed this experience-induced sucrose preference to the postoral learning effects, which was supported by our finding that T1r3 KO mice, like WT mice, learned to prefer a CS+ flavor paired with IG sucrose infusions. Trpm5 KO mice are also reported to display little or no licking response to sucrose in brief access tests but develop preferences for concentrated sugar solutions in 24-h 2-bottle tests (Zhang et al. 2003; Damak et al. 2006). As with T1r3 KO mice, the long-term sugar preference is attributed to postoral effects, which is supported by the IG glucose-conditioned CS+ preference observed in Trpm5 KO mice (Sclafani and Ackroff 2012b). This study extended the analysis of the acquired sugar preferences in sweet-ageusic T1r3 and Trpm5 KO mice by comparing their 24-h intake response to 3 monosaccharide sugars as well as their response to starch.
Glucose preference
Based on the ability of T1r3 KO mice to develop significant sucrose preferences in 24-h 2-bottle tests (Damak et al. 2003; Zhao et al. 2003; Zukerman et al. 2009a, 2009b), we predicted that they would also develop strong preferences for glucose in long-term tests. Experiment 1 revealed a 24-h preference profile for glucose in naïve T1r3 KO mice that was similar to that previously observed for sucrose (Zukerman et al. 2009a). The T1r3 KO mice were initially indifferent to dilute glucose solutions (0.5–4%) but strongly preferred concentrated solutions (8–32%), and then preferred 0.5–32% glucose solutions when offered them again in Test 2. It should be noted that an earlier study by Damak et al. (2006) reported near-normal 24-h glucose preferences in T1r3 KO mice, but these animals had prior experience with sucrose solutions. As the present findings demonstrate, prior experience with 1 sugar can significantly increase the preference for other sugars in KO mice.
The glucose preference response of the Trpm5 KO mice was similar to that of the T1r3 KO mice except that their glucose preference threshold was 16% in Test 1, and they displayed somewhat reduced intakes and preferences in Test 2. Conceivably, the Trpm5 KO mice may have a more severe sweet ageusia than T1r3 KO mice, which have the intact T1r2 component of the sweet receptor. Recent findings, however, revealed similar deficits in the licking response to glucose in T1r3 KO, T1r2 KO, and T1r2 + T1r3 double KO mice (Treesukosol et al. 2011). Alternatively, Trpm5 KO mice may be less responsive to the postoral conditioning effects of glucose. There is evidence for postoral sugar conditioning in both KO models, but their sensitivity to postoral sugar reward effects have not been directly compared (Sclafani and Ackroff 2012b).
Fructose preference
Although sugar-naïve T1r3 KO and Trpm5 KO mice developed strong preferences for glucose, they were indifferent to fructose up to 8% and avoided 16% and/or 32% concentrations. As previously noted, we expected the KO mice to be indifferent to fructose given their sweet taste deficit and the finding that WT mice do not prefer a flavor paired with IG infusions of 8% or 16% fructose (Sclafani and Ackroff 2012a). Together, these results indicate that the preference KO mice display for concentrated sucrose is due primarily to the postoral reward actions of the glucose released by sucrose digestion in the gut. The failure of naïve KO mice to display any preference for fructose also indicates that the small residual gustatory nerve response to sugars observed in T1r3 KO and Trpm5 KO mice does not, by itself, account for their preference for concentrated glucose or sucrose solutions (Damak et al. 2003; Zhao et al. 2003; Damak et al. 2006; Ohkuri et al. 2009). Note that the WT mice increased their preference for dilute fructose solutions from Test 1 to 2, which might suggest a postoral conditioning effect of the sugar. However, WT mice given repeated tests with ascending concentrations of saccharin were also observed to increase their preference from the first to second tests (Sclafani 2006). Saccharin does not have any known postoral conditioning effects so it appears that familiarization with a sweet taste per se is sufficient to enhance subsequent preference in WT mice.
The unexpected finding of Experiment 1 was that the T1r3 KO and Trpm5 KO Fructose groups avoided the 16–32% fructose solutions. We replicated this finding in naïve female and male T1r3 KO mice tested with 8–32% fructose solutions (Supplementary Figure 4). Conceivably, concentrated fructose solutions may have an off-taste to mice lacking T1r3 or Trpm5 sweet taste signaling proteins, but unpublished findings discount this notion. That is, we observed that naïve water-restricted T1r3 KO and Trpm5 KO mice licked similar amounts of fructose, glucose, and water at 16% and 32% sugar concentrations during 1-min 2-choice tests (unpublished data). Rather, it would appear that the concentrated fructose solutions had an aversive or “discomforting” postoral effect that caused the KO mice to avoid it relative to water. Note that the WT mice reduced their fructose preference from 97% to 79% as sugar concentration increased from 8% to 32%. At a still higher concentration (43.2%), rats were found to drink more water than fructose although more glucose than water in 24-h 2-bottle tests (percent intakes ~38% vs. 72%, respectively) (Cagan and Maller 1974). Prolonged access to concentrated fructose solutions may produce strong satiation signals due to rapid gastric emptying and slower intestinal absorption, relative to glucose (Moran 2009).
In marked contrast to the sugar-naïve KO mice, when the T1r3 KO and Trpm5 KO mice in the Glucose groups were offered fructose for the first time in Test 3, they did not avoid the 16–32% fructose solutions. Rather, they significantly preferred all fructose concentrations to water, although their fructose intakes were considerably below those of their glucose intakes in Test 2. Furthermore, the mice in the fructose KO groups, after developing a glucose preference in Test 3, also significantly preferred all fructose concentrations to water in Test 4. Thus, glucose experience had a profound influence on the fructose preference of KO mice whether it occurred before or after their first exposure to fructose. Clearly, the postoral actions of fructose do not prevent the T1r3 KO and Trpm5 KO mice from preferring this sugar in all situations.
Galactose preference
Unlike glucose and fructose, galactose has received relatively little attention in rodent taste studies. In mammals, galactose stimulates the chorda tympani nerve (CTN) less than fructose and glucose (Noma et al. 1971; Jakinovich Jr and Goldstein 1976). Consistent with these results, the B6 WT mice in this study displayed relatively low preferences for galactose compared with their preferences for glucose and fructose. Similar preference profiles were reported in rats (Richter and Campbell 1940). In contrast, the naïve T1r3 KO and Trpm5 KO mice showed stronger preferences for galactose than for fructose although not nearly as strong as their glucose preferences. Because the sugar preferences of the KO mice presumably reflect post oral rather than taste effects, the present findings indicate that galactose has a postoral reward effect in T1r3 KO and Trpm5 KO mice. In Test 1, both KO groups preferred 8% galactose to water, and in Test 2, the Trpm5 KO and T1r3 KO mice preferred the sugar at 4–8% and 2–16% concentrations, respectively. Nevertheless, the KO mice, like the WT mice, consumed much less galactose than glucose. In addition, all 3 groups drank more water than 32% galactose in Tests 1 and 2. This compares to the early report that rats avoided galactose at concentrations of 19% and higher (Richter and Campbell 1940). The reduced intake of concentrated galactose solutions by mice and rats presumably reflects their limited ability to metabolize this sugar (Berman et al. 1978; Solberg and Diamond 1987; Niewoehner and Neil 1992). The significant increase in water intake displayed by the WT mice and KO mice when drinking 16% and 32% galactose suggests galactosuria, that is, excretion of galactose or its metabolite in the urine, as observed in studies of mice and rats fed high-galactose diets (Rancour et al. 1979; Solberg and Diamond 1987). Yet, despite their avoidance of 32% galactose in Test 1, the WT mice and KO mice displayed increased intakes and/or preferences for 0.5–16% galactose in Test 2. This indicates that impaired metabolism of the concentrated galactose solutions did not condition an aversion (“dislike”) to the sugar’s sweet taste in the WT mice or non-sweet flavor elements in the KO mice. As previously discussed, animals may learn to limit their intake of poorly digested (e.g., lactose) or metabolized (e.g., galactose) sugars, but they do not acquire taste aversions because the sugars do not produce upper intestinal malaise (i.e., nausea) (Sclafani et al. 1999).
The sugar preference results obtained with the T1r3 KO and Trpm5 KO mice indicate that glucose and galactose, but not fructose, have rewarding postoral actions in mice. This is consistent with the findings obtained in a conditioned place preference study conducted with outbred mice (ddY strain) (Matsumura et al. 2010). The mice learned a preference for a test chamber (“place”) that was associated with a gastric gavage of 0.2 mL of 20% glucose or galactose, but not fructose, compared with an alternative chamber associated with a saline gavage. On the other hand, we recently reported that IG self-infusions of galactose failed to condition a flavor preference in WT mice (Sclafani and Ackroff 2012a). The IG galactose flavor conditioning results would appear to conflict with the galactose preferences displayed by the KO mice in Experiment 2. The apparent conflict may be explained by the differential galactose intakes observed in the present and IG studies. The KO and WT mice consumed as much as 9–13g/day of the 8% galactose solution in the 2-bottle tests, whereas in the IG study, the WT mice consumed about 23g/day of a net 8% galactose solution when drinking flavored water (the CS+) paired with matched infusions of IG 16% galactose. Intakes were high in the IG study because the mice were attracted to the palatable CS solutions that contained 0.2% saccharin, which may have induced the mice to self-infuse an excessive amount of galactose. The high galactose intakes, in turn, may have had metabolic effects that cancelled out any postoral reward actions of the sugar. According to this analysis, IG galactose may condition a CS+ flavor preference if the training paradigm induces lower galactose intakes, which we have confirmed in a recent study (unpublished data).
Starch preference
In addition to their extensively studied sugar preferences, mice also prefer complex carbohydrates including maltodextrin (e.g., polycose) and pure starch (Feigin et al. 1987; Bachmanov et al. 2001a; Sclafani et al. 2007). A previous study (Sclafani et al. 2007) from our laboratory demonstrated that Trpm5 KO mice fail to prefer polycose or starch (corn starch) at 0.5–4% concentrations. However, the Trpm5 KO mice developed strong preferences for 8–32% polycose solutions similar to the preferences they displayed for 8–32% glucose in Experiment 1. This study extended the analysis of starch preference by testing Trpm5 KO and T1r3 KO mice with 0.5–32% starch solutions. The 2 KO groups differed substantially in their starch preferences, which contrast with their similar sugar preference deficits. The T1r3 KO mice were similar to WT mice in increasing their starch preference as concentration increased in Test 1, and both groups displayed near-total starch preferences and elevated intakes in Test 2. On the other hand, the Trpm5 KO mice did not prefer starch at any concentration in Test 1. In Test 2, only half of the Trpm5 KO mice preferred starch to gum although with additional 1-bottle training with 8% starch eventually all the Trpm5 KO mice acquired a preference for starch even at the 0.5% concentration.
This and previous findings indicate that the T1r3 receptor component, although essential for normal sugar preferences, does not mediate starch or maltodextrin preferences in mice (Treesukosol et al. 2009; Zukerman et al. 2009a; Treesukosol et al. 2011). The Trpm5 channel, however, is required for starch, maltodextrin, and sugar preferences in naïve mice (Sclafani et al. 2007). Furthermore, Trpm5 KO mice displayed an impaired ability to develop a starch preference with experience. In 24-h 2-choice tests, Trpm5 mice acquired preferences for glucose, galactose, sucrose, and polycose at 4–8% concentrations (this study and Damak et al. 2006; Sclafani et al. 2007), but failed to prefer starch at 0.5–32% concentrations in Test 1. Only after 1-bottle training did all the Trpm5 KO mice prefer starch to the gum vehicle. The greatly impaired starch preference of Trpm5 KO mice may have occurred because deletion of the Trpm5 channel disrupts the orosensory detection of starch more than that of sugar or maltodextrin. Starch detection may have also been impaired because the mice were given the choice of the starch + gum solution versus a gum solution rather than plain water, which presumably reduced the discriminability of the 2 fluids. However, a subsequent experiment revealed that naïve Trpm5 KO mice did not prefer an 8% starch + gum solution to plain water, although they did so after 1-bottle starch training as in this study (unpublished data). Note that the naïve B6 WT mice displayed similar preference thresholds (2%) for starch, glucose, and fructose despite the fact that the starch + gum solution was paired with a gum solution. Furthermore, a previous conditioned aversion study revealed that rats have a lower detection threshold for starch (0.025%) than for sucrose (0.05%) (Ramirez 1991c). Little is known about how mice and rats detect starch at such low concentrations, but these findings imply a critical role for Trpm5 signaling in taste cells. The presence of amylase, the enzyme that digests starch, in some taste cells may be involved in the transduction process by which this complex carbohydrate activates the taste cells (Merigo et al. 2009).
Carbohydrate acceptance
There are multiple dimensions to the appetite for carbohydrates and other nutrients (Sclafani 1987; Spector and Glendinning 2009). Early 24-h 2-bottle studies of sweet ageusic KO mice focused exclusively on preference for sugar versus water, and absolute intake data were not reported (Damak et al. 2003; Zhao et al. 2003; Damak et al. 2006). However, as reported in this study and elsewhere (Sclafani et al. 2007; Zukerman et al. 2009a, 2009b), T1r3 KO and Trpm5 KO mice show deficits in both the relative intake (preference) and absolute intake (acceptance) of carbohydrate solutions, and these deficits can be dissociated. In the case of glucose, despite displaying near-total preferences for glucose in Test 2, the T1r3 KO and Trpm5 KO mice consumed significantly less glucose than did WT mice, particularly at the 8% concentration that stimulated maximal solution intakes. Similar results were reported with KO mice tested with concentrated sucrose and polycose solutions (Sclafani et al. 2007). There are several potential explanations for the disparate preference and acceptance displayed by the KO mice relative to the WT mice. First, previous IG conditioning studies with WT mice indicate that the postoral actions of sugar and polycose condition strong preferences for CS+ flavors, but inherently preferred flavors (e.g., saccharin-sweetened grape flavor), produce higher intakes than initially unpreferred flavors (e.g., unsweetened grape flavor) (Sclafani and Glendinning 2003; Sclafani 2007; Sclafani et al. 2010). Thus, KO mice may acquire strong preferences for glucose and sucrose based on a learned association between the sugars’ residual flavor cues (odor and texture) and postoral nutritive feedback, but their intakes are submaximal because the KO mice lack the inherent attraction to the sugars’ sweet taste. Another, non-mutually exclusive explanation is that the KO mice have an impaired ability to absorb and/or metabolize concentrated carbohydrate sources because these processes involve the action of T1r3 and/or Trpm5 in the intestinal tract and pancreas (Jang et al. 2007; Margolskee et al. 2007; Brixel et al. 2010; Geraedts et al. 2012; Kyriazis et al. 2012). However, this does not account for the reduced consumption of 8% glucose by the KO mice given that they were able to consume more sugar (solute) when offered the 16–32% solutions. Also, experienced T1r3 KO mice consumed as much 8% starch (Experiment 3) and 8% polycose (Zukerman et al. 2009a) as did WT mice, so glucose absorption and metabolism does not appear to be a limiting factor for T1r3 KO mice (see also Glendinning et al. 2012). Starch and polycose differ from glucose, however, in that T1r3 KO mice show little or no taste deficits for these non-sweet carbohydrates (Experiment 3) (Treesukosol et al. 2009; Zukerman et al. 2009a; Treesukosol et al. 2011), which supports the idea that sweet ageusia accounts for the reduced glucose and sucrose acceptance displayed by T1r3 KO mice. A third possibility is that the sweet ageusia of T1r3 KO and Trpm5 KO mice disrupts the normal sweet taste elicited cephalic phase digestive responses (CPDR), which contribute to the normal postoral processing of sugars (Smeets et al. 2010). There is as yet no evidence that starch or polycose taste elicits unlearned (or learned) CPDR in rodents, so the role of CPDR in the acceptance of these carbohydrates by sweet ageusic KO or normal WT mice is not known (Sclafani 1991; Tonosaki 2007).
Given the reduced glucose acceptance displayed by the T1r3 KO and Trpm5 KO mice, it is not surprising that the KO mice also underconsumed fructose relative to the WT mice. What is notable about the fructose data, however, is that after experience with glucose, the KO mice displayed near-total preferences for fructose but, unlike WT mice, did not increase their fructose intake as concentration increased. Apparently, the conditioned preference for the non-sweet flavor stimuli provided by fructose was insufficient to stimulate intake in KO mice as sugar concentration increased. The increased fructose acceptance by the WT mice can be attributed solely to the sweet taste of the sugar given that B6 mice show no preference or increased acceptance to a CS+ flavor paired with IG fructose infusions (Sclafani and Ackroff 2012a).
Unlike glucose and fructose, the KO mice displayed normal or even enhanced acceptance of galactose solutions in Test 2 except at the highest concentration. This may have occurred in part because the minimally sweet taste of galactose to the WT mice limited their appetite for this sugar at 0.5–16% concentrations. Why the KO mice underconsumed 32% galactose relative to WT mice in Test 2 is not known; both oral and postoral factors may have contributed to their reduced intake at this concentration.
In addition to the sugar intake differences observed among the WT, T1r3 KO, and Trpm5 KO mice, this study also revealed within-strain differences in the intakes of the 3 sugars. One consistent finding is that all 3 strains consumed substantially more glucose than fructose or galactose whether expressed as solution or energy intake (Supplementary Figures 1–3). The KO mice also consumed more galactose than fructose, whereas the WT mice consumed more fructose at intermediate concentrations (2–8%) but not at higher concentrations. Previous studies also reported higher glucose than fructose intakes in B6 WT mice (Bachmanov and Beauchamp 2008; Glendinning et al. 2010). This differential intake cannot be attributed to glucose having a sweeter taste given that 1) fructose produces stronger gustatory nerve responses than glucose in WT mice (Damak et al. 2003) (see Supplementary Figure 5); 2) WT mice consumed more glucose than fructose when the sugars were self-administered by IG infusions paired with flavored saccharin solutions (Sclafani and Ackroff 2012a); and 3) sweet-ageusic T1r3 KO and Trpm5 KO mice consume more glucose than fructose (Experiment 1). Instead, glucose overconsumption appears to be driven by the postoral appetite stimulatory actions of this sugar (Zukerman et al. 2011; Sclafani and Ackroff 2012b). The finding that T1r3 KO and Trpm5 KO mice consumed more galactose than fructose is a novel finding indicating that galactose has a postoral stimulatory action on sugar intake but one much less potent than that of glucose.
Experiential effects on sugar preference
This study along with previous work (Zhao et al. 2003; Damak et al. 2006; Zukerman et al. 2009a, 2009b) revealed profound experiential effects on the carbohydrate preferences of taste-impaired T1r3 KO and Trpm5 KO mice. In particular, naive KO mice that were indifferent to dilute sucrose, glucose, polycose, and/or starch solutions displayed near-total preferences for these solutions following 24-h exposure to concentrated carbohydrate solutions. As discussed above, this experience-induced preference for simple and complex carbohydrates in KO mice can be explained as a conditioned response to non-taste flavor cues reinforced by the postoral actions of glucose or galactose. It is also possible that the weak residual taste nerve responses to concentrated sugars displayed by T1r3 KO and Trpm5 KO mice contribute to the conditioned preference. However, some data indicate that a residual nerve response to sugar is not essential for experience-induced preference. Unlike single T1r3 KO and T1r2 KO mice, T1r2 + T1r3 KO double KO mice showed no nerve response to sucrose (Zhao et al. 2003), yet double KO mice, like single KO mice preferred sucrose to water after 24-h experience with the sugar (Zhao et al. 2003; Zhao 2005). The present findings further indicate that an experience-induced preference for 1 sugar generalizes to another sugar. Thus, glucose-experienced T1r3 KO and Trpm5 KO mice displayed near-total preferences for fructose, in contrast to naïve KO mice that failed to prefer fructose at any concentration tested. Conceivably, 24-h experience with concentrated glucose solutions may enhance the sugar-sensitivity of gustatory nerves in KO mice and thereby increase their sugar preferences in subsequent tests. However, we observed no differences in the CTN responses to glucose or fructose in naïve versus glucose-experienced T1r3 KO mice (Supplementary Figure 5). Similarly, unpublished data revealed no difference in the CTN response to sucrose in sucrose-naïve and experienced T1r2 + T1r3 double KO mice (Zhao 2005). Thus, the available evidence indicates that experience-induced sugar preferences of KO mice are mediated by non-gustatory stimuli (odor and texture) (Zukerman et al. 2009b).
In contrast to the present findings, de Araujo and coworkers (2008) reported that sucrose or glucose experience did not induce sugar preferences in Trpm5 KO mice, although the mice learned to prefer a sipper tube side paired with sugar (Ren et al. 2010). de Araujo et al. (2008) proposed that differences in the residual taste sensitivities of the Trpm5 KO mice used in their laboratory and ours might account for the discrepant results. However, as noted above, experience-induced sucrose preferences were observed in T1r2 + T1r3 double KO mice with no residual taste nerve responses (Zhao 2005). Rather, procedural differences might be a more important factor. In particular, de Araujo et al. (2008) measured sugar preferences using water- and food-restricted Trpm5 KO mice tested 10min/day, whereas we used ad libitum-fed mice tested 24h/day. It may be that longer test sessions are needed for taste-impaired KO mice to differentiate between the orosensory features of a sugar solution versus water. It is also possible that postoral effects influence the fluid choice of KO mice during long test sessions; that is, the KO mice may drink more from the sugar bottle as they experience the postoral reward effects of the sugar. However, this does not readily explain the significant preferences displayed by glucose-experienced Trpm5 KO mice for fructose solutions, which lack postoral reward actions (Sclafani and Ackroff 2012a) or for very dilute (0.5%) glucose solutions that presumably have minimal postoral effects (Zukerman et al. 2013). Further research is needed that directly compares experiential effects on short- and long-term sugar preferences in the same animals.
Conclusions
The importance of sweet taste signaling to sugar appetite is well established, and the sugar preference and acceptance deficits displayed by sweet-ageusic KO mice provide compelling confirmatory evidence. At the same time, the ability of the KO mice to develop preferences for concentrated glucose, sucrose, and galactose solutions demonstrates that there is more to sugar appetite than sweet taste. A postoral contribution to sugar appetite has been demonstrated by many studies showing that IG sugar infusions condition flavor preferences and stimulate intake, a process referred to as appetition to distinguish it from the intake satiating effects of sugars (Sclafani 2012; Sclafani and Ackroff 2012b). Although T1r3 and Trpm5 are critical to the appetite-stimulating effect of sugars in the mouth, gut T1r3 and Trpm5 appear to have little or no role in postoral sugar appetition as indicated by the ability of T1r3 KO and Trpm5 KO mice to develop preferences for orally consumed or IG-infused sugars (Sclafani and Ackroff 2012b). Rather, gut glucose/galactose sensors, such as SGLT1, and hepatic-portal glucose sensors are implicated in sugar appetition, as well as that induced by complex carbohydrates (maltodextrin and starch) that are digested to glucose (Sclafani 2012; Sclafani and Ackroff 2012b).
In addition to sugars, rodents and some other species are attracted to maltodextrin and starch, which early behavioral and electrophysiological findings indicated is mediated by receptors other than the sweet receptor (Sclafani 2004). The normal preference for starch displayed by T1r3 KO mice (Experiment 3) and for polycose by T1r2 KO and T1r3 KO mice provide compelling new evidence for this view (Treesukosol et al. 2009; Zukerman et al. 2009a; Treesukosol et al. 2011; Glendinning et al. 2012). In addition, the starch preference deficit displayed by Trpm5 KO mice (Experiment 3) and the differential starch versus polycose deficits displayed by gustducin KO mice (Sclafani et al. 2007) are consistent with the view that separate oral receptors mediate maltodextrin and starch tastes (Ramirez 1991b; Sclafani 2004). The identities of the putative maltodextrin and starch taste receptors in rodents remain to be established. Recent reports indicate that humans orally detect maltodextrins, as evidenced by improved motor performance, although they report no specific taste sensation (Jeukendrup and Chambers 2010; Gant et al. 2010), which suggests that, like rodents, humans may have taste receptors for complex carbohydrates.
Supplementary material
Supplementary Figures 1–5 can be found at http://www.chemse.oxfordjournals.org/
Funding
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases [grant DK-031135 to A.S.] and National Institute of Deafness and Other Communications Disorders [grants DC-03055 and DC-03155 to R.F.M.].
Supplementary Material
Acknowledgement
The authors thank Karen Ackroff for her helpful comments on this paper.
References
- Ackroff K, Sclafani A, Axen KV. 1997. Diabetic rats prefer glucose-paired flavors over fructose-paired flavors. Appetite. 28(1):73–83 [DOI] [PubMed] [Google Scholar]
- Ackroff K, Touzani K, Peets TK, Sclafani A. 2001. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol Behav. 72(5):691–703 [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Beauchamp GK. 2008. Amino acid and carbohydrate preferences in C57BL/6ByJ and 129P3/J mice. Physiol Behav. 93(1-2):37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. 2001a. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol Behav. 72(4):603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. 2001b. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 26(7):905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman WF, Rogers SR, Bautista JO, Segal S. 1978. Galactose and glucose metabolism in the isolated perfused suckling and adult rat liver. Metabolism. 27(12):1721–1731 [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. 2008. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 7(1):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasser SM, Norman MB, Lemon CH. 2010. T1r3 taste receptor involvement in gustatory neural responses to ethanol and oral ethanol preference. Physiol Genomics. 41 232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brixel LR, Monteilh-Zoller MK, Ingenbrandt CS, Fleig A, Penner R, Enklaar T, Zabel BU, Prawitt D. 2010. TRPM5 regulates glucose-stimulated insulin secretion. Pflugers Arch. 460(1):69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagan RH, Maller O. 1974. Taste of sugars: brief exposure single-stimulus behavioral method. J Comp Physiol Psychol. 87(1):47–55 [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. 2010. The cell biology of taste. J Cell Biol. 190(3):285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, et al. 2006. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 31(3):253–264 [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. 2003. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 301(5634):850–853 [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. 2008. Food reward in the absence of taste receptor signaling. Neuron. 57(6):930–941 [DOI] [PubMed] [Google Scholar]
- Feigin MB, Sclafani A, Sunday SR. 1987. Species differences in polysaccharide and sugar taste preferences. Neurosci Biobehav Rev. 11(2):231–240 [DOI] [PubMed] [Google Scholar]
- Gant N, Stinear CM, Byblow WD. 2010. Carbohydrate in the mouth immediately facilitates motor output. Brain Res. 1350 151–158 [DOI] [PubMed] [Google Scholar]
- Geraedts MC, Takahashi T, Vigues S, Markwardt ML, Nkobena A, Cockerham RE, Hajnal A, Dotson CD, Rizzo MA, Munger SD. 2012. Transformation of postingestive glucose responses after deletion of sweet taste receptor subunits or gastric bypass surgery. Am J Physiol Endocrinol Metab. 303(4):E464–E474 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Glendinning JI, Beltran F, Benton L, Cheng S, Gieseke J, Gillman J, Spain HN. 2010. Taste does not determine daily intake of dilute sugar solutions in mice. Am J Physiol Regul Integr Comp Physiol. 299(5):R1333–R1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Gillman J, Zamer H, Margolskee RF, Sclafani A. 2012. Contribution of taste to carbohydrate-induced overeating and obesity in mice. 107:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakinovich W, Jr, Goldstein IJ. 1976. Stimulation of the gerbil’s gustatory receptors by monosaccharides. Brain Res. 110(3):491–504 [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. 2007. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 104(38):15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeukendrup AE, Chambers ES. 2010. Oral carbohydrate sensing and exercise performance. Curr Opin Clin Nutr Metab Care. 13(4):447–451 [DOI] [PubMed] [Google Scholar]
- Kyriazis GA, Soundarapandian MM, Tyrberg B. 2012. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci USA. 109(8):E524–E532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush IE. 1989. The genetics of tasting in mice. VI. Saccharin, acesulfame, dulcin and sucrose. Genet Res. 53(2):95–99 [DOI] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. 2007. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 104(38):15075–15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura S, Yoneda T, Aki S, Eguchi A, Manabe Y, Tsuzuki S, Inoue K, Fushiki T. 2010. Intragastric infusion of glucose enhances the rewarding effect of sorbitol fatty acid ester ingestion as measured by conditioned place preference in mice. Physiol Behav. 99(4):509–514 [DOI] [PubMed] [Google Scholar]
- Merigo F, Benati D, Cecchini MP, Cristofoletti M, Osculati F, Sbarbati A. 2009. Amylase expression in taste receptor cells of rat circumvallate papillae. Cell Tissue Res. 336(3):411–421 [DOI] [PubMed] [Google Scholar]
- Moran TH. 2009. Fructose and satiety. J Nutr. 139(6):1253S–1256S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewoehner CB, Neil B. 1992. Mechanism of delayed hepatic glycogen synthesis after an oral galactose load vs. an oral glucose load in adult rats. Am J Physiol. 263(1 Pt 1):E42–E49 [DOI] [PubMed] [Google Scholar]
- Noma A, Goto J, Sato M. 1971. The relative taste effectiveness of various sugars and sugar alcohols for the rat. Kumamoto Med J. 24(1):1–9 [PubMed] [Google Scholar]
- Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. 2009. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol. 296(4):R960–R971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez I. 1991a. Chemoreception for an insoluble nonvolatile substance: starch taste? Am J Physiol. 260(1 Pt 2):R192–R199 [DOI] [PubMed] [Google Scholar]
- Ramirez I. 1991b. Does starch taste like polycose? Physiol Behav. 50(2):389–392 [DOI] [PubMed] [Google Scholar]
- Ramirez I. 1991c. Thresholds for starch and polycose are lower than for sucrose in rats. Physiol Behav. 50(4):699–703 [DOI] [PubMed] [Google Scholar]
- Ramirez I. 1994. Glucose polymer taste is not unitary for rats. Physiol Behav. 55(2):355–360 [DOI] [PubMed] [Google Scholar]
- Rancour NJ, Hawkins ED, Wells WW. 1979. Galactose oxidation in liver. Arch Biochem Biophys. 193(1):232–241 [DOI] [PubMed] [Google Scholar]
- Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. 2010. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 30(23):8012–8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP, Campbell KH. 1940. Taste thresholds and taste preferences of rats for five common sugars. J Nutr. 20 31–46 [Google Scholar]
- Sclafani A. 1987. Carbohydrate taste, appetite, and obesity: an overview. Neurosci Biobehav Rev. 11(2):131–153 [PubMed] [Google Scholar]
- Sclafani A. 1991. Starch and sugar tastes in rodents: an update. Brain Res Bull. 27(3-4):383–386 [DOI] [PubMed] [Google Scholar]
- Sclafani A. 2004. The sixth taste? Appetite. 43(1):1–3 [DOI] [PubMed] [Google Scholar]
- Sclafani A. 2006. Enhanced sucrose and polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiol Behav. 87(4):745–756 [DOI] [PubMed] [Google Scholar]
- Sclafani A. 2007. Fat and sugar flavor preference and acceptance in C57BL/6J and 129 mice: experience attenuates strain differences. Physiol Behav. 90(4):602–611 [DOI] [PubMed] [Google Scholar]
- Sclafani A. Forthcoming 2012. Gut-brain nutrient signaling: appetition vs. satiation. Appetite. http://dx.doi.org/10.1016/j.appet.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. 2012a. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav. 106(4):457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. 2012b. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 302(10):R1119–R1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. 1993. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol. 265(2 Pt 2):R320–R325 [DOI] [PubMed] [Google Scholar]
- Sclafani A, Fanizza LJ, Azzara AV. 1999. Conditioned flavor avoidance, preference, and indifference produced by intragastric infusions of galactose, glucose, and fructose in rats. Physiol Behav. 67(2):227–234 [DOI] [PubMed] [Google Scholar]
- Sclafani A, Glass DS, Margolskee RF, Glendinning JI. 2010. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 299(6):R1643–R1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Glendinning JI. 2003. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav. 79(4-5):783–788 [DOI] [PubMed] [Google Scholar]
- Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. 2007. Fat and carbohydrate preferences in mice: the contribution of alpha-gustducin and Trpm5 taste-signaling proteins. Am J Physiol Regul Integr Comp Physiol. 293(4):R1504–R1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets PA, Erkner A, de Graaf C. 2010. Cephalic phase responses and appetite. Nutr Rev. 68(11):643–655 [DOI] [PubMed] [Google Scholar]
- Solberg DH, Diamond JM. 1987. Comparison of different dietary sugars as inducers of intestinal sugar transporters. Am J Physiol. 252(4 Pt 1):G574–G584 [DOI] [PubMed] [Google Scholar]
- Spector AC, Glendinning JI. 2009. Linking peripheral taste processes to behavior. Curr Opin Neurobiol. 19(4):370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonosaki K, Hori Y, Shimizu Y, Tonosaki K. 2007. Relationships between insulin release and taste. Biomed Res. 28(2):79–83 [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Blonde GD, Spector AC. 2009. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol. 296(4):R855–R865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Smith KR, Spector AC. 2011. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. J Neurosci. 31(38):13527–13534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ. 1962. Statistical principles in experimental design. New York:McGraw Hill; [Google Scholar]
- Wright EM, Loo DD, Hirayama BA. 2011. Biology of human sodium glucose transporters. Physiol Rev. 91(2):733–794 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. 2003. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 112(3):293–301 [DOI] [PubMed] [Google Scholar]
- Zhao GQ. 2005. Studies of the mammalian sweet and umami taste receptors [dissertation]. [San Diego: ]: University of California; [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. 2003. The receptors for mammalian sweet and umami taste. Cell. 115(3):255–266 [DOI] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, Sclafani A. 2011. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol. 301(6):R1635–R1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, Sclafani A. 2013. Post-oral glucose stimulation of intake and conditioned flavor preference in C57BL/6J mice: a concentration-response study. Physiol Behav. 109 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. 2009a. T1R3 taste receptor is critical for sucrose but not polycose taste. Am J Physiol Regul Integr Comp Physiol. 296(4):R866–R876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Touzani K, Margolskee RF, Sclafani A. 2009b. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses. 34(8):685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.