Abstract
Acute kidney injury is common in critically ill children and renal replacement therapies provide a life saving therapy to a subset of these children. However, there is no Food and Drug Administration approved device to provide pediatric continuous renal replacement therapy (CRRT). Consequently, clinicians adapt approved adult CRRT devices for use in children due to lack of safer alternatives. Complications occur using adult CRRT devices in children due to inaccurate fluid balance (FB) between the volumes of ultrafiltrate (UF) removed and replacement fluid (RF) delivered. We demonstrate the design and validation of a pediatric fluid management system for obtaining accurate instantaneous and cumulative FB. Fluid transport was achieved via multiple novel pulsatile diaphragm pumps. The conservation of volume principle leveraging the physical property of fluid incompressibility along with mechanical coupling via a crankshaft was used for FB. Accuracy testing was conducted in vitro for 8-hour long continuous operation of the coupled UF and RF pumps. The mean cumulative FB error was <1% across filtration flows from 300 mL/hour to 3000 mL/hour. This approach of FB control in a pediatric specific CRRT device would represent a significant accuracy improvement over currently used clinical implementations.
Keywords: continuous renal replacement therapy, pediatric, acute kidney injury
Introduction
Acute kidney injury (AKI) is commonly seen in critically ill children, the origins of which may be traced to a wide range of conditions such as inborn errors of metabolism, sepsis, congenital heart defects, bone marrow and organ transplantation, and to a lesser extent from multiple organ dysfunction syndrome (MODS).1 In addition, children requiring extracorporeal membrane oxygenation (ECMO) during cardiopulmonary bypass2 or due to respiratory failure may have renal insufficiency, renal failure, and edema.3,4 The introduction of continuous hemofiltration methods for providing extracorporeal renal function, first conceptualized by Kramer et al. over 30 years ago,5 has led to extensive research on both modalities of therapy and design of devices for providing continuous renal replacement therapy (CRRT) for the care of AKI patients. Substantial advancements in the technology and increasing clinical evidence of the reduction in mortality6,4 have led to CRRT emerging as the preferred method of AKI treatment in many pediatric critical care units in the United States.7 However, the specific modalities of CRRT used in pediatric patients are still largely debated and somewhat institution-biased.8,9,10
The challenges with pediatric CRRT, as compared to adult CRRT, primarily stem from the developmental and physiological variability associated with neonates, infants, children and adolescents. The extracorporeal blood volume used in current adult CRRT devices, exceeds 15% of the total blood volume in many children, posing a significant health hazard to the smaller patients due to risks associated with clotting, hypotension, hypothermia and transfusion complications.8 Additionally, the FB between the removed ultrafiltrate (UF) and infused replacement fluid (RF) is of critical importance in pediatric patients.11 Errors in FB accumulate quickly due to the continuous nature of this therapy, and the smaller pediatric patient is more rapidly clinically symptomatic from the resultant fluid overload or dehydration. Commercial devices designed for adults have been noted to provide significantly inaccurate FB and report erroneous values of the fluid removed,9 and are thus not ideal for use in the pediatric population.
As an example of the safety concerns in using commercial CRRT devices in children, the United States Food and Drug Administration (FDA) published a public health notification in 2005 for class I recall of the Gambro Prisma® CRRT device.12 In the 2006 update to the above notification, the FDA suggested that the errors in FB during usage of the device could be as high as ±60 ml/hour. It must be noted that patient injury in the instances described in the recall occurred not entirely due to equipment failure, but also because operators failed to respond appropriately to alarms activated by the Prisma system. It is important to stress that the cumulative effect of even minor levels of fluid imbalance is hazardous to neonatal and pediatric patients due to their relatively small sizes. Additionally, it was reported that 9 deaths and 11 serious injuries occurred as a result of the excessive fluid removal problem. Following the recall, part of the corrective action mandated by the FDA involved affixing a warning label informing the user that the CRRT device was not approved for use on patients weighing less than 20 kg.
As there is currently no FDA-approved CRRT device that has been designed specifically for treatment of pediatric AKI patients, this decision puts many pediatric physicians and hospitals in a difficult position. Many continue to use these commercially available adult CRRT devices due to lack of a safer pediatric-specific alternative. The work presented herein addresses this challenging and urgent clinical need via the design and development of a novel fluid management system for a pediatric Kidney Injury and Dysfunction Support (KIDS)–CRRT device. The following sections of the paper describe the device assembly, fluid management mechanism, circuit operation, and in vitro experimental results validating the accurate FB achieved from the device.
Methods
Overall device design
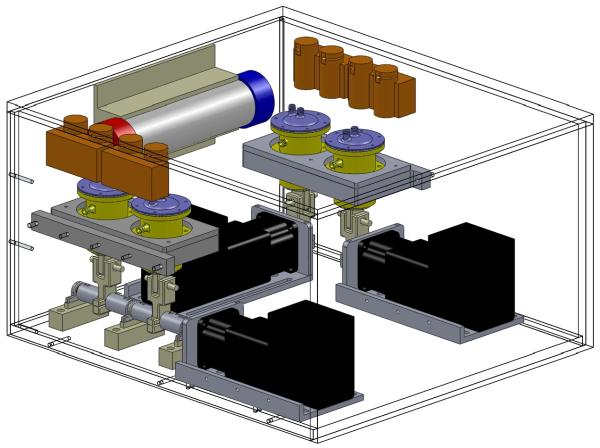
Fig. 1 shows a schematic representation of the final KIDS-CRRT prototype design. Fluid transport was achieved using pulsatile diaphragm pumps with a working stroke volume (SV) of approximately 7 mL. Pump flow was achieved by periodic pressure on a 1.6 mm thick deformable membrane (natural gum rubber, shore hardness 40A) bounded along its circumference by a rigid dome-shaped lid with two ports for the entry and exit of fluid. Hydraulic fluid pressure was limited to 50 mm Hg. The assembly of the device consisted of 3 individual pumps for transporting blood, UF and RF across the circuit. Negative or positive FB can be provided by the use of a 4th offset pump that adds or removes RF prior to mixing into the purified bloodstream. Unidirectional flow through the circuit was obtained via the use of electronically controlled solenoid pinch valves at the input and output of every pump. The assembly for implementation of CVVH is shown in Fig. 1, however the modular pump design can be adapted to simply add an additional dialysate pump (not shown) to the circuit for providing counter-current diffusive (CVVHD) modality. In keeping with the modular approach, the disposables are designed to allow any size, type, or brand hemofilter to be used with our device to ensure appropriate match between equipment and patient needs. All components above the level of the piston (Fig 2), including the rolling diaphragm pump, deforming bladder, lid, and tubing are intended for one time use and disposable.
Figure 1.
The KIDS-CRRT device for pediatric continuous renal replacement therapy.
Figure 2.
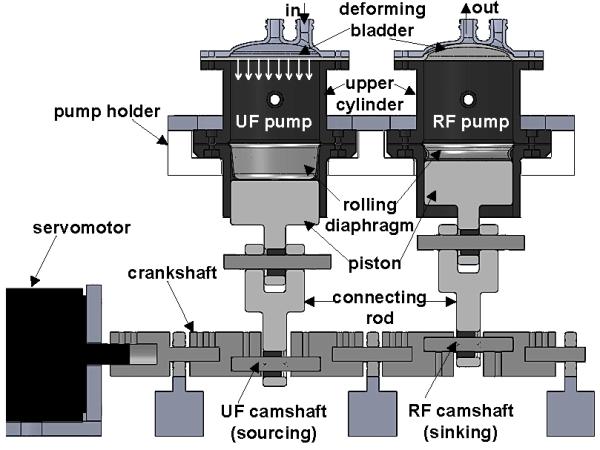
Schematic showing the conservation of volume fluid balance (FB) method implemented via crankshaft-based mechanical coupling of ultrafiltrate (UF) and replacement fluid (RF) pumps. The UF pump is shown in sourcing position and RF in sinking position, with the inflow/outflow ports indicated. The next half of the pumping cycle results in UF sinking and RF sourcing. An approximate indication of the position of the deforming bladder is shown for both pumps, along with the direction of UF pump bladder motion.
Fluid management system
The fluid management principle investigated here exploits the physical property of incompressibility seen across all fluid media used in CRRT. As such, two identically constructed positive displacement pumps containing equal SVs of incompressible fluids should provide equal volumetric outputs when subject to the same mechanical effort. A similar method was validated for CRRT on ECMO using a syringe-pump system,13 however that design was not used due to a desired lower working fluid volume and excessive heat generation.
The UF and RF pumps are coupled mechanically via a crankshaft to an Ethernet/internet protocol servomotor (Lexium® 32, Schneider Electric SA, France) that drives both pumps in tandem (Fig 2). The servomotor shaft encoder controls via a programmable logic controller (PLC) solenoid-operated pinch valves (Bio-Chem Valve Inc., NJ, USA) based on the angular position of the motor shaft to ensure unidirectional flow. The camshaft of each pump is offset in phase by 180 degrees. The UF/RF pumps are identical in construction, function, and hydraulic fluid volumes. The mechanical coupling forces the volumetric input and output through each bladder compartment to be equal, and precise control of FB is thus achieved.
Circuit operation
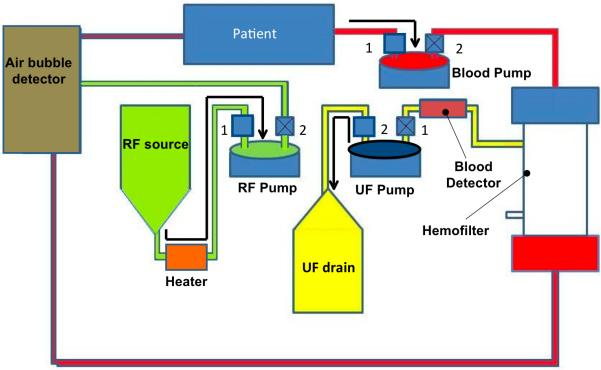
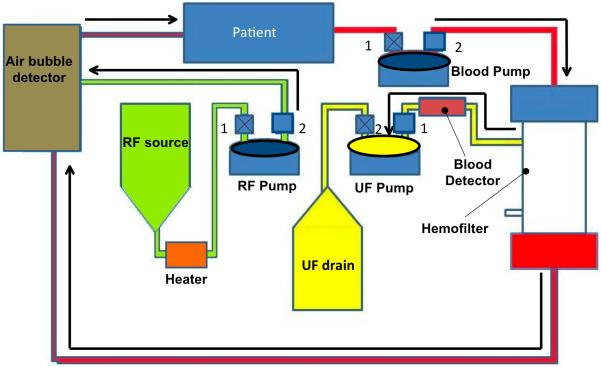
The functioning of the KIDS-CRRT circuit occurs in two cycles (Fig. 3) similar to an automobile internal combustion engine, thus effectively generating a pulsatile flow through the circuit. At any pump in the circuit during any point of time, only 1 pinch valve (for either input or output) is open to prohibit mixing of inflow and outflow. The 1st cycle (top panel of Fig. 3) starts with the filling of blood pump from the source (patient), during which the UF pump is expelling fluid into the drain bag and the RF pump is filling from the replacement fluid source. The 2nd cycle (bottom panel of Fig. 3) shows the 2nd cycle where the blood pump is ejecting the blood volume obtained in the 1st cycle into the inflow port of the hemofilter, while the UF pump is filling from extracting the ultrafiltrate produced by the hemofilter.
Figure 3.
Schematic of the cyclic operation of KIDS CRRT device comprising of two stages. For each pump, 1 and 2 are used to denote the inlet and outlet pinch valves, respectively. Valves with an X are in the closed position, while valves without an X are in the open position. The top panel (a) shows the 1st cycle of events that include filling of blood pump (valve 1 open, valve 2 closed), ejection of UF to drain bag (valve 2 open, valve 1 closed) and filling of RF pump from the RF source (valve 1 open, valve 2 closed). The bottom panel (b) shows the subsequent ejection cycle of blood pump (valve 1 closed, valve 2 open), ejection of RF to purified blood coming from the hemofilter (valve 1 closed, valve 2 open) and filling of the UF pump (valve 1 open, valve 2 closed). Arrows are used to indicate the direction of flow within the circuit. In each part of the cycle, the pumps that are in ejection stage are highlighted with an oval ellipse with bold outline.
A blood detection alarm is built into the pathway of UF fluid for safety. The RF pump simultaneously expels an equal amount of replacement fluid into the purified blood stream that is emanating from the exit port of the hemofilter. The mixture is passed through a bubble detector and warmed to body temperature before returning to the patient. As configured in Figure 3, the system provides for only an even fluid balance. However, the additional offset pump (not shown in Fig. 3) included in the circuit can either pump or remove replacement fluid ejected by the RF pump prior to mixing with the post-hemofilter blood stream.
Anticoagulation for the CRRT circuit, if needed, is provided by bedside IV pumps delivering anticoagulant solution to a standard luer lock connection on the pre-treatment limb of the disposables. The disposables also contain a port for anticoagulation monitoring and a port on the post-treatment limb for anticoagulation reversal and may be used with either heparin or citrate based anticoagulation protocols. A separate anticoagulation system is not built into the KIDS device and will be the focus of future development efforts. To detect failure of pinch valves during device operation, an indirect method is available using detection of excessive servomotor drive current. This method can be simultaneously used to monitor and detect occlusions in the flow or twisted tubing. The KIDS device does not have any inbuilt mechanism for detecting kinking of the central venous line and thus does not offer any benefit over current CRRT devices on this matter.
Validation of fluid management system
The in vitro validation experiments used water as the fluid medium for UF and RF pumps. Two different validation tests were conducted on the prototype. The first set of measurements consisted of acquiring volumetric readings for UF and RF pumps in standalone operation every 15 minutes by measuring the weight of fluids on a scale precise to 0.1 grams for 8 hours of continuous device operation. Cumulative pump and FB errors in the stand-alone operation were calculated using the relations below, where Vprogram is the expected volume output from programmed input, and Vpump is the measured volume pump output.
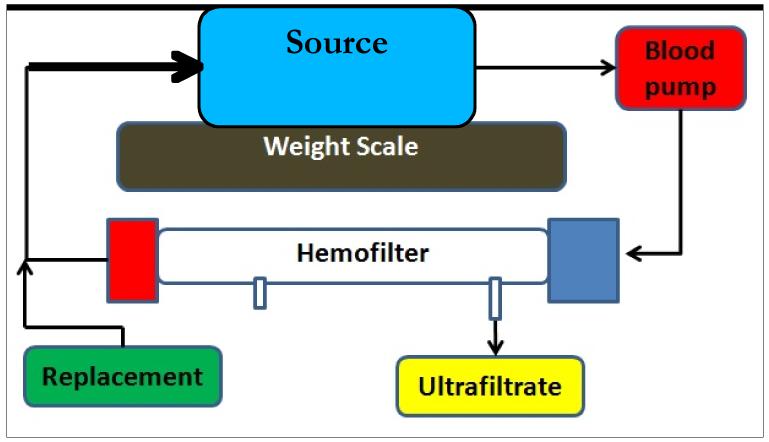
The second set of experiments for in vitro validation was conducted simulating the closed-loop CRRT circuit operation of the KIDS device (Fig. 4). A Renaflo II HF 700 hemofilter (MEDIVATORS, Minneapolis, MN, USA) with priming volume of 58 mL was used in this setup. For this phase of testing, water was used as the fluid medium throughout the circuit. Though this represents a simplification for the blood portion of the circuit, the objective of these experiments were to simply characterize the mechanical performance of the fluid management system without including coagulation related complications from using blood in the extracorporeal circuit. The source container of water was placed on a weight scale precise to 0.1 grams. All pumps were primed in the same manner. The initial weight of the source container was noted at the start of the experiment, and the device was allowed to run continuously for 8 hours. At the end of the test, the weight of the source was measured and the difference from the initial weight was determined. The output error was calculated as the ratio of the difference between the final volume and the initial volume to expected volumetric output at the end of 8 hours based on the programmed flow rate of UF/RF pumps.
Figure 4.
Experimental setup used for accuracy measurement of the fluid management system in the confines of a closed-loop CRRT circuit. The arrows indicate the flow direction within the circuit. Note that a common source was used for source of RF and sink of UF.
Flow control implementation
A common issue amongst positive displacement pumps is their dependence on driving pressure head. Changes in driving pressure affect the output SV of the pump, causing inaccurate fluid delivery. In the context of FB within CRRT, RF and UF working pressures are different and vary continuously during clinical application due to catheter and membrane dynamics. Thus, the pressure dependence of the pump flow characteristics is a non-trivial problem that needs to be addressed for obtaining accurate fluid management in CRRT. A novel solution for flow control using the valve timing was proposed for use in KIDS CRRT device, as illustrated in the crank circle diagrams in Fig. 5. As described above, solenoid valves were used in the KIDS device for ensuring unidirectional flow. In the baseline operation of the pumps, the inflow valve opens when the piston reaches the maximum height, referred here as top dead center (TDC), which is equivalent to minimum volume within the bladder compartment of the pump. The outflow valve closes when the inflow valve opens and vice versa. The outflow valve opens when the piston has reached the minimum height measured from the device base, referred here as bottom dead center (BDC). The opening and closing positions of the valves can thus be adjusted by varying the relative position of the crankshaft with respect to TDC and BDC.
Figure 5.
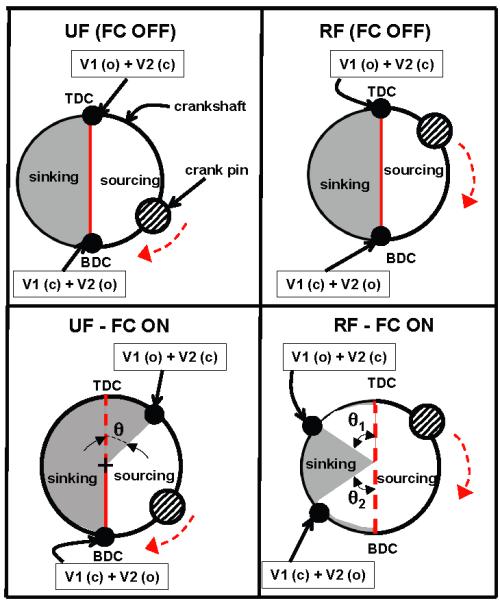
Crank circle diagram for illustrating flow control technique employed in UF and RF pumps in KIDS CRRT device via manipulation of valve timing. The top panels are for the nominal (no flow control) case and the bottom panels show the flow control implementation. The shaded regions are used for sinking phase of the pump cycle.
By varying the angle between the opening/closing point of the valve, herein referred to as the dwell angle, precise control of the volume of fluid delivered by the pump can be achieved. Though the UF and RF pumps in KIDS device were mechanically coupled, implementation of this method allows independent control of their outputs. This flow control strategy is non-unique to this application and can be translated to other pumping systems (roller pumps, centrifugal pumps) seen in extracorporeal support devices. For providing perfect fluid balance, this flow control concept of adjustable valve timing is employed simultaneously with our novel fluid management system design consisting of mechanically coupled UF and RF pumps. The offset pump was installed to provide negative or positive fluid balance, when needed, and can be used to remove or add RF based on clinical prescription into the patient return line.
Results
SV variation with source pressure and valve timing
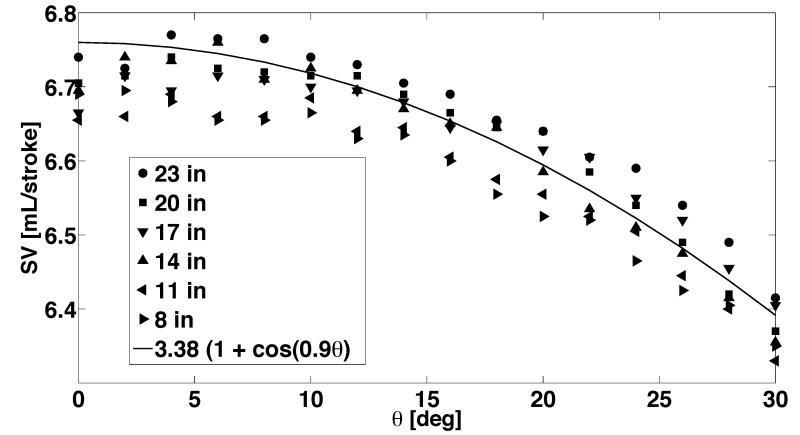
Figure 6 shows experimental assessment of SV of the KIDS UF pump as affected by varying placement of the source fluid reservoir and valve dwell angle. These experiments characterize the sensitivity of SV to advancement or postponement of valve opening/closing events. The flow control method for UF pump depicted in Fig. 5 (bottom left panel) was used for these measurements, so as to allow for earlier closure of inflow valve and opening of outflow valve. In general, a nonlinear decrease in SV with valve dwell angle can be observed across all the different source pressure conditions. This nonlinearity is expected due to the inherent periodic motion of the crankshaft during a pumping cycle. A nonlinear fit to the different conditions is also shown, which has cosine dependence. Interestingly, the governing equations for piston motion also contain the same cosine dependence. The ability to predict SV changes with dwell angle a priori allows for the downstream implementation of this novel flow control strategy in the device, so as to automate the correction of SV with any changes in operating pressure conditions encountered during the operation of the device.
Figure 6.
Variation of the stroke volume of the KIDS-CRRT pump as a function of valve dwell angle and source pressure head (height of source water column from the pump).
FB accuracy in stand-alone CRRT mode
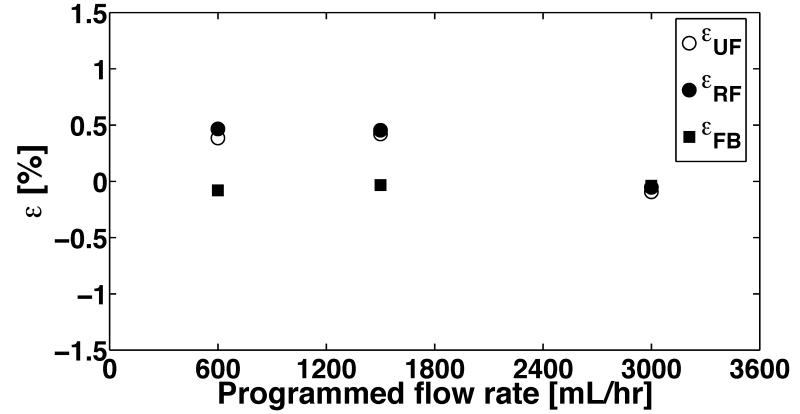
Representative FB assessment results (Fig. 7) in stand-alone operation demonstrate little discrepancy between the UF and RF pumps for filtration flow rates of 300 mL/hour-3000 mL/hour. The RF pump source pressure was set to 50 mmHg to simulate a low-pressure bag in the clinical setting. The UF pump source pressure was maintained at 0 mm Hg to simulate the low dynamic pressure range (0-10 mmHg) measured at the UF source port. The dwell scheme shown in Fig. 5 was used for limiting SV of RF pump, but no flow control was used for UF pump. The value of RF dwell angle θ1 and θ2 was in the range of 6-9 degrees. Our implementation had less than 1% total error after 8-hour period in stand-alone operation across a range of flow rates (Fig. 8).
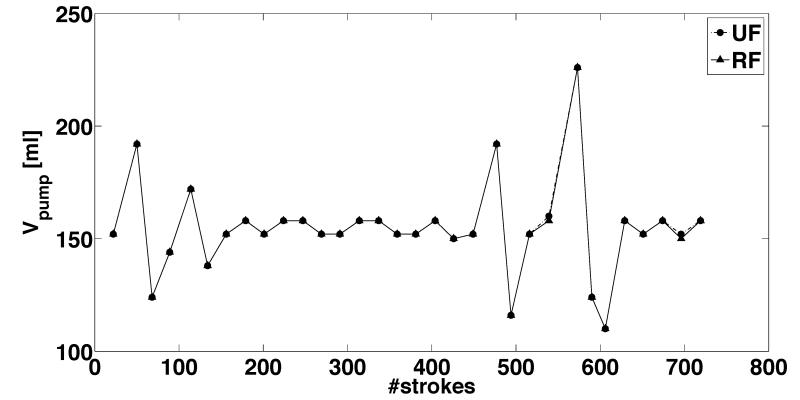
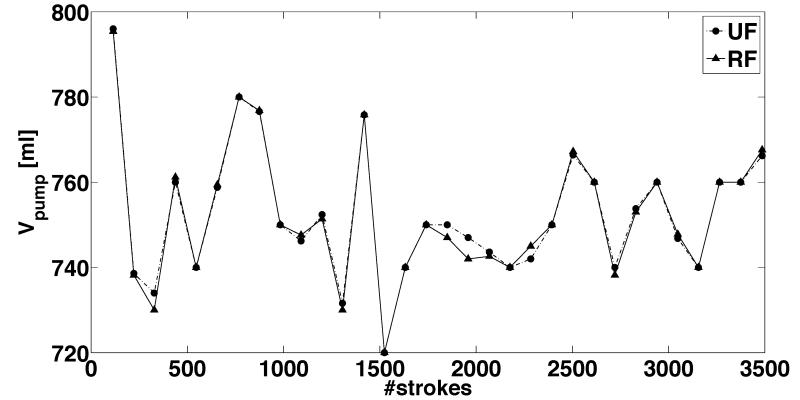
Figure 7.
Actual measurements from representative 8-hour long tests showing the temporal variation of the outputs from ultrafiltrate (UF) and replacement fluid (RF) pumps with a stroke volume of 6.9 ml for programmed flow rates of (a) 600 mL/hour and (b) 3000 mL/hour. Each data point corresponds to approximately 15 minutes of real-time operation. The source pressure head for the RF pump in this test was held constant at 50 mmHg, while the UF pump sourced from a constant 0 mmHg pressure head. The outflow pressure head for both the UF and RF pumps was equal. The pumps were continuously operated for 8-hour duration during the course of these measurements. The strokes of the piston motion driving the bladder for periodic suction and ejection of fluid is used as a surrogate for time period of pump operation.
Figure 8.
Cumulative output volumetric errors of the ultrafiltrate (UF) and replacement fluid (RF) pumps measured at the end of 8-hour long continuous operation. The operating pressure head for each pump and stroke volume are the same as in Fig. 7. The cumulative fluid balance (FB) error is calculated using actual volume outputs of the pumps measured at the end of 8-hour period compared to the expected volume (at 8 hours) estimated from the programmed flow rate.
Closed-loop CRRT circuit testing
The results of cumulative performance assessment over 8-hour long period of the closed-loop CRRT circuit are shown in Table 1. The ratio of filtration to blood flow was maintained at 20% for most of the experiments (except 300 ml/hr UF/RF flow rate), and optimizing timing between UF and blood pump by driving them out of phase minimized UF pump source pressure. Across the entire range of test conditions with filtration rates from 300 to 1800 mL/hr, the fluid balance error was consistently under 1%. This represents a significant improvement in accuracy compared to existing CRRT devices.
Table 1.
Parametric conditions used and results obtained from in vitro accuracy assessment tests of fluid management system in closed-loop CRRT circuit for 8 hours operation.
| Source pressure [mm Hg] |
Blood pump flow rate [ml/min] |
UF/RF flow rate [ml/hr] |
Output error [ml] |
Output error [%] |
|---|---|---|---|---|
| 0 | 15 | 300 | 3.7 | 0.15 |
| 0 | 50 | 600 | 39 | 0.81 |
| 5 | 50 | 600 | 35 | 0.73 |
| 0 | 150 | 1800 | 79 | 0.55 |
| 5 | 150 | 1800 | 50 | 0.35 |
Note that the output errors were computed after the end of 8-hour long period of continuous operation of the device.
Discussion
Device design and operational features
Departing from the conventional design employed in current CRRT devices, the KIDS CRRT device was designed specifically for pediatric patients. The working extracorporeal fluid volume used in the circuit (including the tubing) is 34 mL, which represents over 100 mL reduction compared to the current CRRT devices. Current CRRT devices lack the provision for alarms appropriate for the pediatric setting. A significant issue concerns with the lack of availability of integration of current CRRT devices with extracorporeal technologies, and overriding alarms are often necessary to achieve this purpose. The KIDS device circuit includes alarm profiles for neonatal, pediatric and adolescent patients, activated by sensors for monitoring blood-detection in the UF as well as for air bubble detection in the purified blood with RF infusion prior to delivery into the patient. The KIDS device can thus be easily integrated with an ECMO system or operated in stand-alone configuration.
Although the device operation described is for the CVVH modality of prescribing CRRT, other modalities can be easily realized via minor modifications and are available as a part of the complete device configuration. All the pumps used in the device were exactly similar in their basic construction, as a modular design renders ease in manufacturing and simultaneously alleviates the need for engineering/technical expertise among nursing personnel assembling the unit. In addition, the device design was developed to be flexible enough to accommodate patient-specific needs for providing negative FB and allow the physician to choose the method of prescribing ultrafiltration.15 It should be noted that the materials used for the described device were chosen based on availability, price, biocompatibility, and material properties and do not represent the optimal final design. Studies are ongoing to determine optimal material selection to maximize patient safety and device reliability.
Comparison to existing fluid management systems for CRRT
Several methods of fluid management in CRRT have been used to the present day, including: (a) monitoring filtrate fluid and infusing a matched-volume of RF hourly, (b) use of separate pumps for UF and RF that are identically calibrated, and (c) volumetric balancing.16 The filtrate is extracted in method (a) either using height adjustment of the collecting bag or via a separate IV pump. This method is not typically favored in pediatric CRRT realm due to the slow nature of the process. The use of identically calibrated pumps for UF and RF is currently the most commonly seen balancing mechanism seen in CRRT devices. The volume from each pump is individually monitored using scales to measure fluid weight as a secondary measurement. Corrections in pump flow rates are performed based on the estimated relative imbalance. While this is not as much of an issue with larger patients, the scales tend to be fairly inaccurate for smaller fluid volumes and are highly sensitive to environmental factors including temperature and vibration. Manufacturers have noted this issue and developed hemofiltration sets with higher precision, such as the HF20 set for the PrismaFlex (Gambro AB, Sweden). A large amount of data exists concerning the safe and effective use of this set in the pediatric population.17-19 However, a recently developed neonatal renal replacement device (CarpeDiem, Belco, Mirandola, Italy) with improved scale accuracy has been reported.20
Volumetric balancing has been described previously16 by enclosing the UF and RF within the same larger compartment separated by an impermeable flexible membrane. UF and RF movement was driven by inertia in the pulsatile blood flow, gravitational head, and membrane tension. While Mann’s original device was designed for adult application, substantial reduction in extracorporeal pump volume (25 mL) would be necessary for use in the pediatric population. Our system has an extracorporeal pump volume of 7 mL if one of the novel pumps is used as a blood pump. Concern also exists against using independent UF and RF pumps or two electrically linked pumps for FB to avoid excessive patient overload/dehydration, should one of the pumps fail.16,21,22 Our mechanically coupled FB design gives highest priority to patient safety, followed by accuracy. A novel benefit of our device is the multi-stage approach to ensure perfect fluid balance under varying physiological pressure conditions, through the simultaneous use of mechanically coupled UF and RF pumps and flow control implementation via variable valve timing. Lastly, the offset pump can be used to provide flexibility in the clinical prescription of negative of positive fluid balance and thereby accommodating patient-specific treatment within the design.
Conclusion
The fluid management system described in this manuscript has been designed and validated for flow conditions seen in pediatric CRRT. The current design provides high accuracy, low-volume fluid management between UF and RF instantaneously. The fluid incompressibility based principle implemented with a mechanical linkage between UF and RF pumps functions both as a fault-tolerant mechanism to guarantee a range of FB error superior to adult CRRT devices and a safety mechanism against single pump failure induced injury. Future work will examine accuracy of the device in healthy vertebrate animals to validate the design in vivo.
Acknowledgements
This research was supported by an ARRA Challenge Grant, NIDDK -1RC1DK086939, awarded to MLP (Emory University) & APY (Georgia Institute of Technology).
Footnotes
DISCLOSURES:
1 patent approved and multiple patents pending. ARRA Challenge Grant, NIDDK - 1RC1DK086939. Emory University & Georgia Institute of Technology
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstein SL, Somers MJG, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67:653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 2.Picca S, Ricci Z, Picardo S. Acute kidney injury in an infant after cardiopulmonary bypass. Semin Nephrol. 2008;28:470–476. doi: 10.1016/j.semnephrol.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Weber TR, Kountzman B. Extracorporeal membrane oxygenation for nonneonatal pulmonary and multiple organ failure. J Pediatr Surg. 1998;33:1605–1609. doi: 10.1016/s0022-3468(98)90590-5. [DOI] [PubMed] [Google Scholar]
- 4.Hoover NG, Heard M, Reid C, et al. Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med. 2008;34:2241–2247. doi: 10.1007/s00134-008-1200-y. [DOI] [PubMed] [Google Scholar]
- 5.Kramer P, Wigger W, Rieger J, et al. Arteriovenous haemofiltration: A new and simple method for the treatment of over-hydrated patients resistant to diuretics. Klin Wochenschr. 1977;55:1121–1122. doi: 10.1007/BF01477940. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein SL. Advances in pediatric renal replacement therapy for acute kidney injury. Seminars in Dialysis. 2011;24:187–191. doi: 10.1111/j.1525-139X.2011.00834.x. [DOI] [PubMed] [Google Scholar]
- 7.Warady BA, Bunchman T. Dialysis therapy for children with acute renal failure: survey results. Pediatr Nephrol. 2000;15:11–13. doi: 10.1007/s004670000420. [DOI] [PubMed] [Google Scholar]
- 8.MacLaren G, Butt W. Controversies in paediatric continuous renal replacement therapy. Intensive Care Med. 2009;35:596–602. doi: 10.1007/s00134-009-1425-4. [DOI] [PubMed] [Google Scholar]
- 9.Santiago MJ, Sánchez A, López-Herce J, et al. The use of continuous renal replacement therapy in series with extracorporeal membrane oxygenation. Kidney Int. 2009;76:1289–1292. doi: 10.1038/ki.2009.383. [DOI] [PubMed] [Google Scholar]
- 10.Ricci Z, Ronco C, Picardo S. CRRT in series with extracorporeal membrane oxygenation in pediatric patients. Kid Int. 2010;77:469–470. doi: 10.1038/ki.2009.495. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins R. Special issues with continuous renal replacement therapy in pediatric patients. Seminars in Dialysis. 1996;9:179–183. [Google Scholar]
- 12.Food and Drug Administration [Last accessed, 2/4/2013];Gambro Renal Products Prisma® Continuous Renal Replacement System, Class 1 Recall. 2005 Retrieved from: http://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm063687.htm.
- 13.Sucosky P, Prasad LP, Paden ML, et al. Assessment of current continuous hemofiltration systems and development of a novel accurate fluid management system for use in extracorporeal membrane oxygenation. J Med Devices. 2008;2:035002-1–035002-8. [Google Scholar]
- 14.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard J, Mehta RL. Volume management in continuous renal replacement therapy. Seminars in Dialysis. 2009;22:146–150. doi: 10.1111/j.1525-139X.2009.00561.x. [DOI] [PubMed] [Google Scholar]
- 16.Manns M, Polaschegg HD, Schlaeper C, et al. The acu-men®: A new device for continuous renal replacement therapy in acute renal failure. Kidney Int. 1998;54:268–274. doi: 10.1046/j.1523-1755.1998.00959.x. [DOI] [PubMed] [Google Scholar]
- 17.Santiago MJ, López-Herce J. Prismaflex HF20 for continuous renal replacement therapy in critically ill children. Artif Organs. 2011;35:1194. doi: 10.1111/j.1525-1594.2011.01367.x. [DOI] [PubMed] [Google Scholar]
- 18.Rödl S, Marschitz I, Mache CJ, Koestenberger M, Madler G, Rehak T, Zobel G. One-year safe use of the Prismaflex HF20(®) disposable set in infants in 220 renal replacement treatment sessions. Intensive Care Med. 2011;37:884–5. doi: 10.1007/s00134-011-2147-y. [DOI] [PubMed] [Google Scholar]
- 19.Rödl S, Marschitz I, Mache CJ, Koestenberger M, Madler G, Zobel G. Continuous renal replacement therapy with Prismaflex HF20 disposable set in children from 4 to 15 kg. ASAIO J. 2011;57:451–5. doi: 10.1097/MAT.0b013e31822d2132. [DOI] [PubMed] [Google Scholar]
- 20.Ronco C, Garzotto F, Ricci Z. CA.R.PE.DI.E.M. (Cardio-Renal Pediatric Dialysis Emergency Machine): evolution of continuous renal replacement therapies in infants. A personal journey. Pediatr Nephrol. 2012;27:1203–1211. doi: 10.1007/s00467-012-2179-8. doi: 10.1007/s00467-012-2179-8. [DOI] [PubMed] [Google Scholar]
- 21.Roberts M, Winney RJ. Errors in fluid balance with pump control of continuous hemodialysis. Int J Artif Organs. 1992;15:99–102. [PubMed] [Google Scholar]
- 22.Sonemann K, Niedenthal A, Russ A, et al. Automated fluid balance in continuous hemodialysis with blood safety module BSM 22/VPM. Contrib Nephrol. 1991;93:1S4–192. doi: 10.1159/000420215. [DOI] [PubMed] [Google Scholar]