Abstract
Background
Maternal smoking during pregnancy (SDP) has been extensively studied as a risk factor for adverse offspring outcomes and is known to co-occur with other familial risk factors. Accounting for general familial risk factors has attenuated associations between SDP and adverse offspring outcomes, and identifying these confounds will be critical to elucidating the relationship between SDP and its psychological correlates.
Methods
The current study aimed to disentangle the relationship between maternal SDP and co-occurring risk factors (maternal criminal activity, drug problems, teen pregnancy, educational attainment, and cohabitation at childbirth) using a population-based sample of full- (n=206,313) and half-sister pairs (n=19,363) from Sweden. Logistic regression models estimated the strength of association between SDP and co-occurring risk factors. Bivariate behavioral genetic models estimated the degree to which associations between SDP and co-occurring risk factors are attributable to genetic and environmental factors.
Results
Maternal SDP was associated with an increase in all co-occurring risk factors. Of the variance associated with SDP, 45% was attributed to genetic factors and 53% was attributed to unshared environmental factors. In bivariate models, genetic factors accounted for 21% (non- drug-, non-violence-related crimes) to 35% (drug-related crimes) of the covariance between SDP and co-occurring risk factors. Unshared environmental factors accounted for the remaining covariance.
Conclusions
The genetic factors that influence a woman’s criminal behavior, substance abuse, and her offspring’s rearing environment also influence SDP. Therefore, the intergenerational transmission of genes conferring risk for antisocial behavior and substance misuse may influence the associations between maternal SDP and adverse offspring outcomes.
Keywords: smoking, pregnancy, antisocial, criminal, drug, behavior genetic
Nicotine is the most commonly abused substance by mother’s during pregnancy (22.9%; Office of Applied Studies, 2007), and maternal smoking during pregnancy (SDP) is robustly associated with numerous adverse outcomes in offspring making it a significant public health concern. These outcomes include perinatal health problems, such as lower birth weight (Rice et al., 2009; Thapar et al., 2009), spontaneous abortion, fetal mortality, and sudden infant death syndrome (see Ernst et al., 2001 for review). Maternal SDP is also associated with psychological problems, such as cognitive delays (Batty et al., 2006; Lambe et al., 2006; Lundberg et al., 2010), poorer stress coping (Kuja-Halkola et al., 2010), attention-deficit hyperactivity disorder (ADHD; Lindblad & Hjern, 2010; Thapar et al., 2009), conduct disorder (CD; Brion et al., 2010; Silberg et al., 2003), antisocial behavior (ASB; D'Onofrio et al., 2010a; Paradis et al., in press; Rice et al., 2009), and substance use disorders (Brennan et al., 2002). Research has consistently supported a causal relationship for SDP with many perinatal health problems, but evidence has been inconsistent for its relationship with psychological problems (Rice et al., 2009; Thapar et al., 2009).
Several studies evaluating the associations between SDP and psychological problems have investigated potential confounds. These studies have shown that SDP is not an isolated risk factor, but rather mothers engaging in SDP also have lower levels of educational attainment (Gilman et al., 2008a), less annual income (Maughan et al., 2004; Monuteaux et al., 2006), more substance use problems (Batty et al., 2006), engagement in ASB (Maughan et al., 2004), and a greater probability of having children with men engaging in ASB (Maughan et al., 2004). Thus, to fully test whether these relationships are causal, more rigorous studies accounting for confounds between SDP and psychological outcomes have been necessary (Rutter et al., 2001).
Among the research accounting for such confounds, there appears to be a pattern in which studies accounting for specific, measured confounds (e.g., parental education and socioeconomic status) show attenuated but still significant associations between SDP and psychological outcomes (Ekblad et al., 2010; Espy et al., 2010; Kandel et al., 1994; Langley et al., 2007; Neuman et al., 2007; Wakschlag et al., 2010; Wakschlag et al., 2006; Weissman et al., 1999; Wiebe et al., 2009). But, studies accounting for general, unmeasured familial confounds (i.e., capturing all genetic and environmental factors) show these associations to be fully attenuated (D'Onofrio et al., 2010a; D'Onofrio et al., 2010b; Gilman et al., 2008b; Kuja-Halkola et al., 2010; Lindblad & Hjern, 2010; Lundberg et al., 2010; Silberg et al., 2003). For example, Silberg and her colleagues (2003) found a model of intergenerational transmission of CD liability (i.e., offspring liability due to the presence of maternal CD) to better fit data than a model of direct effects from SDP on offspring CD liability. Consistent with these findings, in vitro fertilization studies, in which mothers were not biologically related to the offspring but provided the prenatal and postnatal environments, have found no relationship between SDP and ADHD or ASB (Rice et al., 2009; Thapar et al., 2009). Thus, what was once considered a causal relationship seems better explained by familial confounds.
The quasi-experimental research suggests that the specific, measured confounds explicitly included in many epidemiological studies are only part of the picture. Identifying the familial confounds will be critical to elucidating the relationship between SDP and its psychological correlates, allowing research to move beyond the uncertainty of the nature of these relationships (Rutter et al., 2001). The aim of the current study is to facilitate the identification of such familial confounds by determining the degree to which genetic and environmental factors account for the relationship between SDP and behavioral correlates of maternal SDP – maternal ASB, substance use problems, and other maternal risk factors for offspring (teen pregnancy, cohabitation status, and low level of education).
The current study disentangled these relationships using a large, population-based sample, which is particularly beneficial for investigating low base rate behavior (e.g., 0.9% of women are convicted of violent crimes; Frisell et al., 2011). In addition, family members with varying degrees of genetic relatedness will be identified to test multivariate behavioral genetic models. To our knowledge, only one other published study has included SDP in a multivariate behavioral genetic model (Agrawal et al., 2008). In that study, genetic factors accounted for 34% of the variance in SDP and 42% of the covariance between SDP and nicotine dependence. Given that the phenotypes in the current study (e.g., externalizing outcomes) are less related to SDP than nicotine dependence, we hypothesize genetic factors will account for a smaller, but significant, proportion of the covariance in all multivariate behavioral genetic models. This hypothesis is consistent with multivariate research showing externalizing disorders to have a common underlying factor that is primarily comprised of genetic influences (Krueger et al., 2002). This hypothesis also is consistent with a passive gene-environmental correlation, wherein mothers are providing the prenatal environment, as well as the postnatal environment and genetic transmission of other risk factors (Plomin et al., 1977).
Methods
Sample
We analyzed a population-based sample, based on data from multiple nationwide registers maintained by Swedish government agencies and research institutes. The information in these registers was linked using a unique identification number assigned to each individual. In addition, identification numbers of family members (e.g., biological parents, offspring) were available, allowing familial relations (e.g., sibling) and genetic relatedness (e.g., sharing one or both parents) to be determined.
Birth Data
The Multi-Generation Register contains identifying information (e.g., identification number) of the biological and adoptive parents of each child born in Sweden since 1932 (Statistics Sweden, 2006). The Swedish Medical Birth Register contains data collected throughout the pregnancy and at childbirth for over 99% of all births in Sweden since 1973 (Centre for Epidemiology, 2003). Data were merged from the Swedish Medical Birth Register and Multi-Generation Register to match each mother with her children and pregnancy/childbirth data (e.g., maternal SDP, maternal age at birth, cohabitation status) and to match each mother with her own parents and sisters.
Data for Co-Occurring Risk Factors
The National Crime Register, held by the National Council for Crime Prevention, contains information about the nature of every conviction in Sweden since 1973, including data on the number of offenses, date of the crime, and sentencing. The Hospital Discharge Register contains information about the nature of hospitalizations in Sweden since 1973, including psychiatric diagnoses from the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10; World Health Organization, 1992) (Centre for Epidemiology, 2005). The Register of Education contains information about the highest level of educational attainment for each individual since 1990 (Statistics Sweden). Data were merged from the National Crime Register, Hospital Discharge Register, and Register of Education to obtain data for maternal criminal, psychiatric, and educational phenotypes, respectively.
Data for Exclusion Criteria
The Cause of Death Register, kept by the National Board of Health and Welfare, contains information about all registered deaths since 1952. The Migration Register, held by Statistics Sweden, contains information from registered migrations, including dates of immigrating to, or emigrating from, Sweden. Data were merged from the Cause of Death Register and Migration Register to determine which individuals were deceased or had emigrated and should be excluded from data analyses.
Total Sample
Several inclusion criteria were applied to the sample. First, given this study’s focus on maternal SDP, participants were restricted to females with at least one biological child born after SDP data became available in 1982. Birth-related data (e.g., SDP, maternal age) were retained from the first childbirth of each mother. There were 1,600,609 mothers for whom such data were available. Second, mothers born after 1995 were excluded from analyses, as they had not yet entered the high-risk period for some co-occurring risk factors (e.g., substance use problems) as of the last wave of data collection. Finally, individuals belonging to a multiple birth set (e.g., twins, triplets), or who either were deceased or had emigrated out of Sweden as of the last wave of data collection, were excluded from analyses. Therefore, the current sample was comprised of mothers who were born before 1995, had given birth in Sweden after 1982, and were still living in Sweden as of 2009. There were 1,193,080 mothers meeting exclusion criteria.
The identification numbers of each mother’s parents (i.e., maternal and paternal identification numbers) were then used to construct families. First, mothers with common maternal identification numbers were grouped into maternal families (i.e., sisters with the same mother were grouped together). There were 924,946 maternal families available in the data set. Second, the two oldest sisters of each maternal family were identified and retained for subsequent steps. That is, each family consisted of the two oldest sisters who had at least one biological child. Finally, paternal identification numbers were used to determine the genetic relatedness of each sister pair. Full sisters were identified as having the same paternal identification number (i.e., sharing 50% of segregating genes), and half sisters were identified as having different paternal identification numbers (i.e., sharing 25% of segregating genes). Sisters without paternal identification numbers (i.e., for whom genetic relatedness was unknown) or who were adopted into different families (i.e., sister pairs that may not have been raised together) were excluded from analyses.
In total, there were 225,676 maternal families with a sister pair meeting all criteria, of which there were 206,313 (91.42%) full- and 19,363 (8.58%) half-sister pairs. Mothers’ average age at the end of follow-up (2009) was 44.02 (SD=8.60).
Variables
Maternal Smoking During Pregnancy
Maternal SDP was assessed via self-report at the first antenatal visit and measured on a 3-point ordinal scale as a nonsmoker (0 cigarettes per day), moderate smoker (1–9 cigarettes per day), or heavy smoker (10+ cigarettes per day). Self-reports of SDP during antenatal visits have been shown to be valid compared to retrospective self-reports (i.e., after pregnancy; Jacobson et al., 2002) and bioassays (e.g., serum cotinine levels; Pickett et al., 2009). For example, a large majority (94%) of maternal self-reports of nonsmoking are in agreement with serum cotinine levels (Lindqvist et al., 2002). In the current sample, 14.17% of mothers engaged in moderate SDP and 7.58% engaged in heavy SDP (21.76% of the total sample). Notably, mothers from half-sister pairs reported considerably higher rates of any SDP (33.91%) than those from full-sister pairs (20.62%), which reflects an increased prevalence of environmental risk factors (e.g., lower SES) and adverse offspring outcomes (e.g., poorer educational outcomes) in blended families (Ginther & Pollak, 2004).
Criminal Convictions
Criminal histories were based on the Swedish Penal Code. Convictions were categorized as violent, drug, substance-related driving, or other offenses. In addition, the date of conviction was used to determine the individual’s age when the crime was committed. To simplify analyses, only data for the first conviction of each type of criminal offense was retained for each individual.
Violent crime was defined as attempted/completed murder, manslaughter, and filicide, aggravated assault, gross violation of a person’s integrity, kidnapping and illegal constraint, illegal coercion and threat, harassment, aggravated robbery, aggravated arson, and/or threats or violence against an officer. Drug crime was defined as offenses related to the manufacturing and/or distribution of illicit drugs. Driving crime was defined as offenses related to operating a motor vehicle under the influence of a controlled substance. Finally, other crimes consisted of any non-violent and non-drug related conviction. In the current sample of mothers, there was at least one lifetime conviction related to violent crime in 1.11%, drug crime in 0.74%, driving crime in 1.03%, other crime in 8.65%. In total, 11.07% had at least one conviction of any type.
Psychiatric Hospitalizations
Diagnoses during psychiatric hospitalizations were based on the ICD-10. Only hospitalizations related to alcohol- or drug-use were analyzed, as internalizing disorders have not been associated with SDP (Brion et al., 2010). Again, the date discharged from the hospital was used to determine the individual’s age when hospitalized, and only the first psychiatric discharge for alcohol- or drug-related hospitalizations was retained. In the current sample, there was at least one lifetime hospitalization in 1.33% related to alcohol use, 0.95% related to drug use, and 1.92% related to any substance use.
Other Maternal Risk Factors
Maternal teen pregnancy status was determined by the mother’s age at the birth of her first child. The average age of first childbirth was 27.04 (SD=4.95), and 4.56% of mothers had teen pregnancies. Maternal cohabitation status was based on whether the mother reported living with her spouse or partner at the time of her first childbirth. Of the mothers in the current study, 6.86% reported not living with a spouse or partner.
Education was based on the highest level of educational attainment for each mother. The Register of Education categorizes each person into one of seven levels. Low level of educational attainment was assessed by combining the first two categories (no education beyond primary and lower secondary school, 9.50% in the current study).
Data Analysis
Logistic Regression
First, logistic regression models were used to identify the strength of association between SDP and co-occurring risk factors. Logistic regression models were conducted using SAS, version 9.2 (SAS Institute Inc., Cary, North Carolina). PROC SURVEYLOGISTIC was used to account for familial clustering. Dummy-coded variables were created to compare moderate and heavy SDP to no smoking. In addition, polychoric correlations were used to determine the within- and cross-sister associations involving SDP and co-occurring risk factors, with full- and half-sister dyads being analyzed separately to help explore the degree to which genetic and environmental factors may influence each trait and the associations with SDP (Neale & Cardon, 1992).
Univariate Behavioral Genetic Analyses
Second, structural equation models (SEMs) were fitted to estimate the degree to which variance in each phenotype is associated with additive genetic (A), common environmental (C), and unshared environmental (E) factors. This is done by using genetically-informed data and imposing variance and covariance constraints, from which latent variables are assumed to represent the biometrical (ACE) factors (e.g., constraining sibling correlations of the A factors to 0.5 for full siblings and 0.25 for half siblings). Thus, behavioral genetic models estimated the covariances between full- (calculated as 0.5*A+C) and half-sibling pairs (calculated as 0.25*A+C) and the percentage of phenotypic variance attributable to the biometrical factors. This approach is similar to that of the classical twin study (Neale & Cardon, 1992; Prescott, 2004).
Given that all phenotypes were categorical, thresholds were estimated instead of means for all manifest variables. Finally, age at the last wave of data collection was included as a covariate for all phenotypes. All behavioral genetic analyses were conducted using Mplus, version 6.1 (Muthén & Muthén, 2010).
Bivariate Behavioral Genetic Analyses
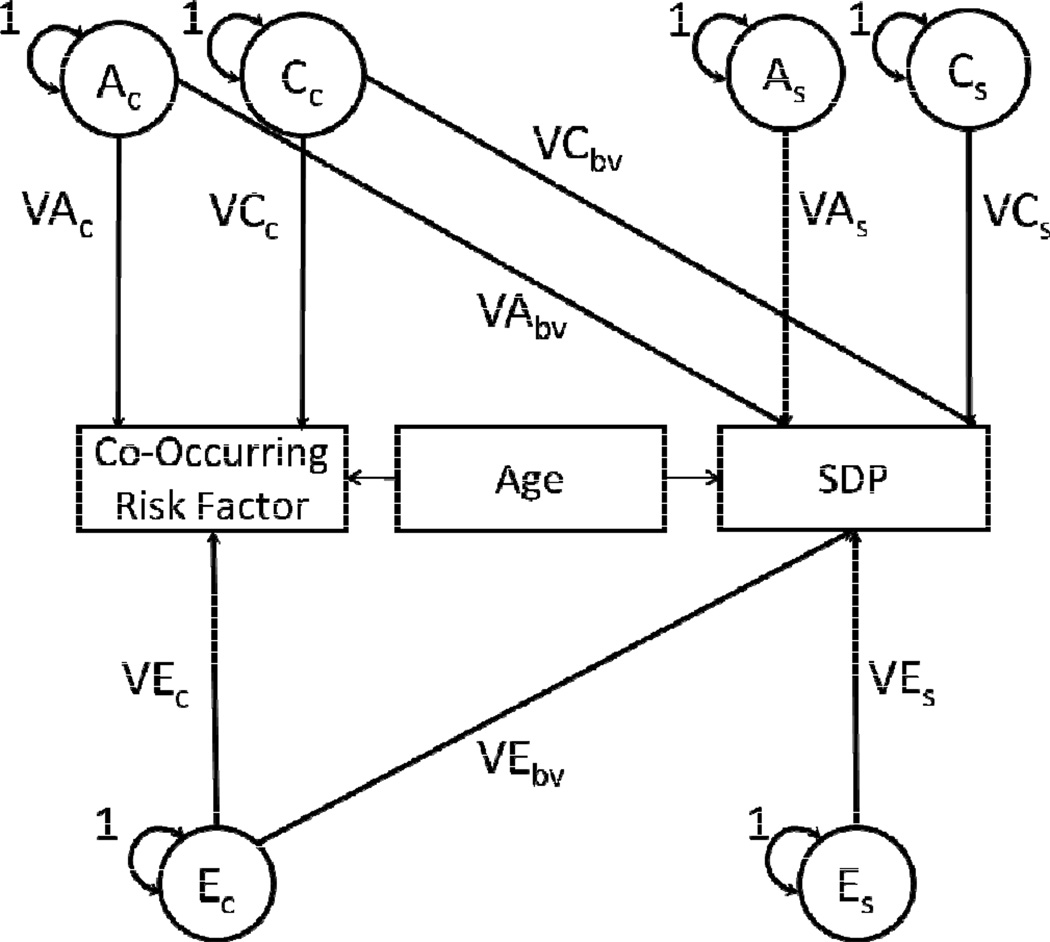
Finally, SEMs were fitted to estimate the degree to which covariances between SDP and co-occurring risk factors are associated with the biometrical factors. The bivariate model was based on the Cholesky decomposition approach (see Figure 1), from which three triangular matrices containing parameter estimates for the biometrical factors are derived (Loehlin, 1996; Neale & Cardon, 1992). In bivariate models, each matrix contains three elements, two on the diagonal accounting for the variance in each phenotype (e.g., SDP and a co-occurring risk factor) and one on the off-diagonal accounting for the covariance between both phenotypes. Thus, the variances and covariance are decomposed into the biometrical factors. Model constraints and parameterizations were similar to those used in the univariate model, and age was again included as a covariate for both phenotypes.
Figure 1. Bivariate behavioral genetic model of genetic and environmental factors accounting for covariance in maternal smoking during pregnancy and behavioral correlates.
Bivariate behavioral genetic models using the Cholesky decomposition approach involving maternal smoking during pregnancy (SDP) and other co-occurring risk factors.
NOTE: Both SDP and the co-occurring risk factor entered into each model are regressed on mother’s age, to account for risk/opportunity for the phenotype to occur (e.g., criminal conviction, psychiatric hospitalization). Models were used to obtain estimates of the proportion of covariance between SDP and each co-occurring risk factor associated with genetic and environmental factors. Standard errors were derived from the equations provided in the caption, which were entered into a Model Constraint command in Mplus. Adapted from Mplus User’s Guide, examples 5.19 and 7.28 (Muthen & Muthen, 2010).
Shared Environment (C) was fixed to zero in bivariate models. Parameter estimates were calculated as follows: A=VAc*VAbv, E=VEc*VEbv, Covariance = A + C(0), H2 = A / (A+E) = proportion of covariance attributed to genetic factors, E2 = E / (A+E) = proportion of covariance attributed to environmental factors.
Results
Association Measures
Frequencies of all co-occurring risk factors by level of SDP engagement, and the corresponding odds ratios obtained from logistic regression models, are displayed in Table 1. Odds ratios indicated the risk factors were significantly more likely to occur in mothers engaging in moderate or heavy SDP, relative to those engaging in no SDP. The largest effects were for crimes and hospitalizations related to substance abuse (OR = 7.5–13.6), and the smallest effects were for other crimes (i.e., non-drug, non-violent convictions; OR = 2.2). Interestingly, teen pregnancy was more strongly associated with moderate SDP than heavy SDP, which may be due to teenagers having less time to acquire more severe smoking habits than older mothers.
Table 1.
Frequencies and odds ratios of co-occurring risk factors as a function of smoking during pregnancy in mothers.
| Frequency | Odds Ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| Behavioral Correlate | No SDP | Moderate SDP | Heavy SDP | Moderate SDP | Heavy SDP | |||
| Criminal Convictions | ||||||||
| Violent | 0.6% | 2.6% | 3.9% | 4.9 (4.6, 5.3) | 7.3 (6.7, 7.8) | |||
| Drug | 0.2% | 2.1% | 3.2% | 8.8 (8.0, 9.6) | 13.6 (12.4, 15.0) | |||
| Driving | 0.5% | 2.5% | 3.9% | 5.4 (5.0, 5.8) | 8.6 (8.0, 9.3) | |||
| Other | 7.3% | 12.8% | 14.8% | 1.9 (1.8, 1.9) | 2.2 (2.2, 2.3) | |||
| Any | 8.4% | 18.7% | 23.5% | 2.5 (2.5, 2.6) | 3.3 (3.2, 3.4) | |||
| Psychiatric Diagnoses | ||||||||
| Alcohol | 0.7% | 2.9% | 4.8% | 4.4 (4.2, 4.7) | 7.6 (7.1, 8.1) | |||
| Illicit Drug | 0.4% | 2.3% | 3.6% | 5.5 (5.1, 5.9) | 8.8 (8.2, 9.6) | |||
| Any Substance | 1.0% | 4.3% | 6.8% | 4.6 (4.4, 4.9) | 7.5 (7.1, 8.0) | |||
| Other Maternal Characteristics | ||||||||
| Teen Pregnancy | 3.3% | 9.4% | 8.6% | 3.1 (3.0, 3.2) | 2.8 (2.7, 2.9) | |||
| Non-cohabitation | 4.8% | 12.2% | 16.9% | 2.7 (2.6, 2.8) | 4.0 (3.9, 4.2) | |||
| Low Educational Attainment | 6.4% | 18.2% | 23.2% | 3.3 (3.2, 3.4) | 4.4 (4.3, 4.6) | |||
NOTE: Odds ratios are calculated relative to the non-SDP group.
Polychoric correlations of SDP with co-occurring risk factors are displayed in Table 2 using within-sister (e.g., correlations of a mother’s engagement in SDP with her own criminal convictions) and cross-sister phenotypic correlations for full- and half-sister pairs (e.g., correlations of a mother’s engagement in SDP with her sister’s criminal convictions). The strongest within-sister correlations were for drug-related convictions (r=0.43), driving convictions (r=0.38), and substance-related psychiatric hospitalizations (r=0.37–0.39). As expected, the strongest cross-sister correlate of each mother’s SDP was her sister’s engagement in SDP (r=0.45 for full, r=0.24 for half). All cross-sister correlations were higher for full- than half-sister pairs, suggesting genetic factors influence SDP and the association between SDP and each risk factor.
Table 2.
Cross-trait polychoric correlations of smoking during pregnancy with co-occurring risk factors, using within- and cross-sister.
| Criminal Conviction | Psychiatric Hospitalization | Other Maternal Risk Factors | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDP | Violent | Drug | Driving | Other | Any | Drug | Alcohol | Any | Teen Pregnancy | No Cohabitation | Low Education | |
| Correlation Type | ||||||||||||
| Within-Sister | * | 0.36 | 0.43 | 0.38 | 0.19 | 0.30 | 0.37 | 0.38 | 0.39 | 0.26 | 0.32 | 0.37 |
| Full Sisters | 0.45 | 0.23 | 0.25 | 0.23 | 0.12 | 0.18 | 0.21 | 0.22 | 0.22 | 0.16 | 0.17 | 0.26 |
| Half Sisters | 0.24 | 0.11 | 0.13 | 0.09 | 0.08 | 0.11 | 0.08 | 0.12 | 0.11 | 0.06 | 0.06 | 0.13 |
NOTE: All correlations were conducted with the three-category SDP measure.
Univariate Behavioral Genetic Analyses
Estimates of the proportion of variance associated with genetic and environmental factors in SDP and co-occurring risk factors are displayed in Table 3. The variance in SDP was primarily influenced by additive genetic (45%) and unshared environmental factors (53%), with shared environment having a nonsignificant influence (2%). All co-occurring risk factors were most strongly associated with unshared environmental factors (i.e., environmental factors affecting sisters differently), which accounted for at least 50% of the variance in all phenotypes. In addition, a substantial amount of variance in all co-occurring risk factors was due to genetic factors, ranging from 19% (non-cohabitation) to 42% (low level of educational attainment). Shared environmental factors (i.e., environmental factors affecting siblings similarly) were associated with any convictions (6%), teen pregnancy (7%), non-cohabitation (8%), and low level of educational attainment (4%), but showed negligible influences on all other phenotypes. Notably, common environmental factors were near 10% for some phenotypes (e.g., violence- and drug-related convictions), but these were low base rate occurrences and had large standard errors resulting in estimates that were not significantly different from zero.
Table 3.
Parameter estimates of genetic and environmental factors accounting for the variance of SDP and co-occurring risk factors from univariate behavioral genetic models.
| Behavioral Correlate | Additive Genetic | Shared Environment | Unshared Environment | |
|---|---|---|---|---|
| Smoking During Pregnancy | 0.45 (0.02) | 0.02 (0.01)NS | 0.53 (0.01) | |
| Criminal Convictions | ||||
| Violent | 0.29 (0.09) | 0.09 (0.06)NS | 0.63 (0.04) | |
| Drug | 0.30 (0.09) | 0.11 (0.06)NS | 0.59 (0.04) | |
| Driving | 0.24 (0.15)NS | 0.05 (0.09)NS | 0.71 (0.07) | |
| Other | 0.25 (0.05) | 0.00 (0.03)NS | 0.75 (0.02) | |
| Any | 0.24 (0.04) | 0.06 (0.02) | 0.70 (0.02) | |
| Psychiatric Diagnoses | ||||
| Alcohol | 0.39 (0.09) | 0.00 (0.05)NS | 0.60 (0.04) | |
| Illicit Drug | 0.28 (0.10) | 0.09 (0.06)NS | 0.63 (0.04) | |
| Any Substance | 0.40 (0.07) | 0.00 (0.04)NS | 0.60 (0.03) | |
| Other Maternal Characteristics | ||||
| Teen Pregnancy | 0.37 (0.04) | 0.07 (0.02) | 0.56 (0.01) | |
| Non-cohabitation | 0.19 (0.06) | 0.08 (0.03) | 0.73 (0.03) | |
| Low Educational Attainment | 0.42 (0.03) | 0.04 (0.02) | 0.55 (0.01) | |
NOTE: standard errors are provided in parentheses
denotes parameter estimate non-significant from zero
Bivariate Behavioral Genetic Analyses
Estimates of the proportion of covariance between SDP and co-occurring risk factors associated with genetic and environmental factors are displayed in Table 4. Shared environment was fixed to zero for all models because of its negligible influence on SDP and to ensure interpretable parameter estimates across all models (e.g., sums of the biometrical parameters would account for 100% of the covariance in each model).
Table 4.
Parameter estimates of genetic and environmental factors accounting for the covariance for SDP with co-occurring risk factors from bivariate behavioral genetic models
| Behavioral Correlate | Additive Genetic | Unshared Environment | |
|---|---|---|---|
| Criminal Convictions | |||
| Violent | 0.33 (0.01) | 0.67 (0.01) | |
| Drug | 0.35 (0.01) | 0.65 (0.01) | |
| Driving | 0.30 (0.01) | 0.70 (0.01) | |
| Other | 0.21 (0.01) | 0.80 (0.01) | |
| Any | 0.27 (0.00) | 0.73 (0.00) | |
| Psychiatric Diagnoses | |||
| Alcohol | 0.31 (0.01) | 0.70 (0.01) | |
| Illicit Drug | 0.32 (0.01) | 0.69 (0.01) | |
| Any Substance | 0.32 (0.01) | 0.68 (0.01) | |
| Other Maternal Characteristics | |||
| Teen Pregnancy | 0.34 (0.00) | 0.66 (0.00) | |
| Non-cohabitation | 0.28 (0.01) | 0.72 (0.01) | |
| Low Educational Attainment | 0.35 (0.00) | 0.66 (0.00) | |
NOTE: standard errors are provided in parentheses
denotes parameter estimate non-significant from zero
Unshared environmental factors accounted for the largest proportion of covariance between SDP and all co-occurring risk factors. Additive genetic factors were also associated with a significant proportion of the covariance between SDP and all phenotypes, as these estimates ranged from 21% (non-violence-, non-drug-related offenses) to 35% (drug-related convictions). The proportion of covariance attributed to genetic factors was particularly high for substance-related phenotypes (30%–35%), including driving related convictions (i.e., driving under the influence) and substance-related psychiatric hospitalizations. Shared genetic liability also accounted for a relatively large proportion of the covariance between SDP and other co-occurring risk factors (28%–35%), such as low maternal educational attainment. In sum, the genetic factors that influence a woman’s criminal behavior, substance abuse, and the environment she provides for her offspring also influence SDP.
Discussion
The current study used multivariate behavioral genetic models to elucidate the relationship between maternal SDP and co-occurring familial risk factors, which previous studies have indicated are familial in nature. The co-occurrence of SDP and these risk factors was largely attributed to environmental factors (accounting for 65% – 80% of the covariance), with genetic factors playing a significant role and accounting for the remaining covariance. As expected, genetic factors were associated with a smaller proportion of covariance between SDP and co-occurring risk factors (21% – 35%) than previously shown for SDP and nicotine dependence (42%; Agrawal et al., 2008).
Maternal SDP may be a proxy of behavioral dysregulation, such as problems with delayed gratification (Metcalfe & Mischel, 1999) or an inability to control one’s own behavior (e.g., dyscontrol; Widiger & Sankis, 2000), which may manifest in SDP and the other risk factors included in the current study (e.g., CD, substance abuse; Barkley, 1997; Lau et al., 1995). These results are consistent with previous research showing externalizing disorders (e.g., CD, ASPD, drug dependence) to cluster under a single latent factor (Krueger & Markon, 2006; Lahey et al., 2009). Notably, an effect of SDP has not extended to emotional dysregulation (e.g., internalizing disorders; Brion et al., 2010; Wakschlag et al., 2006), suggesting that the risk factors associated with SDP are specific to behavioral dysregulation (Huijbregts et al., 2008; Thapar et al., 2003). Furthermore, poor inhibition and difficulty delaying gratification may be similar to the cognitive deficits found in ADHD (Solanto et al., 2001) and low levels of educational attainment. Therefore, many of the genetic effects involved in SDP and adverse psychological phenotypes are likely acting on executive functioning (e.g., decision making, planning), and the environmental effects may be counter to the benefits of social and emotional learning programs (Payton et al., 2000).
To our knowledge, only one other multivariate behavioral genetic study involving SDP has been conducted to date. Agrawal and her colleagues (2008) conducted a twin study in which 42% of the covariance between the SDP and nicotine dependence was attributed to common genetic factors. Those findings are comparable to the substance-related phenotypes analyzed in the current study, in which the proportion of covariance attributed to genetic factors was slightly smaller (30%–35%). Notably, multiple substance-related phenotypes in the current study were of those most strongly associated with the genetic influences of SDP. Although a common factor representing liability for substance abuse has been identified (as opposed to several, drug-specific factors; Han et al., 1999), the covariance attributed to genetic factors in Agrawal et al. (2008) may include influences on nicotine sensitivity and, thus, contribute to a larger proportion of covariance.
An important strength of the current analyses stems from using a large, population-based sample, in which numerous co-occurring risk factors of public interest are available. Many of the co-occurring risk factors included in the current study are rare events, requiring a large sample for adequate power in identifying effects. For example, violent criminal acts are rare occurrences in females (e.g., 1.1% of the current sample), as are substance-related psychiatric hospitalizations (e.g., 1.9% of the current sample). Notably, even with a large sample of 225,676 families in the current study, phenotypes with a low base rate occurrence had relatively large standard errors for biometrical factors (e.g., see Table 3).
Multiple measures were also available across the domains of interest in the current study – criminal convictions, psychiatric hospitalizations, and other characteristics of an offspring's rearing environment. This allows parameter comparisons to be made to identify consistencies or outliers. For example, all maternal characteristics (maternal teen pregnancy, cohabitation, educational attainment) had similar degrees of genetic influence shared with SDP. On the other hand, there was an unexpectedly large difference between the proportions of covariance attributed to genetic factors for other crimes (i.e., non-violence-, non-drug-related; 21%) relative to violence- (34%) and drug-related crimes (35%). Whether this finding is anomalous or indicative of differences in causal underpinnings warrants further investigation.
An inherent characteristic of studies on maternal SDP, which is a limitation to the current study, is the use of a sample restricted to females. Specifically, there are gender differences for the heritability of externalizing behaviors, with genetic factors having a strong influence on these phenotypes in men (Hicks et al., 2007). Given these differences, the estimates of the genetic and environmental contributions to the covariance between SDP and externalizing phenotypes may not apply to males. Additional research is therefore needed to disentangle how the intergenerational transmission of maternal SDP may confer risk for adverse outcomes in male offspring.
These findings also do not identify specific genetic or environmental factors that contribute to SDP and/or co-occurring risk factors, and research in the fields of molecular genetics, neuroscience, and the social sciences will be needed to further the progress in this area. For example, several gene-environment interactions have been identified that involve SDP and may hint to genes influencing both SDP and these adverse psychological phenotypes (Lotfipour et al., 2009; Neuman et al., 2007; Wiebe et al., 2009). A basic understanding of how these and other genes influence SDP and behavioral dysregulation will likely be critical to advance understanding in this area.
Gene-environment interactions may also involve specific environmental factors, which moderate the heritability of phenotypes and/or the covariance among phenotypes associated with genetic factors. For example, a polymorphism protecting against alcohol dependence in Japanese populations has been identified (i.e., ALDH), but this protective effect has declined as per capita alcohol consumption increased in Japan (Higuchi et al., 1994). In the current study, data span a period when SDP became increasingly deviant (as the public awareness of the consequences of SDP increased), and, consequently, the relationship between SDP and co-occurring risk factors may have changed. We investigated this possibility, and the associations between SDP and co-occurring risk factors were moderated by secular changes. This interaction may be due to marked changes in the prevalence of SDP over this period (from 32% in 1982 to 7% in 2009). However, there was no difference in the heritability of SDP when our sample was split into two cohorts, those giving birth in 1982–1991 (47%) and those giving birth in 1992–2009 (46%).
A final limitation concerns the phenotypes used in the current study. Behavioral dysregulation manifests in numerous phenotypes that range greatly in severity, but many phenotypes in the current study reflect severe dysregulation (e.g., violent crime). The current findings, therefore, cannot be applied to less severe co-occurring risk factors, and future research should fill in these blanks.
Alternative Models of SDP
Slotkin (1998) proposed three pathways through which SDP may influence a developing organism: (1) direct effects on the maternal-fetal unit, such as hypoxia, vascular effects, placental effects, and malnutrition in offspring; (2) neurodevelopmental insults causing behavioral dysregulation (for instance, via nicotine exposure); and (3) environmental risk factors co-occurring with SDP, including lower parental educational attainment. Much evidence has supported a causal link between SDP and effects on the maternal-fetal unit (e.g., increased pregnancy-related problems; Cnattingius, 2004; Johansson et al., 2009). Rigorously controlling for familial factors (e.g., the co-occurring environmental risk factors Slotkin described), however, has attenuated the effect of SDP on neurobehavioral outcomes, which is inconsistent with the presence of neurodevelopmental effects (e.g., psychological problems; Knopik, 2009). The current study suggests another pathway through which SDP is associated with offspring psychological problems—co-occurring genetic risk factors that influence SDP and the environment in which offspring are reared. Thus, what Slotkin posited to be an effect of neurodevelopmental insults may be, at least partially, due to shared genetic liability. However, the lack of a direct effect of SDP on psychological outcomes does not negate the fact that SDP can lead to fatal consequences for offspring via direct effects on the maternal-fetal unit.
Implications
The aim of the current study was to further disentangle the relationship between SDP and co-occurring familial risk factors. The current results suggest that the genetic factors that influence a woman’s criminal behavior, substance abuse, and the environment she provides for her offspring also influence SDP. Thus, it is possible that the intergenerational transmission of genes conferring risk for ASB and substance misuse, at least partially, influence the associations between maternal SDP and adverse offspring outcomes.
Acknowledgments
Funding for the study was provided by the National Institute of Child and Human Development (Grant: HD061817). Preliminary analyses were presented at the Behavior Genetics Association Conference (June 2011, Newport, Rhode Island).
Footnotes
Declaration of Interest
None
References
- Agrawal A, Knopik VS, Pergadia ML, Waldron M, Bucholz KK, Martin NG, Heath AC, Madden PAF. Correlates of cigarette smoking during pregnancy and its genetic and environmental overlap with nicotine dependence. Nicotine and Tobacco Research. 2008;10:567–578. doi: 10.1080/14622200801978672. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–65. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Batty GD, Der G, Deary IJ. Effect of maternal smoking during pregnancy on offspring's cognitive ability: Empirical evidence for complete confounding in the US National Longitudinal Survey of Youth. Pediatrics. 2006;118:943–950. doi: 10.1542/peds.2006-0168. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Grekin ER, Mortensen EL, Mednick SA. Relationship of maternal smoking during pregnancy with criminal arrest and hospitalization for substance abuse in male and female adult offspring. American Journal of Psychiatry. 2002;159:48–54. doi: 10.1176/appi.ajp.159.1.48. [DOI] [PubMed] [Google Scholar]
- Brion MJ, Victora C, Matijasevich A, Horta B, Anselmi L, Steer C, Menezes AMB, Lawlor DA, Smith GD. Maternal smoking and child psychological problems: Disentangling causal and noncausal effects. Pediatrics. 2010;126:e57–e65. doi: 10.1542/peds.2009-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Epidemiology. The Swedish Medical Birth Register - A summary of content and quality. 2003 [Google Scholar]
- Centre for Epidemiology. The Swedish Hospital Discharge Register. 2005 [Google Scholar]
- Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine and Tobacco Research. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman C, Grann M, Neiderhiser JM, Långström N, Lichtenstein P. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: A population-based study in Sweden. Archives of General Psychiatry. 2010a;67:529–538. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman C, Neiderhiser JM, Långström N, Lichtenstein P. A quasi-experimental study of maternal smoking during pregnancy and offspring academic achievement. Child Development. 2010b;81:80–100. doi: 10.1111/j.1467-8624.2009.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad M, Gissler M, Lehtonen L, Korkeila J. Prenatal smoking exposure and the risk of psychiatric morbidity into young adulthood. Archives of General Psychiatry. 2010;67:841–849. doi: 10.1001/archgenpsychiatry.2010.92. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:630–642. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Espy KA, Fang H, Johnson C, Stopp C, Wiebe SA, Respass J. Prenatal tobacco exposure: Developmental outcomes in the neonatal period. Developmental Psychology. 2010;47:153–169. doi: 10.1037/a0020724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisell T, Lichtenstein LN, Långström N. Violent crime runs in families: A total population study of 12.5 million individuals. Psychological Medicine. 2011;41:97–106. doi: 10.1017/S0033291710000462. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Breslau J, Subramanian SV, Hitsman B, Koenen KC. Social factors, psychopathology, and maternal smoking during pregnancy. American Journal of Public Health. 2008a;98:448–448. doi: 10.2105/AJPH.2006.102772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Gardener H, Buka SL. Maternal smoking during pregnancy and children's cognitive and physical development: A causal risk factor? American Journal of Epidemiology. 2008b;168:522–531. doi: 10.1093/aje/kwn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginther DK, Pollak RA. Family structure and children’s educational outcomes: Blended families, stylized facts, and descriptive regressions. Demography. 2004;41:671–696. doi: 10.1353/dem.2004.0031. [DOI] [PubMed] [Google Scholar]
- Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: Univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: A longitudinal twin study. Journal of Abnormal Psychology. 2007;116:433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi S, Matsushita S, Imazeki H, Kinoshita T, Takagi S, Kono H. Aldehyde dehydrogenase genotypes in Japanese alcoholics. Lancet. 1994;343:741–742. doi: 10.1016/s0140-6736(94)91629-2. [DOI] [PubMed] [Google Scholar]
- Huijbregts SCJ, Warren AJ, de Sonneville LMJ, Swaab-Barneveld H. Hot and cool forms of inhibitory control and externalizing behavior in children of mothers who smoked during pregnancy: An exploratory study. Journal of abnormal child psychology. 2008;36:323–333. doi: 10.1007/s10802-007-9180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Johansson ALV, Dickman PW, Kramer MS, Cnattingius S. Maternal smoking and infant mortality: Does quitting smoking reduce the risk of infant death? Epidemiology. 2009;20:590–597. doi: 10.1097/EDE.0b013e31819dcc6a. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. American Journal of Public Health. 1994;84:1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS. Maternal smoking during pregnancy and child outcomes: Real or spurious effect? Developmental Neuropsychology. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuja-Halkola R, D'Onofrio BM, Iliadou AN, Langstrom N, Lichtenstein P. Prenatal smoking exposure and offspring stress coping in late adolescence: No causal link. International journal of epidemiology. 2010;39:1531–1540. doi: 10.1093/ije/dyq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, D’Onofrio BM, Waldman ID. Using epidemiologic methods to test hypotheses regarding causal influences on child and adolescent mental disorders. Journal of Child Psychology and Psychiatry. 2009;50:53–62. doi: 10.1111/j.1469-7610.2008.01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe M, Hultman C, Torrång A, MacCabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17:524–530. doi: 10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- Langley K, Holmans PA, Van Den Bree M, Thapar A. Effects of low birth weight, maternal smoking in pregnancy and social class on the phenotypic manifestation of Attention Deficit Hyperactivity Disorder and associated antisocial behaviour: investigation in a clinical sample. BMC psychiatry. 2007;7:26–26. doi: 10.1186/1471-244X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau MA, Pihl RO, Peterson JB. Provocation, acute alcohol intoxication, cognitive performance, and aggression. Journal of Abnormal Psychology. 1995;104:150–150. doi: 10.1037//0021-843x.104.1.150. [DOI] [PubMed] [Google Scholar]
- Lindblad F, Hjern A. ADHD after fetal exposure to maternal smoking. Nicotine & Tobacco Research. 2010;12:408–415. doi: 10.1093/ntr/ntq017. [DOI] [PubMed] [Google Scholar]
- Lindqvist R, Lendahls L, Tollbom ÖR, Åberg H, Håkansson A. Smoking during pregnancy: Comparison of self reports and cotinine levels in 496 women. Acta Obstetricia et Gynecologica Scandinavica. 2002;81:240–244. doi: 10.1034/j.1600-0412.2002.810309.x. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. The Cholesky approach: A cautionary note. Behavior Genetics. 1996;26:65–69. [Google Scholar]
- Lotfipour S, Ferguson E, Leonard G, Perron M, Pike B, Richer L, Seguin JR, Toro R, Veillette S, Pausova Z. Orbitofrontal cortex and drug use during adolescence: Role of prenatal exposure to maternal smoking and BDNF genotype. Archives of General Psychiatry. 2009;66:1244–1252. doi: 10.1001/archgenpsychiatry.2009.124. [DOI] [PubMed] [Google Scholar]
- Lundberg F, Cnattingius S, D’Onofrio B, Altman D, Lambe M, Hultman C, Iliadou A. Maternal smoking during pregnancy and intellectual performance in young adult Swedish male offspring. Paediatric and Perinatal Epidemiology. 2010;24:79–87. doi: 10.1111/j.1365-3016.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal smoking and early childhood conduct problems: Testing genetic and environmental explanations of the association. Archives of General Psychiatry. 2004;61:836–843. doi: 10.1001/archpsyc.61.8.836. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: Dynamics of willpower. Psychological review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Monuteaux MC, Blacker D, Biederman J, Fitzmaurice G, Buka SL. Maternal smoking during pregnancy and offspring overt and covert conduct problems: a longitudinal study. Journal of Child Psychology and Psychiatry. 2006;47:883–890. doi: 10.1111/j.1469-7610.2005.01566.x. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide, 6th. 2010 [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Springer Netherlands: 1992. [Google Scholar]
- Neuman RJ, Lobos E, Reich W, Henderson CA, Sun LW, Todd RD. Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biological Psychiatry. 2007;61:1320–1328. doi: 10.1016/j.biopsych.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Office of Applied Studies. The National Survey of Drug Use and Health Report. Rockville, MD: Mental Health Services Administration; 2007. Cigarette use among pregnant women and recent mothers. [Google Scholar]
- Paradis AD, Fitzmaurice GM, Koenen KC, Buka SL. Maternal smoking during pregnancy and criminal offending among adult offspring. Journal of Epidemiology and Community Health. doi: 10.1136/jech.2009.095802. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton JW, Wardlaw DM, Graczyk PA, Bloodworth MR, Tompsett CJ, Weissberg RP. Social and emotional learning: A framework for promoting mental health and reducing risk behavior in children and youth. Journal of School Health. 2000;70:179–185. doi: 10.1111/j.1746-1561.2000.tb06468.x. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Kasza K, Biesecker G, Wright RJ, Wakschlag LS. Women who remember, women who do not: A methodological study of maternal recall of smoking in pregnancy. Nicotine & Tobacco Research. 2009;11:1166–1174. doi: 10.1093/ntr/ntp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Prescott CA. Using the Mplus computer program to estimate models for continuous and categorical data from twins. Behavior Genetics. 2004;34:17–40. doi: 10.1023/B:BEGE.0000009474.97649.2f. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, Hay DF, Van den Bree M, Thapar A. Disentangling prenatal and inherited influences in humans with an experimental design. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2464–2467. doi: 10.1073/pnas.0808798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Pickles A, Murray R, Eaves LJ. Testing hypotheses on specific environmental causal effects on behavior. Psychological Bulletin. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Parr T, Neale MC, Rutter M, Angold A, Eaves LJ. Maternal smoking during pregnancy and risk to boys' conduct disturbance: An examination of the causal hypothesis. Biological Psychiatry. 2003;53:130–135. doi: 10.1016/s0006-3223(02)01477-4. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: Which one is worse? Journal of Pharmacology and Experimental Therapeutics. 1998;285:931–945. [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. Journal of abnormal child psychology. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Statistics Sweden. Educational attainment of the population. [Google Scholar]
- Statistics Sweden. Multi-Generation Register 2005 – A description of contents and quality. Statistics Sweden: Örebro; 2006. [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, Harold G, Hay D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. American Journal of Psychiatry. 2003;160:1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, Van den Bree M, Rutter M, Harold G. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: Evidence from a novel design. Biological Psychiatry. 2009;66:722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, Dukic V, Blaire RJR, Leventhal BL, Cox NJ, Burns JL, Kasza KE, Wright RJ, Cook EHJ. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Molecular Psychiatry. 2010;15:928–937. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Leventhal BL, Pine DS, Pickett KE, Carter AS. Elucidating early mechanisms of developmental psychopathology: The case of prenatal smoking and disruptive behavior. Child Development. 2006;77:893–906. doi: 10.1111/j.1467-8624.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Sankis LM. Adult psychopathology: Issues and controversies. Annual Review of Psychology. 2000;51:377–404. doi: 10.1146/annurev.psych.51.1.377. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Espy KA, Stopp C, Respass J, Stewart P, Jameson TR, Gilbert DG, Huggenvik JI. Gene-environment interactions across development: Exploring DRD2 genotype and prenatal smoking effects on self-regulation. Developmental Psychology. 2009;45:31–44. doi: 10.1037/a0014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1992. [Google Scholar]