Abstract
Increased glutamatergic neurotransmission appears to mediate the reinforcing properties of drugs of abuse, including ethanol (EtOH). We recently reported that the administration of ceftriaxone (CEF), a β-lactam antibiotic known to upregulate glutamate transporter 1 (GLT1) levels/activity, decreased the maintenance of EtOH intake in adult male alcohol-preferring (P) rats. In the present study, we tested whether CEF administration would reduce the acquisition and maintenance of EtOH drinking in adolescent and adult female P rats. The rats were treated with saline or 200 mg/kg ceftriaxone for 7 days (starting at 35 or 75 days old, respectively) followed by the EtOH acquisition test. Five weeks later the effects of CEF were examined regarding the maintenance of EtOH intake. For the maintenance test, half of the animals that received CEF during acquisition received CEF for 7 days and the other half received saline for 7 days. Saline-treated acquisition animals were treated similarly. The results indicated that pretreatment with ceftriaxone reduced the maintenance of EtOH intake in both animals that started as adolescents and those that started as adults. However, the beneficial effect of CEF was more pronounced in rats pretreated with CEF as adults compared with rats pretreated as adolescents. Reductions in EtOH intake by ceftriaxone were paralleled by an upregulation of GLT1 protein levels in both the nucleus accumbens (µ25% in rats starting at both ages) and prefrontal cortex (µ50% in rats starting as peri-adolescents and µ65% in those starting as adults). These findings provide further support for GLT1-associated mechanisms in high alcohol consuming behavior, and hold promise for the development of effective treatments targeting alcohol abuse and dependence.
Keywords: Ceftriaxone, EAAT2, acquisition, maintenance
Introduction
Over half of adult Americans have a family history of alcoholism or alcohol (ethanol) abuse (Alcoholism, 2009), and a subset of this group has this trait in multiple generations. Young men and women are initiating alcohol use earlier and experiencing more alcohol-related problems than ever before (Quine and Stephenson, 1990, Kandel et al., 1997, Nelson et al., 1998, Miller et al., 2001, Pitkanen et al., 2005, Miller et al., 2007, Bava and Tapert, 2010, Gore et al., 2011). This is significant as early onset of alcohol use is a strong predictor of future alcohol dependence (Chou and Pickering, 1992, Anthony and Petronis, 1995, Grant and Dawson, 1997, Hawkins et al., 1997). Additionally, nearly half of all individuals meeting life-time diagnostic criteria for alcohol dependence do so by the age of 21, with this percentage increasing to approximately 65% by the age of 25 (Hingson et al., 2006). The danger of alcohol abuse among youth is compounded by the fact that the brain continues to mature during adolescence and young adulthood [c.f., (Spear, 2010) for an overview]. Thus, it is clear that a greater understanding of alcohol abuse and its consequences among youth is needed. However, the effects of alcohol may, or may not, differ between the peri-adolescent and adult subject. Thus, when addressing this developmental question it is important to evaluate whether observed effects during peri-adolescence are also seen during adulthood.
In important reviews, Spear and colleagues have indicated that the boundaries of adolescence for rats often differ given the parameters (e.g., behavioral vs. neurochemical) examined (Spear and Brake, 1983, Spear, 2000, 2007). Nonetheless, neurochemical and neurobehavioral differences from postweanling through adulthood support an adolescent developmental window of postnatal days (PNDs) 28 to 42 (Spear and Brake, 1983, Spear, 2000, 2007). When assessing the effects of pharmacological pretreatment, during adolescence, on adult behaviors, Spear has suggested that this conservative window (PNDs 28 to 42) could be extended to PND 60 (Spear, 2000, 2004). This extended window allows one to examine the earliest adolescent/pubertal changes in the female rat as well as the latest adolescent/pubertal changes in the male rat. These windows of development correspond with adolescent (a) changes in glutamatergic i N-methyl-D-aspartate (NMDA) receptor binding of the prefrontal cortex (PFC) (Insel et al., 1990); (b) decreased excitatory synaptic transmission in the nucleus accumbens (Acb) relative to juveniles (Kasanetz and Manzoni, 2009); (c) greater cerebral metabolic activity relative to adults (Chugani et al., 1987, Spear, 2000, 2007); and (d) synaptic pruning/remodeling of subcortical regions, in early peri-adolescence, and cortical regions, in later peri-adolescence (Trommer et al., 1996, Casey et al., 2000, Dumas, 2004, Schochet et al., 2008).
Changes in glutamatergic neurotransmission affect many aspects of neuroplasticity associated with alcohol dependence. For example, neuroadaptations in the glutamatergic system appear to mediate ethanol tolerance, dependence, and withdrawal (Krystal et al., 2003). Additionally, the effects of ethanol withdrawal are linked to an increase in extracellular glutamate levels in rats made dependent on ethanol (Rossetti and Carboni, 1995). Ethanol-induced neuroadaptations of the glutamatergic system include alterations in N-methyl-D-aspartate (NMDA) receptor activity (Grant et al., 1990, Sanna et al., 1993, Snell et al., 1996, Chen et al., 1997). For instance, rapid withdrawal from chronic ethanol results in phosphorylation and re-localization of synaptic NMDA receptors (Clapp et al., 2010). In addition, chronic ethanol induced increases in extracellular glutamate levels are associated with enhanced NMDA-receptor sensitivity in the Acb (Siggins et al., 2003).
Importantly, ethanol administration for one week resulted in decreased glutamate uptake in the Acb of male Sprague Dawley rats (Melendez et al., 2005). In addition, chronic ethanol consumption for 20 months down-regulates glutamate uptake in the cerebral cortex of alcohol-preferring cAA rats (Schreiber and Freund, 2000). Extracellular glutamate levels are regulated by several glutamate transporters located in neurons and glia (Gegelashvili and Schousboe, 1997, Seal and Amara, 1999, Anderson and Swanson, 2000). Glutamate transporter 1 [(GLT1), or its human homolog, the excitatory amino acid transporter 2 (EAAT2)], is the primary transporter that regulates the removal of extracellular glutamate in the central nervous system (CNS) (Ginsberg et al., 1995, Rothstein, 1995, Rothstein et al., 1995, Danbolt, 2001, Mitani and Tanaka, 2003).
The role of GLT1 in chemical dependency has been studied in drug abuse models, including those associated with excessive ethanol intake. Functional activation of GLT1 appears to reduce the rewarding effects of cocaine, morphine and methamphetamine (Nakagawa et al., 2005). In addition, upregulation of GLT1 activity by ceftriaxone, a beta-lactam antibiotic, attenuates cue-induced cocaine-seeking behavior (Sari et al., 2009, Knackstedt et al., 2010). Importantly, our laboratory has recently reported that upregulation of GLT1 level/activity by ceftriaxone resulted in a dose-dependent, long-lasting reduction in ethanol intake by adult male alcohol-preferring (P) rats (Sari et al., 2011). In the present study, we investigated the effects of up-regulating GLT1 level, by ceftriaxone, on the acquisition of ethanol intake in both peri-adolescent and adult female P rats. We also investigated the effects of ceftriaxone on the maintenance of ethanol intake in these animals five weeks after the acquisition test. Since ceftriaxone-induced reductions in ethanol intake in male P rats is associated with upregulation of GLT1 levels in the PFC and Acb, the present study also assessed whether ceftriaxone-induced reductions in ethanol intake by female P rats were associated with upregulation of GLT1 expression levels in these two brain reward regions, as well.
Materials and Methods
Animals
Adolescent and adult female P rats (35 and 75 days of age at the start of the experiment, respectively) were used in this study. These rats were obtained from the Indiana Alcohol Research Center breeding colonies (Indianapolis, IN). No more than 2 rats from a litter were included in any condition or interaction between conditions. This was done to avoid litter effects and increase the generalizability of the findings (Holson and Pearce, 1992). Rats were housed in a temperature- (21°C) and humidity- (50%) controlled vivarium which was maintained on a 12h reverse-light/dark cycle (lights on at 2200h). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine and are in accordance with guidelines set by the Institutional Animal Care and Use Committee of the National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals.
Adolescent female P rats
First Treatment Cycle/Acquisition of Ethanol Drinking Phase
Subjects were group-housed at the start of the study. Each rat received 7 consecutive injections (i.p.; 1x/day) with 200 mg/kg ceftriaxone (n = 20), or the equivalent volume (2 ml/kg) of vehicle (sterile saline; n = 19). Subjects had free access to food and water throughout the experiment. The day following the first treatment cycle (i.e., 42 days of age), subjects were individually housed in hanging wire-mesh cages. Animals were given 24-hr concurrent access to 15% and 30% v/v ethanol. Measures of ethanol (g/kg) and water (ml/kg) intake as well as body weights (g) were recorded 5 days/wk (Mon.-Fri.) for the next 5 weeks.
Second Treatment Cycle/Maintenance of Ethanol Drinking Phase
During Week 6, subjects were reassigned to groups for a second injection cycle (1x/day for 7 consecutive days). Approximately half of the rats were exposed to the same treatment that they had received during the first treatment cycle, whereas the remaining rats were exposed to the opposite treatment, yielding four treatment groups (n = 9–10/group): vehicle-vehicle [VEH-VEH]; ceftriaxone-vehicle [CEF-VEH]; vehicle-ceftriaxone [VEH-CEF]; and ceftriaxone-ceftriaxone [CEF-CEF]. During the second treatment cycle, defined here as the maintenance test phase, ethanol (g/kg) and water (ml/kg) intakes, as well as body weights were recorded daily. Ethanol (15% and 30% v/v), food, and water remained freely available.
Adult female P rats
First Treatment Cycle/Acquisition of Ethanol Drinking Phase
Subjects were group-housed at the start of the study. Each rat received 7 consecutive injections (i.p.; 1x/day) with 200 mg/kg ceftriaxone (n = 18), or the equivalent volume (2 ml/kg) of vehicle (sterile saline; n = 20). Subjects had free access to food and water. The day following the first treatment cycle (i.e., 82 days of age) subjects were housed individually in hanging wire-mesh cages. Animals were given 24-hr concurrent access to 15% and 30% v/v ethanol. Again, food and water were freely available. Measures of ethanol (g/kg) and water (ml/kg) intake, as well as body weights, (g) were recorded five days/wk (Mon.–Fri.) for five weeks.
Second Treatment Cycle/Maintenance of Ethanol Drinking Phase
During Week 6, subjects were reassigned to groups for a second cycle of ceftriaxone or vehicle injections (1x/day for 7 consecutive days). Half of the rats were exposed to the same treatment that they had received during the first treatment cycle, whereas the remaining rats were exposed to the opposite treatment, yielding four treatment groups (n = 9–10/group): vehicle-vehicle [VEH-VEH]; ceftriaxone-vehicle [CEF-VEH]; vehicle-ceftriaxone [VEH-CEF]; and ceftriaxone-ceftriaxone [CEF-CEF]. During the second treatment cycle, ethanol (g/kg) and water (ml/kg) intakes as well as body weights were recorded daily throughout the treatment period.
It is noteworthy that rats were treated similarly at both ages (adolescent and adult). Each rat received 7 consecutive injections (i.p.; 1x/day) with 200 mg/kg ceftriaxone, or the equivalent volume (2 ml/kg) of vehicle at adolescent or adult age.
Brain harvesting
Seven days after the completion of the second treatment cycle, rats were euthanized by carbon dioxide inhalation and decapitated. The brains were removed and frozen at −70°C. The PFC and Acb subsequently were micropunched using a cryostat maintained at −20°C, in order to keep the tissue frozen. After the brain regions were isolated, they were returned to the −70°C freezer until GLT1 protein levels were assayed with the Western blot procedure. We have selected randomly only saline- [VEH-VEH] and ceftriaxone-treated [VEH-CEF] groups at both ages for Western blot analysis.
Western Blot Assay
Western blot procedures to examine the level of GLT1 in the PFC and Acb were performed as described in recent studies (Sari et al., 2009, Sari et al., 2010, Sari et al., 2011). Briefly, proteins were extracted, quantified and equal amounts of each protein from control and treated groups were loaded in 10–20% glycine gel (Invitrogen) and separated in a mini-cell apparatus. Proteins then were electrophoretically transferred onto a nitrocellulose membrane. The membrane was incubated in blocking buffer (3% milk in TBST, 50 mM Tris HCl; 150 mM NaCl, pH7.4; 0.1 % Tween20); and then incubated with primary antibody guinea pig anti-GLT1 (Millipore) diluted 1:5000 in 3% milk in TBST overnight at 4°C. Membranes then were incubated with horseradish peroxidase (HRP)-labeled anti-guinea pig secondary antibody. The same membranes also were assessed for β-tubulin immunoblotting as a loading control. SuperSignal West Pico Chemiluminescence substrate (Pierce) was used to reveal HRP activity. Membranes then were exposed to Kodak BioMax MR film and developed using an SRX-101A developer. Immunoblots showing GLT1 and β-tubulin in the films were analyzed using the MCID system. The data are presented as percentage ratios of GLT1/ β-tubulin, relative to saline-control levels.
Statistical Analyses
First Treatment Cycle/Acquisition of Ethanol Drinking Phase
Three-way (Age × Acquisition Treatment × Acquisition Week) mixed ANOVAs, with the repeated measure being week, were conducted to evaluate the effects of ceftriaxone pretreatment on ethanol and water intake, as well as body weight during the acquisition of ethanol drinking test-phase. Significant interactions and main effects were analyzed further with protected Fisher’s LSD t-tests.
Second Treatment Cycle/Maintenance of Ethanol Drinking Phase
Four-way (Age × Acquisition Treatment × Maintenance Treatment × Test Day) mixed ANOVAs with repeated measures for Treatment and Test Day were conducted to evaluate the effects of ceftriaxone exposure on body weight, water intake, and the maintenance of ethanol drinking across the seven days of treatment. Significant interactions and main effects were analyzed further with appropriate analyses. For instance, following a significant interaction and to determine the effects of ceftriaxone, a priori Dunnett’s t-tests were conducted on each Test Day during the maintenance-phase of the experiment. Alpha was set at 0.05 for all statistics.
Western blot data
The percentage ratios of GLT1/ β-tubulin in PFC and Acb, for each age at acquisition, were analyzed using single-sample t-tests to determine upregulation of protein expression. Because multiple separate analyses were done, alpha was set at p = 0.01 for these analyses.
Results
Effects of ceftriaxone on body weight (g) during acquisition of drinking
The 3-way mixed ANOVA revealed a significant Age × Acquisition Week [F(4,292) = 811.181, p < 0.001] interaction; and significant main effects of Age [F(1,73) = 190.627, p < 0.001] and Acquisition Week [F(4,292) = 2852.860, p < 0.001], but none of the Treatment effects were significant (Data not shown).
Effects of ceftriaxone on body weight (g) during maintenance of drinking
The 4-way mixed ANOVA revealed significant Age × Maintenance Treatment × Test Day [F(6,420) = 2.936, p = 0.008]; Age × Maintenance Treatment [F(1,70) = 4.353, p = 0.041]; Maintenance Treatment × Test Day [F(6,420) = 2.312, p = 0.033]; and Age × Test Day [F(6,420) = 8.234, p < 0.001] interactions and significant main effects of Age [F(1,70) = 37.061, p < 0.001] and Test Day [F(6,420) = 23.974, p < 0.001]. In general, animals that started as adolescents weighed less than animals that started as adults. Also, saline pretreated adolescent rats receiving ceftriaxone during the maintenance test weighed more than the other three groups, which did not differ in body weight; whereas, saline pretreated adult rats receiving ceftriaxone during the maintenance test weighed less than the other three groups, which did not differ in body weight. However, none of the simple main effects of treatment for each test day were significant, and none of the ceftriaxone-treated groups differed from their age-respective control (Data not shown).
Effects of ceftriasxone on water intake (ml/kg) during acquisitiion
The 3-way mixed ANOVA revealed significant Age × Acquisition Treatment × Acquisition Week [F(4,292) = 22.883, p < 0.001]; Age × Acquisition Treatment [F(1,73) = 7.517, p = 0.008]; Age × Acquisition Week [F(4,292) = 24.733, p < 0.001]; and Acquisition Treatment × Acquisition Week [F(4,292) = 18.766, p < 0.001] interactions and significant main effects of Age [F(1,73) = 121.524, p < 0.001]; Acquisition Treatment [F(1,73) = 19.348, p < 0.001] and Acquisition Week [F(4,292) = 71.839, p < 0.001]. In general, adolescent animals consumed more water per kg body weight than adult animals. In addition, whereas ceftriaxone pretreated adolescent animals consumed more water than saline pretreated adolescent animals, ceftriaxone pretreated adult animals did not differ from saline pretreated adult animals in water intake (Data not shown).
Effects of ceftriaxone on water intake (ml/kg) during maintenance of drinking
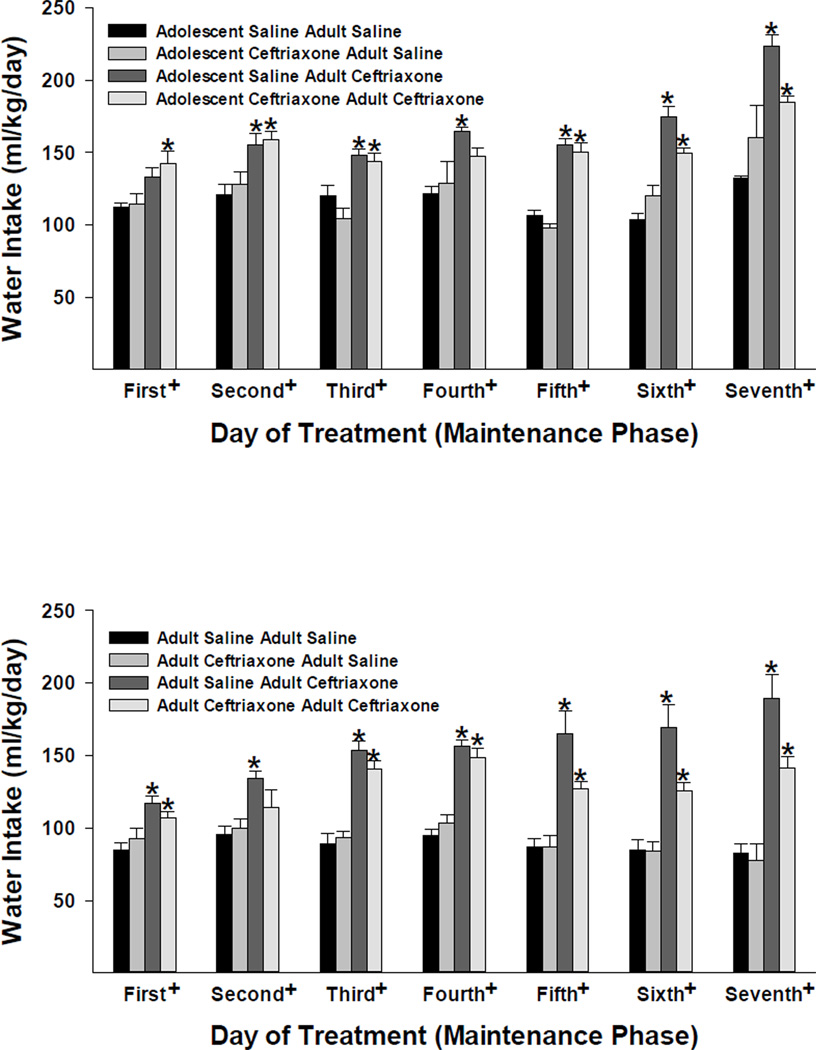
The 4-way mixed ANOVA revealed significant Acquisition Treatment × Maintenance Treatment × Test Day [F(6,414) = 4.624, p < 0.001]; Acquisition Treatment × Maintenance Treatment [F(1,69) = 7.965, p = 0.006]; Acquisition Treatment × Test Day [F(6,414) = 2.564, p = 0.019]; Maintenance Treatment × Test Day [F(6,414) = 13.826, p < 0.001]; and Age × Test Day [F(6,414) = 7.830, p < 0.001] interactions and main effects of Maintenance Treatment [F(1,69) = 134.539, p < 0.001]; Age [F(1,69) = 13.982, p < 0.001] and Test Day [F(6,414) = 26.029, p < 0.001]. In general, ceftriaxone increased water intake with the effect being greater in animals that initiated ethanol intake in adulthood vs. those initiating ethanol in adolescence (Figure 3). In addition, younger adult animals consumed more water than the older adult animals (i.e., those that initiated ethanol drinking in adulthood).
Figure 3.
The effects of ceftriaxone (200 mg/kg) on average (± S.E.M.) water intake (ml/kg/day) for adolescent and adult female P rats during the maintenance of ethanol-drinking test (seven consecutive days). Four groups of animals from each age (adolescence in the top panel and adulthood in the bottom panel) at onset of ethanol-drinking behavior are depicted. The two bars on the left, for each respective test day, indicate data from animals that received saline during the maintenance test, whereas the two bars on the right indicate data from animals that received ceftriaxone during the maintenance test. +, indicates a significant (p < 0.05) main effect of treatment (four groups) for that respective test day. *, indicates a significant (p < 0.05) difference between the respective group and the saline-saline control group (Dunnett’s t-test).
Effects of ceftriaxone on the acquisition of ethanol drinking (g/kg/day)
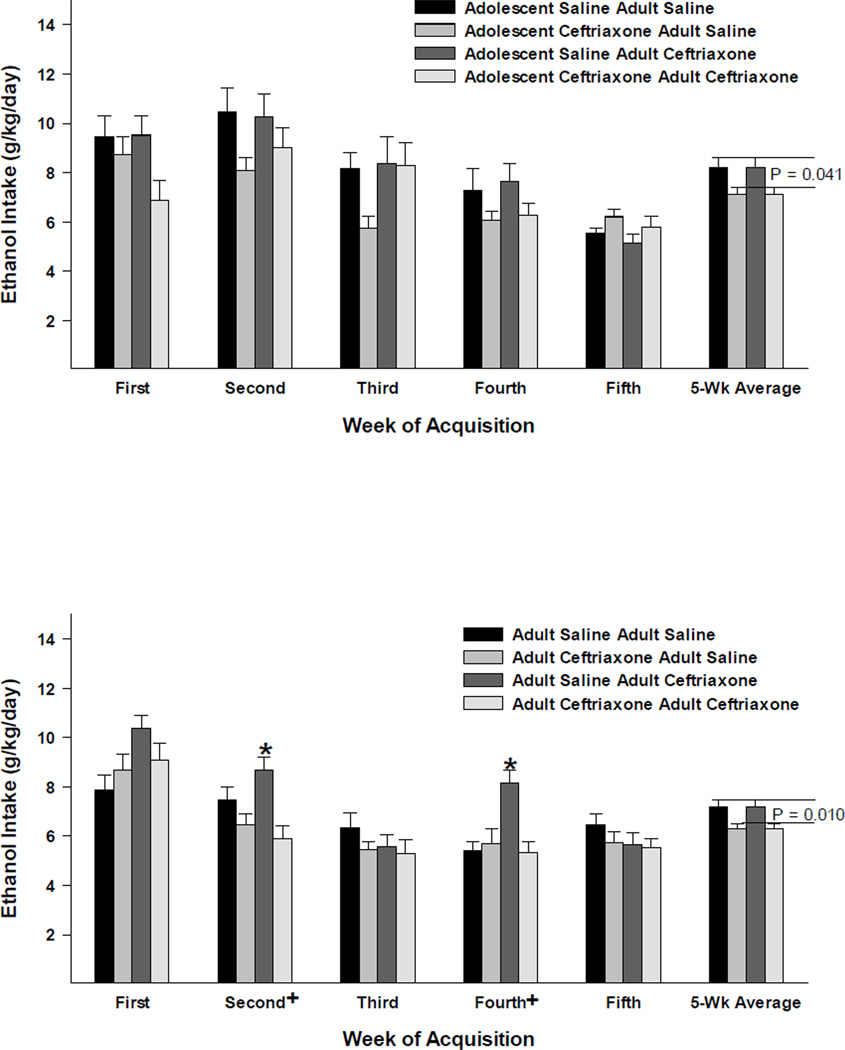
The 3-way mixed ANOVA revealed significant Age × Acquisition Week [F(4,292) = 12.123, p < 0.001]; Acquisition Treatment × Acquisition Week [F(4,292) = 4.405, p = 0.002] interactions and significant main effects of Age [F(1,73) = 8.431, p = 0.005]; Acquisition Treatment [F(1,73) = 6.329, p = 0.014]; and Acquisition Week [F(4,292) = 50.509, p < 0.001]. As seen in Figure 1, in general, ceftriaxone pre-treated animals consumed less ethanol than their saline control counterparts; as depicted by 5-Wk Average for peri-adolescent (upper) and adult (lower) female P rats where those receiving ceftriaxone pre-treatment were compared with those receiving saline pre-treatment regardless of subsequent treatment.
Figure 1.
The effects of ceftriaxone (200 mg/kg) on average (± S.E.M.) ethanol (15% and 30% available concurrently) intake (g/kg/day) for adolescent (upper panel) and adult (lower panel) female P rats during the acquisition (five weeks) of 24-hour free-choice drinking. +, indicates a significant (p < 0.05) simple main effect of treatment [split into four groups according to their pre-treatment before acquisition of ethanol intake and subsequent treatment (as depicted in Figure 2) during maintenance of ethanol drinking] for the respective acquisition week. *, indicates a significant difference between the respective (maintenance) treatment group and the saline-saline control group. The 5-Wk Average depicts the average ethanol intake for the animals that were pretreated with saline vs. ceftriaxone before the acquisition of ethanol intake, with the corresponding p-values reflecting significant simple main effects for treatment across acquisition weeks for each age group. In general, ceftriaxone pre-treated animal consumed less ethanol than those pre-treated with saline and animals initiating ethanol-drinking behavior during adolescence consumed more ethanol than those initiating this behavior in adulthood.
Effects of ceftriaxone on the maintenance of ethanol drinking (g/kg/day)
The 4-way mixed ANOVA revealed significant Age × Acquisition Treatment × Maintenance Treatment × Test Day [F(6,414) = 4.231, p < 0.001]; Age × Acquisition Treatment × Test Day [F(6,414) = 3.082, p = 0.006]; Age × Maintenance Treatment × Test Day [F(6,414) = 3.389, p = 0.003]; Maintenance Treatment × Test Day [F(6,414) = 5.618, p < 0.001]; Age × Test Day [F(6,414) = 5.943, p < 0.001] interactions and significant main effects of Age [F(1,69) = 8.850, p = 0.004]; Maintenance Treatment [F(1,69) = 65.529, p < 0.001]; and Test Day [F(6,414) = 61.856, p < 0.001]. As seen in Figure 2, in animals that initiated ethanol intake during peri-adolescence (upper panel), animals pretreated with saline and then receiving ceftriaxone during the maintenance test had significantly reduced ethanol intake relative to the double-saline control group during the maintenance test days two through seven. However, animals that were pretreated with ceftriaxone and then receiving ceftriaxone during the maintenance test displayed significantly decreased ethanol intake, relative to the double-saline control group, on test days one through three only. In animals that initiated ethanol intake during adulthood (lower panel), animals pretreated with saline and then receiving ceftriaxone during the maintenance test had significantly reduced ethanol intake, relative to the double-saline control group during maintenance test days five through seven only. However, animals that were pretreated with ceftriaxone and then receiving ceftriaxone during the maintenance test displayed significantly decreased ethanol intake, relative to the double-saline control group, on test days three through seven. Thus, ceftriaxone pretreatment in adolescence reduced the subsequent effects of ceftriaxone in adulthood, whereas ceftriaxone pretreatment in adulthood increased the subsequent effects of ceftriaxone in adulthood.
Figure 2.
The effects of ceftriaxone (200 mg/kg) on average (± S.E.M.) ethanol (15% and 30% available concurrently) intake (g/kg/day) for adolescent and adult female P rats during the maintenance of ethanol-drinking test (seven consecutive days). Four groups of animals from each age (adolescence in the top panel and adulthood in the bottom panel) at onset of ethanol-drinking behavior are depicted. The two bars on the left, for each respective test day, indicate data from animals that received saline during the maintenance test, whereas the two bars on the right indicate data from animals that received ceftriaxone during the maintenance test. +, indicates a significant (p < 0.05) main effect of treatment (four groups) for that respective test day. *, indicates a significant (p < 0.05) difference between the respective group and the saline-saline control group (Dunnett’s t-test). #, indicates the result of the Dunnett’s t-test had a p-value of 0.050.
Effects of ceftriaxone on GLT1 expression in PFC and Acb of animals that started either during adolescence or adulthood
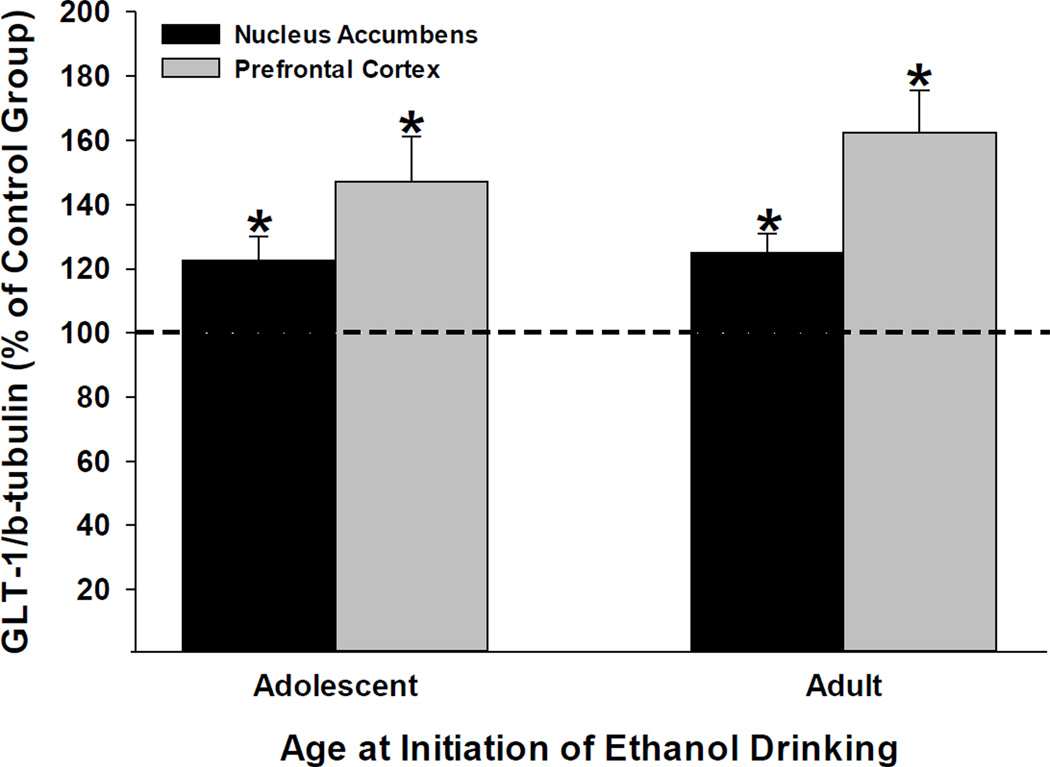
We examined the effects of ceftriaxone on GLT1 expression levels in the PFC and Acb in animals that started ethanol drinking in adolescence as well as those that started drinking in adulthood using Western blot assays (Fig. 4). We have tested only randomly saline- [VEH-VEG] and ceftriaxone-treated [VEH-CEF] groups. Ceftriaxone significantly upregulated GLT1 protein expression in both brain regions in animals that initiated ethanol consumption in adolescence as well as those that initiated ethanol intake in adulthood (t-values > 10, p-values ≤0.002: Figure 4). Note that the protein data were collected from animals 1 week after the maintenance test-phase was completed. Figure 5 indicates there were no differences in β-tubulin protein expression levels between any of the age and treatment groups, which were confirmed by statistical analyses (p’s > 0.5).
Figure 4.
Effects of ceftriaxone on average (± S.E.M.) GLT1 protein expression levels [GLT1/β-tubulin (% of Control Group)] within the nucleus accumbens (left bar) and prefrontal cortex (right bar) from brains harvested seven days after the maintenance test-phase of the experiment was completed. Animals that initiated ethanol intake during adolescence are depicted on the left, whereas animals that initiated ethanol intake during adulthood are depicted on the right. Both the ceftriaxone group (n = 4) and the control group (n = 4) received saline prior to the acquisition of ethanol intake phase of the experiment. *, indicates a significant (p < 0.01) difference between the ceftriaxone-treated group and the 100% control-value (single-sample t-tests). Ceftriaxone induced a significant up-regulation of GLT1 protein expression levels in both the nucleus accumbens and prefrontal cortex regardless of when the animals initiated ethanol drinking.
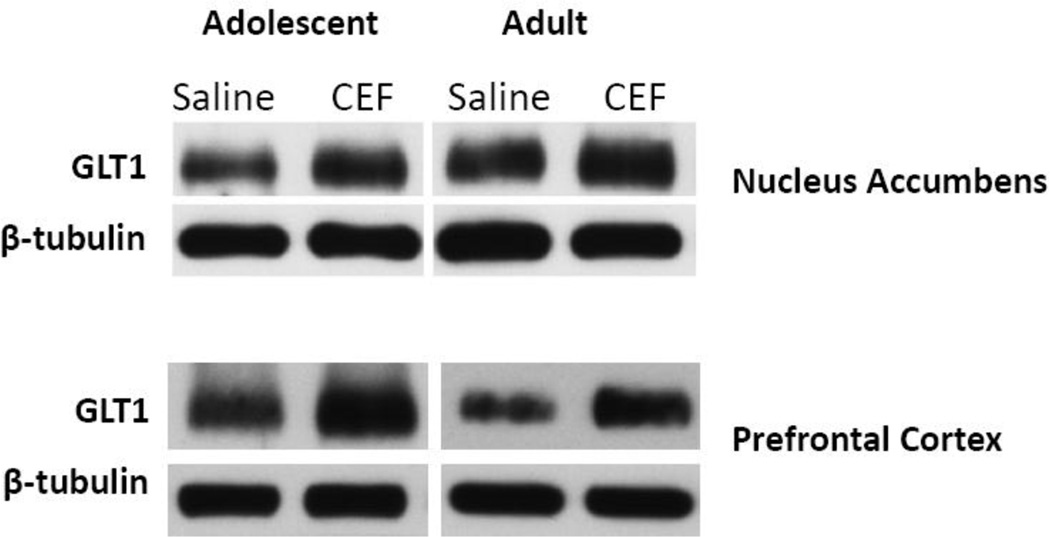
Figure 5.
Effects of ceftriaxone [VEH-CEF] (200 mg/kg, on the right) vs. saline [VEH-VEH] (on the left) on protein expression levels of GLT1 and β-tubulin within the nucleus accumbens (top panel) and prefrontal cortex (bottom panel). Data from animals that initiated ethanol drinking during adolescence are depicted on the left, whereas data from animals that initiated ethanol drinking during adulthood are depicted on the right. Visual and statistical analyses (p’s > 0.5) revealed that β-tubulin levels did not differ between any of the groups.
Discussion
We demonstrated in this study that pretreatment with ceftriaxone during the maintenance phase reduced ethanol intake compared with the double-saline control groups in both animals that started as adolescents and adults. However, ceftriaxone has less effect when it is administered during acquisition. These findings parallel previous findings from our recent study in male P rats (Sari et al., 2011), such that ceftriaxone significantly reduces the maintenance of ethanol intake in both male and female P rats. Similarly, water intake was increased following ceftriaxone treatment in both age groups during maintenance paradigm in these female P rats as compared to saline-treated rats. Moreover, the present results replicated the finding (Sari et al., 2011) that ceftriaxone-induced reductions in ethanol intake and increased water intake in male P rats, and these effects were associated with a significant upregulation of GLT1 expression level in the PFC and Acb. It is noteworthy that a recent study performed in our lab showed that ceftriaxone did not affect sucrose consumption as a motivated behavioral drinking control in male P rats, which suggests the specificity of the drug’s effect on ethanol intake (Sari et al., 2011).
The mesocorticolimbic system, including the two key regions: the PFC (Goldstein and Volkow, 2002) and the Acb (Childress et al., 1999), mediates the development and expression of alcohol abuse and alcoholism. For instance, glutamatergic inputs from the PFC to the Acb and ventral tegmental area (VTA) play an important role in addiction to, and craving for, commonly abused drugs and alcohol (Childress et al., 1999, Goldstein and Volkow, 2002). Thus, the negative association between lower ethanol intake and higher GLT1 expression levels in these brain regions following ceftriaxone treatment supports the role of mesocorticolimbic glutamate in ethanol abuse.
Regarding cocaine abuse, we recently reported that ceftriaxone attenuates cue-induced reinstatement of cocaine-seeking behavior by male rats in a dose-dependent manner (Sari et al., 2009). Similarly, Kalivas and colleagues (Knackstedt, 2010) reported ceftriaxone reduced cue-induced cocaine relapse-like behavior in male rats. Regarding ethanol intake, we recently reported that male P rats treated with ceftriaxone, for five days during the maintenance of drinking, showed a significant dose-dependent reduction in ethanol intake compared to saline-treated rats (Sari et al., 2011). In this previous study (Sari et al., 2011), the attenuation by ceftriaxone had long-lasting effects on ethanol intake in male P rats. It is noteworthy that in these studies (Sari et al., 2009, 2011) the attenuation of cue-induced reinstatement of cocaine-seeking behavior and ethanol intake were negatively associated with upregulation of GLT1 expression levels in the PFC and Acb. In the present study, we tested whether functional activation of GLT1 by ceftriaxone may have similar effects on the maintenance of ethanol consumption by female P rats. The present findings indicate that ceftriaxone induced similar effects on the maintenance of ethanol intake and GLT1 expression levels as those seen in male P rats (Sari et al., 2011). In addition, similar to the findings reported by Sari and colleagues (Sari et al., 2011), ceftriaxone-induced reductions in ethanol intake were associated with increased water intake during ceftriaxone treatment. It appears that these increases in water intake are, at least in part, a compensatory increase in fluid intake coinciding with reductions in ethanol intake. Previous work with the P line of rats indicates that female P rats consume significantly more water than their male counterparts [e.g., (Bell et al., 2006)], which indicates that female P rats may display a greater physiological response to defend optimal levels of hydration. Moreover, the compensatory increase in water intake during ceftriaxone treatment was greater in the present study, which used female P rats, compared with our laboratory’s previous study using male P rats (Sari et al., 2011).
The present study also tested whether peri-adolescent and adult female P rats pretreated with ceftriaxone would display disrupted acquisition of ethanol intake. In fact, pretreatment with ceftriaxone did not affect the acquisition of ethanol intake, compared with controls, in both adolescent and adult rats. However, as noted above, ceftriaxone was effective in reducing the maintenance of ethanol drinking. It is noteworthy that ceftriaxone is less effective in reducing the subsequent maintenance of ethanol intake when rats were pretreated with the drug prior to acquisition during adolescence when compared with those that had received pretreatment prior to acquisition during adulthood. This difference might be due to differences in neurochemistry between these two stages of development. It has been suggested that the functional activity and absolute expression levels of glutamate transporters in the CNS are different between those seen during adolescence versus adulthood (Sims and Robinson, 1999). Moreover, regulation of extracellular levels of glutamate is crucial for glutamate transmission and synaptic plasticity, including that observed during brain maturation (Danbolt, 2001, Diamond, 2005, Thomas et al., 2011). In addition, neuroadaptative plasticity of NMDA receptors is implicated in the enhanced vulnerability to ethanol dependence during adolescence (Carpenter-Hyland and Chandler, 2007). Therefore, because the glutamatergic system is in flux during adolescence, it may be more sensitive to long-term alterations induced by ceftriaxone. In addition, the effects of isolation stress during peri-adolescence, as a critical stage of development, may have altered ceftriaxone’s subsequent efficacy. Thus, future research is needed to determine whether the magnitude of ceftriaxone-induced increases in GLT1 expression levels is similar during peri-adolescence as that observed during adulthood, and whether the stress of single-housing during peri-adolescence interacted with the effects of ceftriaxone to reduce its efficacy when tested later in adulthood. It is noteworthy that ethanol exposure during peri-adolescence can disrupt neuronal plasticity including glutamatergic neurons, and can increase the risk for developing alcohol abuse and dependence (Pascual et al., 2009, Chin et al., 2010, Ehlers and Criado, 2010, Guerri and Pascual, 2010, Nixon et al., 2010).
Although the precise mechanism by which ceftriaxone enhances the expression level of GLT1 is still unknown, there are at least two cellular pathways (which might have direct, indirect, or interactive effects) involved in GLT1 expression. Moreover, these two cellular pathways may either directly or indirectly interact with each other. First, the canonical NF-kB signaling pathway appears to be necessary for ceftriaxone-induced increases in GLT1 levels within astrocytes (Lee et al., 2008). Secondly, the mammalian target for rapamycin (mTOR) and its associated pathway have been implicated in regulating GLT1 expression and glutamate uptake in vitro (Wu et al., 2010). These authors (Wu et al., 2010) found that phosphorylation of mTOR by Akt is involved in increased GLT1 expression (Wu et al., 2010). Further research is needed to determine how ethanol would interact with either, or both, of these pathways and their modulation of GLT1 expression levels.
We conclude here that repeated ceftriaxone treatment consistently reduces the maintenance of ethanol intake by adult male P rats (Sari et al., 2011) were extended to demonstrate this effect in female P rats as well. Also, pretreatment with ceftriaxone during adolescence appears to decrease its efficacy to reduce subsequent maintenance of ethanol intake during adulthood, when compared to those that received pretreatment during adulthood and underwent subsequent treatment during maintenance of ethanol drinking test. It should be noted that while the generalizability of ceftriaxone’s effects across the sexes appears strong for the adult data, the effects of ceftriaxone in peri-adolescent male P rats have not been examined thus far. Research examining the generalizability of ceftriaxone’s effects during peri-adolescence, across the sexes, would be important for a number of reasons including the fact that female mammals tend to mature faster than their male counterparts. Moreover, it is warranted to determine sex differences that might be found during peri-adolescence, and the specific role of a number of different neuromodulatory (e.g., hormone) systems that would be affected by ethanol exposure during this stage.
In summary, ceftriaxone appears to offer some off-label potential for interfering with the development and expression of high ethanol-drinking behavior. This compound may also prove efficacious in providing important information on the role of the glutamatergic system, particularly glutamate transporters, in some of the processes that promote high ethanol-drinking behavior. Further research is needed to determine if ceftriaxone’s ability to reduce alcohol drinking is specific to subjects with a genetic predisposition for high alcohol consumption. Overall, the present results indicate that ceftriaxone offers some promise as a prospective tool for developing treatments targeting and characterizing the neurobiology of alcoholism.
Highlights.
> CEF treatment reduced also the maintenance of ethanol intake. > The beneficial effect of CEF was pronounced in rats pretreated with CEF. > Reductions in ethanol intake were paralleled by an upregulation of GLT1 levels.
Acknowledgements
The presented research was supported in part by AA019458 (Y. Sari) and AA13522 (R.L. Bell).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest None of the authors have real or perceived conflicts of interest.
References
- Alcoholism RSo. Impact of Alcoholism and Alcohol Induced Disease on America. Austin, TX: Research Society on Alcoholism; 2009. [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychology review. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction biology. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav. 2007;86:200–208. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chen X, Michaelis ML, Michaelis EK. Effects of chronic ethanol treatment on the expression of calcium transport carriers and NMDA/glutamate receptor proteins in brain synaptic membranes. J Neurochem. 1997;69:1559–1569. doi: 10.1046/j.1471-4159.1997.69041559.x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin VS, Van Skike CE, Matthews DB. Effects of ethanol on hippocampal function during adolescence: a look at the past and thoughts on the future. Alcohol. 2010;44:3–14. doi: 10.1016/j.alcohol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Chou SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. British journal of addiction. 1992;87:1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of neurology. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Diamond JS. Deriving the glutamate clearance time course from transporter currents in CA1 hippocampal astrocytes: transmitter uptake gets faster during development. J Neurosci. 2005;25:2906–2916. doi: 10.1523/JNEUROSCI.5125-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas TC. Early eyelid opening enhances spontaneous alternation and accelerates the development of perforant path synaptic strength in the hippocampus of juvenile rats. Developmental psychobiology. 2004;45:1–9. doi: 10.1002/dev.20011. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR. Adolescent ethanol exposure: does it produce long-lasting electrophysiological effects? Alcohol. 2010;44:27–37. doi: 10.1016/j.alcohol.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Martin LJ, Rothstein JD. Regional deafferentation down-regulates subtypes of glutamate transporter proteins. J Neurochem. 1995;65:2800–2803. doi: 10.1046/j.1471-4159.1995.65062800.x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore FM, Bloem PJ, Patton GC, Ferguson J, Joseph V, Coffey C, Sawyer SM, Mathers CD. Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet. 2011;377:2093–2102. doi: 10.1016/S0140-6736(11)60512-6. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of substance abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010;44:15–26. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. Journal of studies on alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Archives of pediatrics & adolescent medicine. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain--I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug Alcohol Depend. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Manzoni OJ. Maturation of excitatory synaptic transmission of the rat nucleus accumbens from juvenile to adult. J Neurophysiol. 2009;101:2516–2527. doi: 10.1152/jn.91039.2008. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino Acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Miller ET, Turner AP, Marlatt GA. The harm reduction approach to the secondary prevention of alcohol problems in adolescents and young adults: considerations across a developmental spectrum. In: Monti PM, Colby SM, O'Leary TA, editors. Adolescents, Alcohol, and Substance Abuse: Reaching Teens through Brief Interventions. New York: Guilford Press; 2001. pp. 58–79. [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J Neurosci. 2003;23:7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav Brain Res. 2005;156:233–239. doi: 10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Nelson CB, Heath AC, Kessler RC. Temporal progression of alcohol dependence symptoms in the U.S. household population: results from the National Comorbidity Survey. Journal of consulting and clinical psychology. 1998;66:474–483. doi: 10.1037//0022-006x.66.3.474. [DOI] [PubMed] [Google Scholar]
- Nixon K, Morris SA, Liput DJ, Kelso ML. Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol. 2010;44:39–56. doi: 10.1016/j.alcohol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pitkanen T, Lyyra AL, Pulkkinen L. Age of onset of drinking and the use of alcohol in adulthood: a follow-up study from age 8–42 for females and males. Addiction. 2005;100:652–661. doi: 10.1111/j.1360-0443.2005.01053.x. [DOI] [PubMed] [Google Scholar]
- Quine S, Stephenson JA. Predicting smoking and drinking intentions and behavior of pre-adolescents: The influence of parents, siblings, and peers. Family Sys Med. 1990;8:191–200. [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Rothstein JD. Excitotoxicity and neurodegeneration in amyotrophic lateral sclerosis. Clin Neurosci. 1995;3:348–359. [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Annals of neurology. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Sanna E, Serra M, Cossu A, Colombo G, Follesa P, Cuccheddu T, Concas A, Biggio G. Chronic ethanol intoxication induces differential effects on GABAA and NMDA receptor function in the rat brain. Alcohol Clin Exp Res. 1993;17:115–123. doi: 10.1111/j.1530-0277.1993.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Sari Y, Prieto AL, Barton SJ, Miller BR, Rebec GV. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington's disease. J Biomed Sci. 2010;17:62. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochet TL, Bremer QZ, Brownfield MS, Kelley AE, Landry CF. The dendritically targeted protein Dendrin is induced by acute nicotine in cortical regions of adolescent rat brain. Eur J Neurosci. 2008;28:1967–1979. doi: 10.1111/j.1460-9568.2008.06483.x. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Freund WD. Glutamate transport is downregulated in the cerebral cortex of alcohol-preferring rats. Med Sci Monit. 2000;6:649–652. [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annual review of pharmacology and toxicology. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De Lecea L. Glutamatergic transmission in opiate and alcohol dependence. Ann N Y Acad Sci. 2003;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- Sims KD, Robinson MB. Expression patterns and regulation of glutamate transporters in the developing and adult nervous system. Crit Rev Neurobiol. 1999;13:169–197. doi: 10.1615/critrevneurobiol.v13.i2.30. [DOI] [PubMed] [Google Scholar]
- Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res. 1996;40:71–78. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Ann N Y Acad Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Spear LP. The developing brain and adolescent-typical behavior patterns: An evolutionary approach. In: Romer D, Walker EF, editors. Adolescent Psychopathology and the Developing Brain. New York: Oxford University Press; 2007. pp. 9–30. [Google Scholar]
- Spear LP. The Behavioral Neuroscience of Adolescence. New York: WW Norton; 2010. [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Developmental psychobiology. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Tian H, Diamond JS. The relative roles of diffusion and uptake in clearing synaptically released glutamate change during early postnatal development. J Neurosci. 2011;31:4743–4754. doi: 10.1523/JNEUROSCI.5953-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommer BL, Liu YB, Pasternak JF. Long-term depression at the medial perforant path-granule cell synapse in developing rat dentate gyrus. Brain research Developmental brain research. 1996;96:97–108. doi: 10.1016/0165-3806(96)00104-6. [DOI] [PubMed] [Google Scholar]
- Wu X, Kihara T, Akaike A, Niidome T, Sugimoto H. PI3K/Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochemical and biophysical research communications. 2010 doi: 10.1016/j.bbrc.2010.02.038. [DOI] [PubMed] [Google Scholar]