Abstract
Thymosin beta 4 (Tβ4), a G-actin sequestering peptide, increases oligodendrogenesis and improves functional outcome in models of neurological injury. The molecular mechanisms of Tβ4 mediated oligodendrogenesis are unclear. The p38 mitogen-activated protein kinase (p38MAPK) regulates oligodendrocyte (OL) differentiation and myelin gene expression in other models. Therefore, we investigated p38MAPK signaling pathways. We used primary rat neural progenitor cells (NPCs) and a mouse oligodendrocyte progenitor cell (OPC) line (N20.1 cells) to investigate the molecular mechanisms of Tβ4-enhanced oligodendrogenesis. NPCs were isolated from rat subventricular zone (SVZ) of the lateral ventricles (n=12). Primary NPCs and N20.1 cells were grown in the presence of 0, 25 and 50 ng/ml of Tβ4 (RegeneRx Biopharmaceuticals Inc, Rockville, MD) for 14 days. Quantitative real-time PCR and Western blot data showed significant induction of both expression and phosphorylation of p38MAPK with simultaneous inhibition of phosphorylation of extracellular signal regulated kinase (ERK1), c-Jun N-terminal kinase 1 (JNK1), leading to reduction of phosphorylation of c-Jun, a potent negative regulator of transcription of myelin genes. These effects were reversed with transfection of Tβ4siRNA. Our data indicate that Tβ4 treatment induces OL differentiation by inducing p38MAPK with parallel inactivation of ERK1 and JNK1, thus preventing the accumulation of phosphorylated c-Jun.
Keywords: N20.1 cells, subventricular zone, neruospheres, actin binding proteins
Introduction
Thymosin β4 (Tβ4) is a regenerative 43-amino acid peptide with a molecular weight of 4964 Daltons (Goldstein et al. 2005). Tβ4's fundamental action is sequestration of G-actin monomers which promote cell migration by inhibiting actin-cytoskeletal organization (Huff et al. 2004; Safer et al. 1991; Sanders et al. 1992). Tβ4 has multiple additional biological functions, including inhibiting inflammation and promoting regeneration in both dermal and cardiac injury models (Bock-Marquette et al. 2004; Malinda et al. 1999; Smart et al. 2007). In post-natal and adult murine cardiac myocardium models, Tβ4 regulated vasculogenesis, angiogenesis and arteriogenesis in part by mobilizing, recruiting and promoting the differentiation of progenitor cells (Smart et al. 2007). Moreover, Tβ4 promoted cardiomyocyte survival, improved cardiac function, and reduced scar formation after myocardial infarction in adult mice (Bock-Marquette et al. 2004). Tβ4 may improve cardiac function by increasing cardiomyocyte survival and stimulating epicardial progenitor cells to differentiate into smooth muscle and endothelial cell types to repair damaged myocardium.
Tβ4 treatment improves functional neurological outcome in a rat model of embolic stroke, a mouse model (EAE, experimental autoimmune encephalomyelitis) of multiple sclerosis and a rat model of traumatic brain injury (Morris et al. 2010; Xiong et al. 2010; Zhang et al. 2009). A common observation in these neurological diseases is that Tβ4 targets axonal repair by stimulation of oligoprogenitor cells (OPC) in the SVZ and in the intact white matter. Tβ4 increased the number of mature oligodendrocytes (OLs) leading to an increase in myelinated axons after injury, suggesting that Tβ4 enhances remyelination. Remyelination occurs only from OPCs and not from surviving OLs or from mature surviving OLs adjacent to the injured axons (Franklin 2002; Franklin and Ffrench-Constant 2008; Nait-Oumesmar et al. 2008). Mature OLs are for the most part, unable to migrate or divide. Therefore, Tβ4 is hypothesized to improve neurological outcome by upregulation of OPCs and subsequent axonal remyelination.
The mechanisms of Tβ4 mediated oligodendrogenesis are unclear, however, Chew et al, recently demonstrated in embryonic day 20 rats that p38 mitogen-activated protein kinase (p38MAPK) regulates OPC differentiation and myelin gene expression by suppressing phosphorylated c-Jun activity as accumulation of phosphorylated c-Jun negatively regulates the myelin gene promoter activity in OPCs (Chew et al. 2010). Moreover, p38MAPK upregulation was observed to antagonize c-Jun-N-terminal kinase (JNK1) which phosphorylates c-Jun and is associated with neuronal apoptosis. Following a similar experimental design from Chew et al, (Chew et al. 2010), we hypothesize that Tβ4 treatment upregulates p38MAPK with subsequent suppression of JNK1 activity and phosphorylated c-Jun accumulation in a primary rat subventricular zone (SVZ) neural progenitor cell model, and a mouse OL cell line (N20.1). We demonstrate that Tβ4 treatment induced expression of markers of mature OL, myelin basic protein (MBP) and 2’, 3’-cyclic nucleotide 3’-phosphodiesterase (CNPase) and upregulated p38MAPK activity with subsequent suppression of extracellular signal-regulated kinase (ERK1) and JNK1 phosphorylation. These data indicate that Tβ4 treatment induces OL differentiation by inducing p38MAPK with parallel inactivation of ERK1 and JNK1, thus preventing the accumulation of phosphorylated c-Jun.
Materials and Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital. Discomfort and the number of animals needed to complete the study were minimized.
Preparation of SVZ cells and Tβ4 treatment
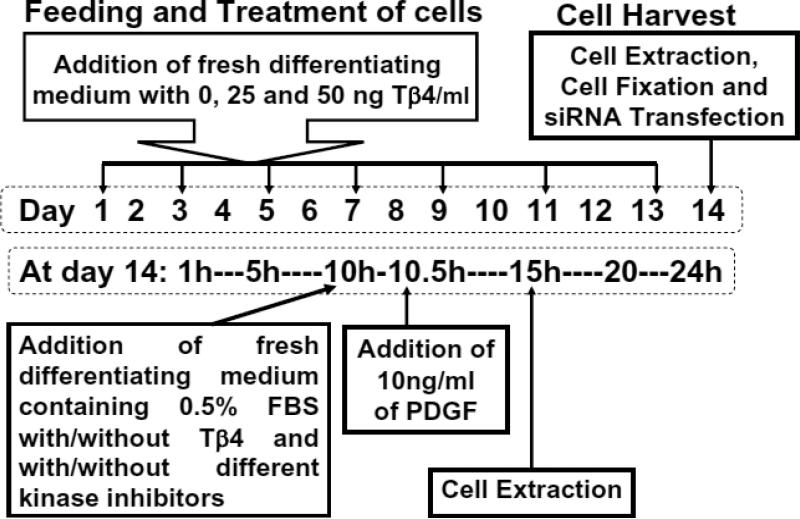
SVZ cells were dissociated from rat brains (n=12), as previously described (Zhang et al. 2004). The SVZ of the adult male rat brain was examined under a microscope (Olympus BX40; Olympus Optical, Tokyo, Japan) and was surgically dissected. SVZ cells were dissociated in DMEM medium containing 20 ng/mL of epidermal growth factor (EGF; R&D system, Minneapolis, MN, USA) and basic fibroblast growth factor (bFGF). Three separate cultures each containing SVZ cells from four rats were grown. The cells were plated at a density of 104cells/cm2 in DMEM medium containing 20ng/mL of EGF and bFGF. The generated neurospheres (primary spheres) were passed by mechanical dissociation and reseeded as single cells at a density of 104cells/cm2 in EGF-containing media. Passage 2 and 3 neurospheres were used in the present study. For the differentiation assay, the neurospheres were grown in differentiating medium (DMEM medium containing 2% fetal bovine serum (FBS)) for an additional 7 days (Liu et al. 2009). The cells (102cells/cm2) were then incubated at 37 °C for 14 days with differentiating medium with 0, 25 or 50 ng/mL of Tβ4 (RegeneRx Biopharmaceuticals Inc, Rockville, MD). The cell cultures were fed with fresh differentiating medium (with and without Tβ4) every 2 days (Fig. 1).
Fig. 1. Schematic diagram of experimental manipulation.
Rat SVZ cells and mouse N20.1 cells were fed with their differentiating medium and treated with 0, 25 and 50 ng/ml of Tβ4 (top box at left). These cells were fed and treated every two days indicated by arrows for 14 days (dotted rectangle box at center). On day 14, these cells were harvested indicated by an arrow as cell extraction for preparation of total RNA and proteins, cell fixation for immunostaining and siRNA transfection for Tβ4 silencing in these cells after the treatment with Tβ4 (top box at right). To determine the effect of kinase inhibitors and PDGF on these cells, the medium was changed with differentiating medium containing reduced FBS (0.5%) along with and without Tβ4 and different kinase inhibitors (rectangle box at left) on day 14 at hour 10 (10AM). After 30min, these cells were treated with 10ng PDGF/ml. These cells were incubated for additional 4.5h and then subjected to preparation of cell extracts for total RNA and proteins at hour 15 (3PM).
Cell cultures and Tβ4 treatment for a premature mouse oligodendrocyte cell line (N20.1)
A premature mouse oligodendrocyte cell line (N20.1) was generously provided by Dr. Anthony Campagnoni (University of California at Los Angeles). N20.1 cell line was obtained from mouse primary cultures of OLs conditionally immortalized by transformation with a temperature-sensitive large T-antigen. N20.1 cells are used to investigate cell proliferation and differentiation, and they are useful models to study the cellular and molecular mechanisms involved in the development, maturation and possibly formation of myelin by OLs in the mammalian brain (Paez et al. 2004). N20.1 cells were grown in Dulbecco's modified Eagle's medium/ F12 with 1% fetal bovine serum and G418 (100 μg/ml) at 39°C, and then were divided into 3 groups: (a) regular cell culture medium for control; (b) 25ng/ml Tβ4; (c) 50ng/ml Tβ4 (Fig. 1). The N20.1 cells (102cells/cm2) were incubated in the presence of Tβ4 for 14 days (Fig. 1).
Immunochemistry
Single immunostaining was performed on N20.1 and SVZ cells fixed with 4% paraformaldehyde. After blocking, a polyclonal antibody against CNPase (AVES, 1:200, chicken) was incubated at room temperature for one hour, rinsed with PBS and then incubated with FITC-conjugated f(ab)2 anti-chicken IgG secondary antibody for 60 minutes, rinsed with PBS and air-dried. Cells were then counterstained with DAPI.
TUNEL assay and Trypan Blue exclusion method
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) stains were performed by using the Apoptosis Detection Kit, ApopTag Peroxidase according to the manufacturer's protocol (EMD Millipore, Billerica, MA, USA), as previously described (Santra et al. 2006b). Briefly, the cells were fixed in 1% paraformadehyde for 10 minutes at room temperature, washed in 2 changes of PBS for 5 min, quenched in 3.0% hydrogen peroxide in PBS for 5 minutes at room temp, washed with PBS twice, equilibrate with equilibration buffer followed by addition of dTd enzymes and incubated in a humidified chamber at 37°C for 1h. The reaction was stopped by Stop solution, washed with PBS for three times and incubated in a humidified chamber for 30 minutes at room temp with anti-digoxigenin peroxidase conjugate. Color was developed after addition of diaminobenzidine (DAB) peroxidase substrate.
To count viable cells in Trypan Blue exclusion method, the cell suspension was mixed 0.4% Trypan Blue solution (Sigma, St Louis, MO, USA) in 1:1 ratio, placed in a hemacytometer and counted the cells stained blue as non-viable or dead cells and the cells excluded blue staining as viable or alive cells for three times for each sample.
Silencing of Tβ4 with siRNA transfection and treatment with PDGF and pharmaceutical inhibitors of signaling molecules
For Tβ4 gene silencing, the SVZ and N20.1 cells the control and were treated with 50ng/ml of Tβ4 for 14 days (Fig. 1) followed by transfection with empty vector as scramble control and the vector containing Tβ4 siRNA expression cassette (Santa Cruz), using Lipofectamine 2000 (Invitrogen) for 18 h, as previously described (Santra et al. 2006c). The medium was replaced and cells were harvested after an additional 72 h, followed by quantitative real-time PCR (QrtPCR) and Western blot analysis. The control and Tβ4 treated rat SVZ and N20.1 cells (14 days) that were additionally treated with human PDGF (10 ng/ml for 4.5 hours) and kinas inhibitors were fed with fresh differentiating medium containing 0.5% FBS for 5 hours just before the preparation of cell extracts for total RNA and protein analysis (Fig. 1). In experiments using kinas inhibitors, the cells were pretreated with p38MAPK specific inhibitor (SB 203580), ERK1 specific inhibitor (328006), JNK specific inhibitor II (SP600125) (Calbiochem, San Diego, CA, USA) for 20–30 min before the addition of PDGF in the medium (Fig. 1).
Quantitative real time PCR
Total RNA extraction and cDNA synthesis were performed, as previously described (Santra et al. 2006a; Santra et al. 2006b; Santra et al. 2006c). The sequences for each primer are listed in Table 1. SYBR green (Life Technologies, Grand Island, NY) QrtPCR amplification was performed for 40 cycles in the following thermal profile: 95°C for 30 s, 60°C for 30s, and 72°C for 45 s. After QrtPCR, dissociation curves and agarose gel electrophoresis were performed to verify the quality of the QrtPCR products. There were no secondary products in our data. Each sample was tested in triplicate and all values were normalized to GAPDH. Values obtained from five independent experiments were analyzed relative to gene expression data using the 2-ΔΔCT method (Livak and Schmittgen 2001).
Table 1.
| Primer | Forward Sequence | Reverse Sequence |
|---|---|---|
| CNPase (m,r) | 5′ -TACTTCGGCTGGTTCCTGAC-3′ | 5′ -GCCTTCCCGTAGTCACAAAA-3′ |
| MBP (m,r) | 5′ -ATGGCATCACAGAAGAGACCCTCA-3′ | 5′ -TAAAGAAGCGCCCGATGGAGTCAA-3′ |
| p38 (r) | 5′ -ATGACGAAATGACCGGCTAC-3′ | 5′- ACAACGTTCTTCCGGTCAAC-3′ |
| p38 (m) | 5′ -GCTGAACAAAGGGAGAGACG-3′ | 5′ -TGCTTTCTCCCCAAATTGAC-3′ |
| JNK1 (r) | 5′ -TTCAATGTCCACAGATCCGA-3′ | 5′ -CTAACCAATTCCCCATCCCT-3′ |
| JNK1 (m) | 5′ -GCCATTCTGGTAGAGGAAGTTTCTC-3′ | 5′ -CGCCAGTCCAAAATCAAGAATC-3′ |
| Jun (r) | 5′ -TGAAGCAGAGCATGACCTTG-3′ | 5′ -CACAAGAACTGAGTGGGGGT-3′ |
| Jun (m) | 5′ -CGCAACCAGTCAAGTTCTCA-3′ | 5′ -GAAAAGTAGCCCCCAACCTC-3′ |
Immunochemical procedures
Total protein extracts from the cells were prepared, as previously described (Santra et al. 2006c). The protein extracts were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis for western blot analysis. Treatment with p38MAPK specific inhibitor (SB 203580), ERK1 specific inhibitor (328006), JNK specific inhibitor II (SP600125) (Calbiochem, San Diego, CA, USA) at the dose of 1μM for 3 h were performed, as previously described (Santra et al. 2006a). For Western blot analysis, goat antiserum for MBP (1:1000; Dako, Carpinteria, CA), monoclonal antibodies (1:1000) for CNPase and p38MAPK, phosphorylated p38MAPK, c-Jun, phosphorylated c-Jun (1:1000; Upstate, Charlottesville, VA, USA), rabbit polyclonal antibodies for JNK1 and phosphorylated JNK1 (1: 2000; Promega Corporation) and mouse monoclonal β-actin (1: 1000–5000; Santa Cruz Biotechnology) were used. Donkey anti-goat, anti-rabbit, and anti-mouse horseradishperoxidase (1: 10,000; Jackson ImmunoResearch Labs, West Grove, PA, USA) were used as secondary antibodies. Each experiment was repeated at least three times.
Statistical analysis
Comparison of data was performed in cell cultures with and without Tβ4. Ratio of Tβ4 versus control was calculated for each variable data including gene expression. One sample t-test was used with estimation of the mean and its 95% confidence limits for all data. Comparison of all variable data was considered to be significant if the mean and its 95% lower bound CI were over 1.5; in the other hand, if the mean and its 95% upper bound CI were less than 0.5. A value of p < 0.05 is considered as significant.
Results
Tβ4 treatment induces oligodendrocyte differentiation in OPCs (N20.1) and rat SVZ cells
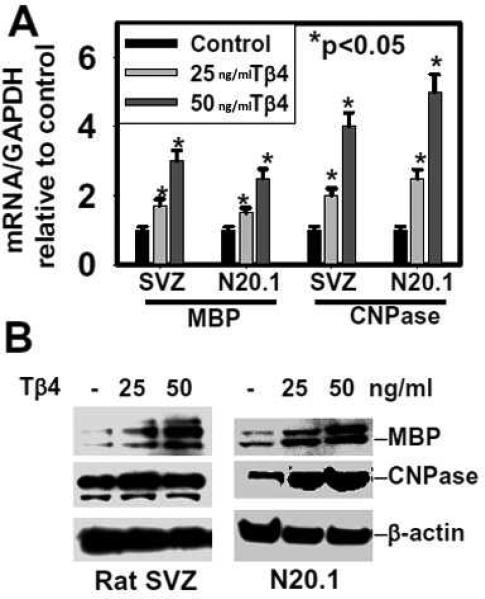
As Tβ4 treatment increases oligodendrogenesis in models of neurological injury, we analyzed expression of myelin genes, MBP and CNPase (i.e. markers of mature oligodendrocytes) in N20.1 and rat primary SVZ neural progenitor cells after Tβ4 treatment by QrtPCR and Western blot analysis. Tβ4 treatment induced gene and protein expression of MBP and CNPase in mouse N20.1 and primary neural progenitor cells in 25ng and 50ng /ml doses of Tβ4 (Figures 2A and 2B). Because Tβ4's main action is to sequester G-actin, an internal control comparing GAPDH to β-actin was performed in both cell systems. No change was observed demonstrating that use of β-actin as a reference in Western blots is valid (data not shown). Immunostaining of CNPase positive cells revealed that Tβ4 increased the number of CNPase positive cells which exhibited multi processes (Fig. 3A). Collectively, these data demonstrate that Tβ4 treatment induces expression of myelin genes MBP and CNPase both in N20.1 and primary neural progenitor cells, indicating that Tβ4 treatment increases OL differentiation.
Fig. 2. Expression of MBP and CNPase in rat SVZ and mouse N20.1 cells after Tβ4 treatment.
Top panel (A) indicates QrtPCR analysis of mRNA of MBP and CNPase in rat SVZ and mouse N20.1 cells after Tβ4 treatment. Panel (B) shows Western blot analysis of protein of MBP and CNPase in rat SVZ and mouse N20.1 cells after Tβ4 treatment. Loading of the samples is normalized with β-actin. Dose of Tβ4 for treatment is shown at the top. Migrations of protein are shown at right.
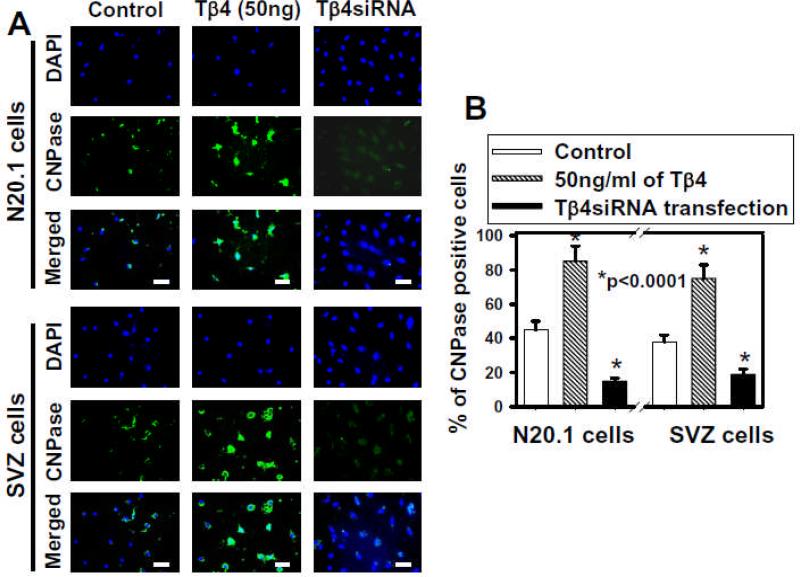
Fig. 3. Immunostaining of mouse N20.1 and rat SVZ cells after Tβ4 treatment.
Panel A shows immunostaining with DAPI and CNPase antibody with resulting merged images in control, 50ng/ml Tβ4 and Tβ4siRNA transfection groups. Panel B demonstrates the percentage (%) of CNPase positive cells in control, 50ng/ml of Tβ4 and Tβ4siRNA transfection. Scale bar of images = 25μm.
To determine what proportion of the cells differentiated into OLs as opposed to cell fate of other cell types, CNPase positive cells were quantified by counting after Tβ4 treatment in mouse N20.1 and rat SVZ cells for 14 days (Fig. 3B). These data showed that when compared to control, Tβ4 treatment significantly increased the number of CNPase cells from ~40% to ~80%. After Tβ4siRNA transfection in both mouse N20.1 and rat SVZ cells, the numbers of CNPase cells were reduced by at least half when compared to control (Fig. 3B).
Tβ4 treatment has no effect on neuroblast and astrocyte differentiation in rat SVZ cells
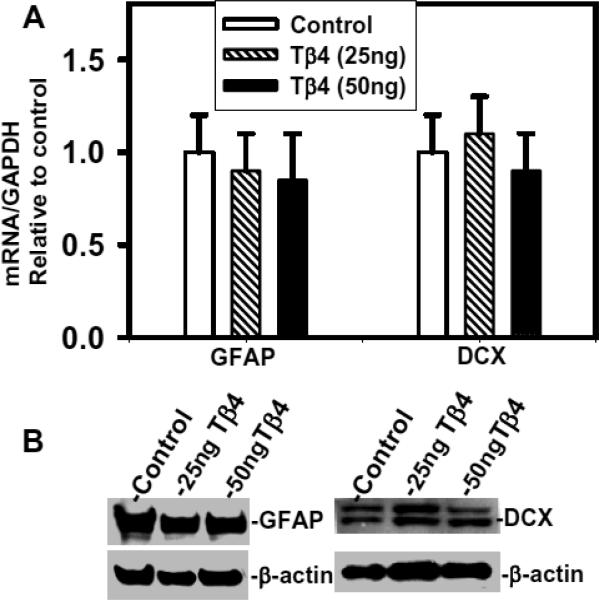
The primary SVZ neural progenitor cells differentiate into OPCs, neuroblasts and astrocytes under normal physiological condition (Hermann et al. 2009; Menn et al. 2006; Sanai et al. 2005). QrtPCR and Western blot analysis revealed that Tβ4 treatment had no significant effect on expression of astrocyte marker- GFAP and neuroblast marker- doublecortin (DCX) in SVZ cells (Fig. 4A and 4B). These data indicate that Tβ4 treatment does not affect astrocyte and neuroblast differentiation in SVZ cells in culture.
Fig. 4. Expression of GFAP and DCX in primary rat SVZ cells after Tβ4 treatment.
Top panel (A) indicates QrtPCR analysis of GFAP and DCX in control rat SVZ cells, 25ng Tβ4 and 50ng Tβ4. Panel (B) shows Western blot analysis of GFAP and DCX in rat SVZ cells after Tβ4 treatment. Loading of the samples is shown at the top of the lane as control rat SVZ cells, Tβ4 25ng and Tβ4 50ng. Migration of proteins is shown at right. Loading of the samples is normalized with β-actin. No significant differences between groups.
Effect of Tβ4 treatment on apoptosis in N20.1 and rat SVZ cells
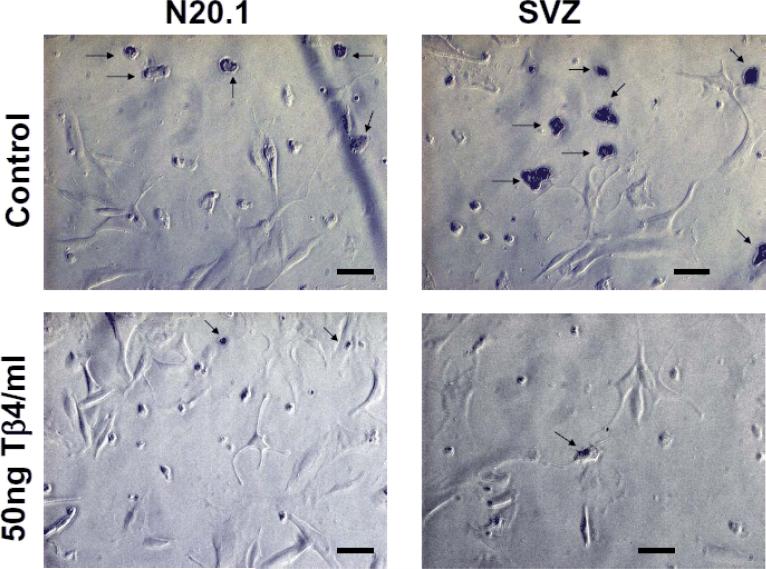
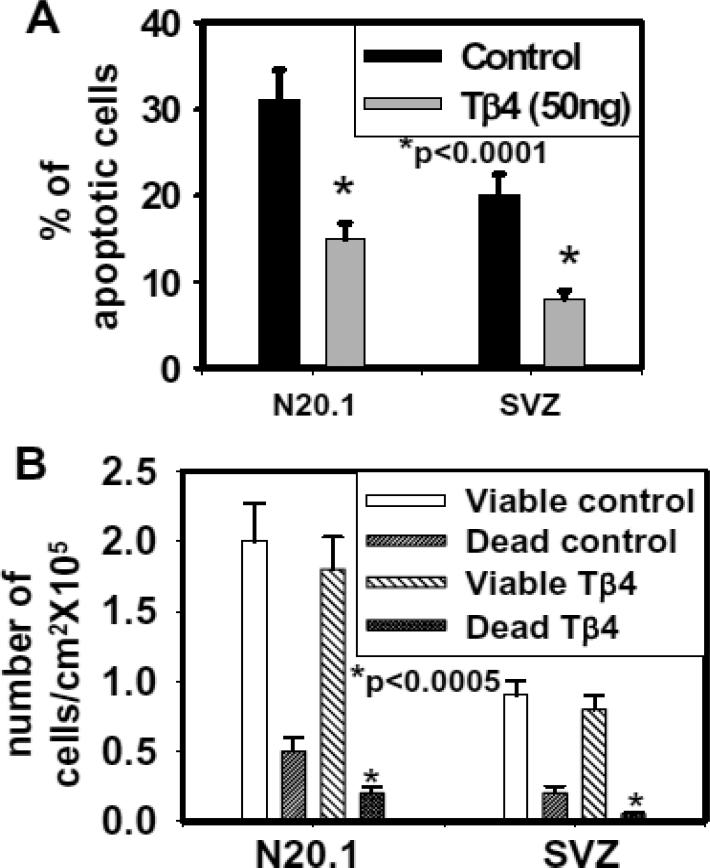
To determine the effect of Tβ4 treatment on apoptosis in cell culture, TUNEL assay was performed after the treatment with 50ng Tβ4/ml in mouse N20.1 and rat SVZ cells. Apoptotic cells were quantified by positive and negative cells for TUNEL staining in mouse N20.1 and rat SVZ cells. These data showed that Tβ4 treatment significantly reduced apoptosis in N20.1 and rat SVZ cells (Fig. 5 and 6A). Using the Trypan Blue exclusion method to determine the number of total viable and non-viable (dead) cells after Tβ4 treatment in N20.1 and rat SVZ cells, we found that there was no significant difference between total numbers of viable cells/mm2 after Tβ4 treatment (Fig. 6B). In contrast, total number of non-viable (dead) cells/mm2 was significantly reduced after Tβ4 treatment indicating consistency with TUNEL assay (Fig. 6B).
Fig. 5. Tβ4 treatment inhibits apoptosis in rat SVZ and mouse N20.1 cells.
TUNEL assay was performed in rat SVZ and mouse N20.1 cells after the treatment with 50 ng Tβ4/ml. Apoptotic cells after TUNEL staining are indicated by arrows. Scale bar of images = 50μm
Fig. 6. Quantitative analysis of apoptotic and viable cells in mouse N20.1 and rat SVZ cells.
Panel A indicates quantitative analysis of apoptotic cells in control and the cells treated with 50 ng/ml of Tβ4 measured by counting DAB positive and negative cells after TUNEL staining. Panel-B shows the Trypan Blue Exclusive Method for detection of viable and non-viable (dead) cells in control and 50 ng/ml of Tβ4 treated rat SVZ and N20.1 cells.
Effect of Tβ4 treatment on p38MAPK in N20.1 and rat SVZ cells
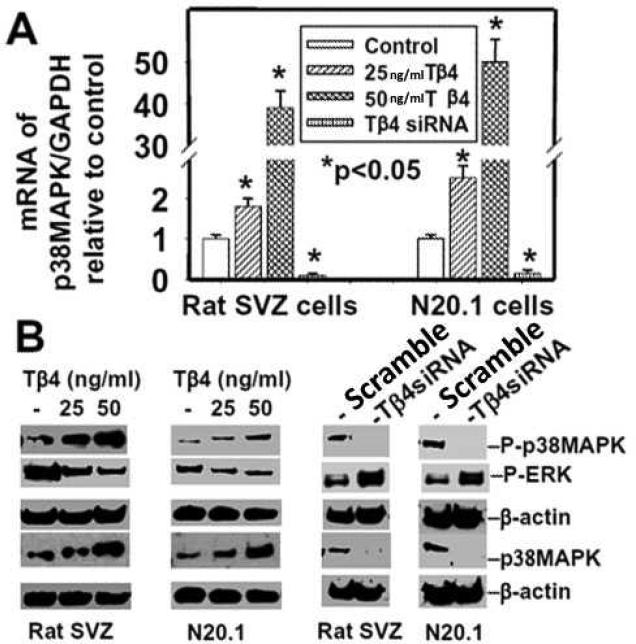
Many extracellular and intrinsic factors regulate OL development, but their signaling pathways remain poorly understood. The p38MAPK-dependent pathway is implicated in OL differentiation (Baron et al. 2000). We therefore investigated the effect of Tβ4 treatment on p38MAPK expression and activity in N20.1 and rat SVZ neural progenitor cells. These cells were treated with Tβ4 (25 and 50 ng/ml) for 2 weeks followed by QrtPCR and Western blot analysis. These data showed that Tβ4 treatment at both doses significantly induced p38MAPK expression in mRNA and protein levels in N20.1 and SVZ cells (Fig. 7A, B). Phosphorylation/activity of p38MAPK was also increased in mouse N20.1 and rat SVZ cells after the treatment (Fig. 7B). Tβ4siRNA transfection reversed the effect of Tβ4 on induction of expression and activity of p38MAPK.
Fig. 7. Expression and phosphorylation of p38MAPK in rat SVZ and mouse N20.1 cells after Tβ4 treatment.
Top panel (A) indicates QrtPCR analysis of mRNA of p38MAPK in rat SVZ and mouse N20.1 cells after Tβ4 treatment. Bottom panel (B) shows Western blot analysis of protein of p38MAPK and phosphorylated p38MAPK (p-p38MAPK) in rat SVZ and mouse N20.1 cells after Tβ4 treatment at left (2 blots) and after Tβ4siRNA transfection at right (2 blots). Loading of the samples is normalized with β-actin. Treatment with Tβ4 and transfection with Tβ4siRNA are shown at the top. Migrations of protein are shown at right.
Inhibition of ERK1 activity in rat SVZ and N20.1 cells after Tβ4 treatment
The antagonistic effects between p38MAPK and ERK1 have been demonstrated in mitosis and tumorigenesis (Aguirre-Ghiso et al. 2003; Chao and Yang 2001). We investigated the effect of Tβ4 treatment on ERK1 activity in OL differentiation in mouse N20.1 and rat SVZ neural progenitor cells. These cells were treated with Tβ4 (25 and 50 ng/ml) for 2 weeks followed by QrtPCR and Western blot analysis. The activity/phosphorylation of ERK1 (p-ERK1) was significantly reduced in mouse N20.1 and rat SVZ neural progenitor cells in 25ng and 50ng /ml doses of Tβ4 (Fig. 7B). Tβ4siRNA transfection reversed the effect of Tβ4 on reduction of activity of p-ERK1. These data indicate that Tβ4 inactivates pERK1 expression.
Downregulation of JNK1 in mouse N20.1 and rat SVZ cells after Tβ4 treatment
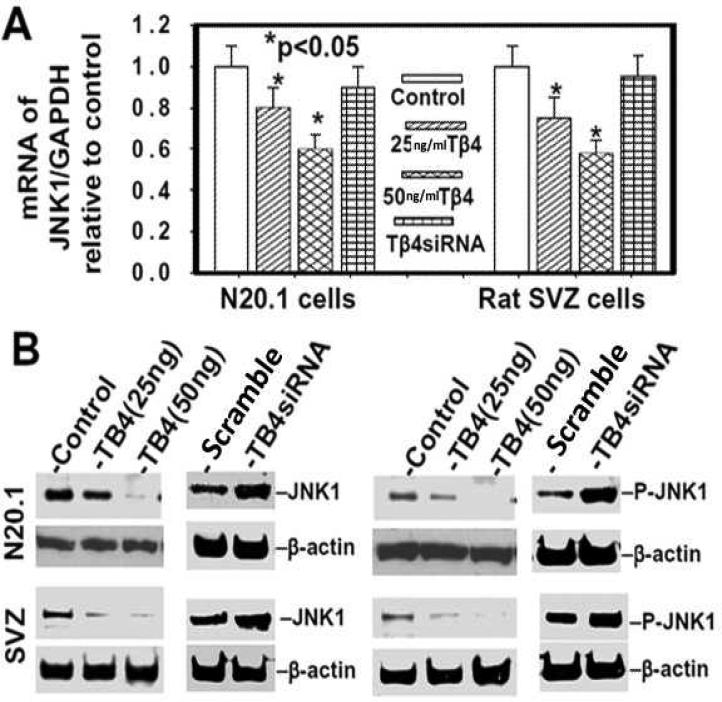
JNK1 phosphorylates c-Jun which binds to the MBP promoter and inhibits myelin gene expression (Parkinson et al. 2008). We investigated the effect of Tβ4 treatment on JNK1 activity in OL differentiation in rat SVZ neural progenitor cells and mouse N20.1 cells. These cells were treated with Tβ4 (25 and 50 ng/ml) for 2 weeks followed by QrtPCR and Western blot analysis. The Tβ4 treatment inhibited expression of JNK1 mRNA and protein levels (Fig. 8A, B) as well as phosphorylated JNK1 in a dose dependent manner (Fig. 8B). Tβ4siRNA transfection reverses this effect of Tβ4 treatment on inhibition of both expression and phosphorylation of JNK1 (Fig. 8A, B). These data indicate that Tβ4 treatment specifically inhibits JNK1 activity.
Fig. 8. Tβ4 treatment down-regulates expression and activation of JNK1 in rat SVZ and mouse N20.1 cells.
Top panel (A) indicates QrtPCR analysis of mRNA of JNK1 in rat SVZ and mouse N20.1 cells after Tβ4 treatment. Bottom panel (B) shows Western blot analysis of protein of JNK1 and phosphorylated JNK1 (p-JNK1) in rat SVZ and mouse N20.1 cells after Tβ4 treatment. Loading of the samples is normalized with β-actin. Treatment with Tβ4 and transfection with Tβ4siRNA are shown at the top.
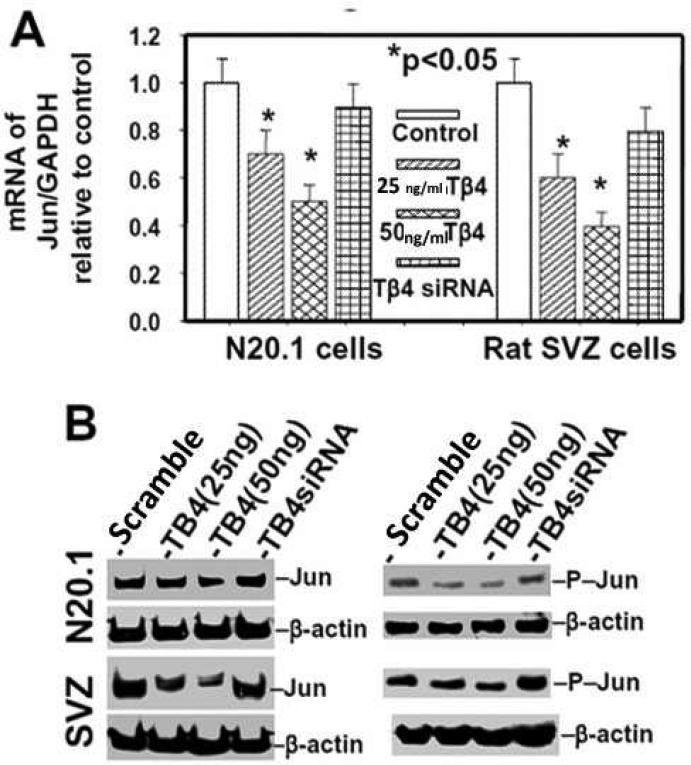
Tβ4 treatment prevents accumulation of c-Jun in mouse N20.1 and rat SVZ cells
We investigated the effect of Tβ4 treatment on expression and activity of c-Jun in N20.1 and rat SVZ neural progenitor cells. These cells were treated with Tβ4 (25 and 50 ng/ml) for 2 weeks followed b y QrtPCR and Western blot analysis. Tβ4 treatment inhibited both expression of c-Jun and phosphorylated c-Jun in 25ng and 50ng /ml doses of Tβ4 (Fig. 9A, B). Tβ4siRNA transfection neutralized the effect of Tβ4 on suppression of expression and phosphorylation of c-Jun (Fig. 9A, B). These data indicate that Tβ4 treatment specifically downregulates expression and activation of c-Jun in rat SVZ neural progenitor cells and mouse N20.1 cells. Among the mitogenic signaling proteins, phosphorylated c-Jun directly binds to the both promoters of MBP and CNPase, acts as a repressor and negatively regulates expression of MBP and CNPase (Parkinson et al. 2008). These data indicate that Tβ4 treatment inactivates c-Jun.
Fig. 9. Tβ4 treatment prevents the expression and activation of c-Jun in rat SVZ and mouse N20.1 cells.
Top panel (A) indicates QrtPCR analysis of mRNA of Jun in rat SVZ and mouse N20.1 cells after Tβ4 treatment. Bottom panel (B) shows Western blot analysis of protein of Jun and phosphorylated c-Jun (p-c-jun) in rat SVZ and mouse N20.1 cells after Tβ4 treatment. Loading of the samples is normalized with β-actin. Treatment with Tβ4 and transfection with Tβ4siRNA are shown at the top.
Effect of PDGF on Tβ4 treated mouse N20.1 and rat SVZ cells
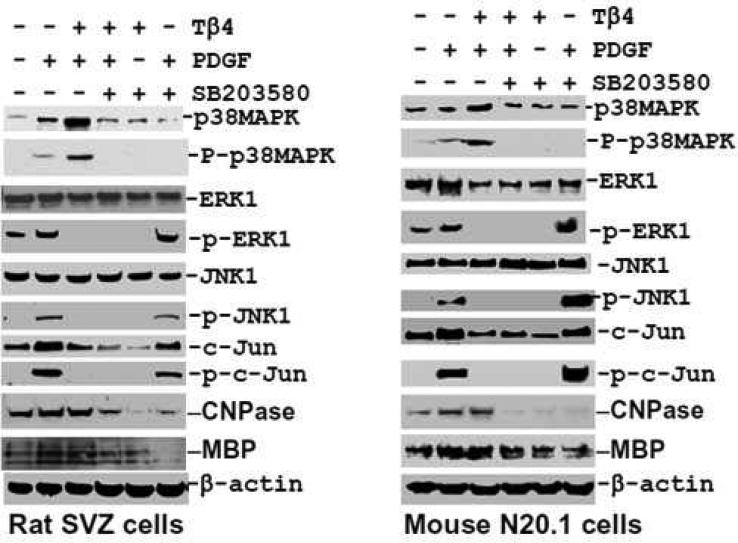
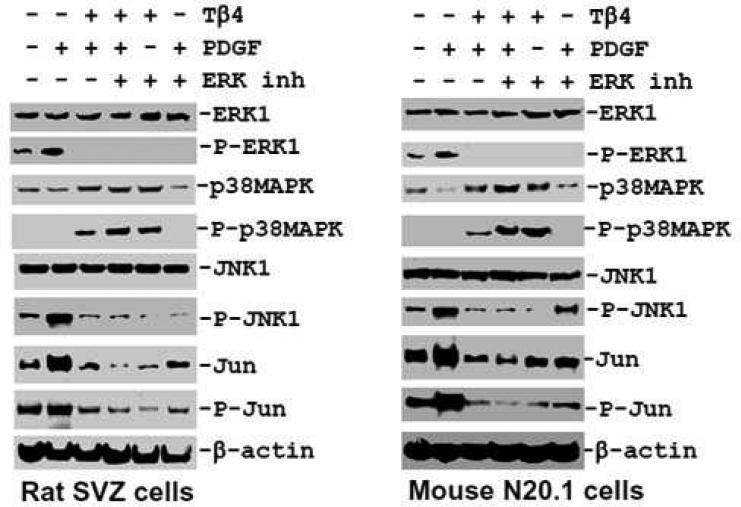
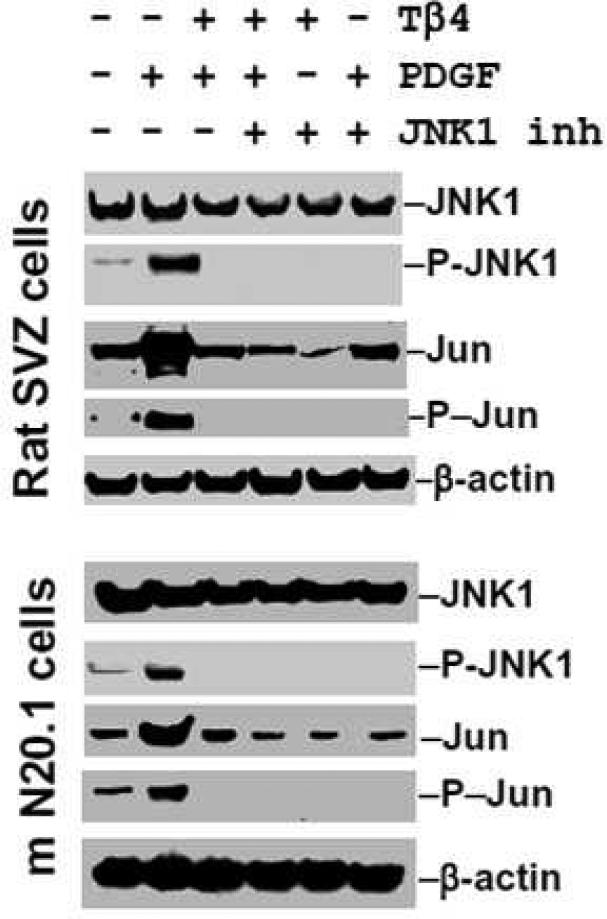
As PDGF influences the phosphorylation of MAPKs e.g. p38MAPK, ERK1 and JNK1 (Chew et al. 2010), we therefore investigated the specific effect of TB4 on PDGF-induced phosphorylation/activation of p38MAPK, ERK1 and JNK1. To determine specificity on phosphorylation/activation of these MAPKs, the specific pharmacological inhibitors which specifically inhibit phosphorylation/activation of p38MAPK, ERK1 and JNK1 were employed in rat SVZ cells and mouse N20.1 cells. PDGF treatment induced phosphorylation/activation of ERK1 in untreated cells in control rat SVZ neural progenitor cells and mouse N20.1 cells (Fig. 10, 11). In contrast, PDGF failed to reverse the inhibitory effect of Tβ4 on phosphorylation of ERK1 and JNK1 in rat SVZ neural progenitor cells and mouse N20.1 cells (Fig. 10, 11). These data suggest that Tβ4 treatment blocks the PDGFRα/ERK1 signaling pathway. Under basal conditions, PDGF acutely induced the phosphorylation of ERK1, p38MAPK, JNK and c-Jun in rat SVZ neural progenitor cells and mouse N20.1 cells (Fig. 10, 11). Expression of p38 MAPK was vastly increased when Tβ4 was added to the PDGF treated cells. PDGF failed to reverse the inhibitory effect of Tβ4 on phosphorylation of ERK1, JNK1 and c-Jun in rat SVZ neural progenitor cells and mouse N20.1 cells (Fig. 10, 11). Addition of the specific inhibitor of p38MAPK had no effect on phosphorylation of ERK1, JNK1 and c-Jun after Tβ4 treatment in N20.1 and rat SVZ neural progenitor cells (Fig. 10). In the same fashion, specific inhibition of ERK1 showed significant down-regulation of phosphorylation of ERK1, JNK1 and c-Jun after Tβ4 treatment (Fig. 11). Finally, inhibition of JNK1 demonstrated no expression of phosphorylated c-Jun in Tβ4 treated cells (Fig. 12). These data indicate that Tβ4 treatment has a direct effect on phosphorylation of ERK1, JNK1 and C-Jun in N20.1 and rat SVZ neural progenitor cells.
Fig. 10. Effect of Tβ4 in p38MAPK signaling pathways in rat SVZ and mouse N20.1 cells.
Western blot analyses show p38MAPK phosphorylation after combination treatment with PDGF, Tβ4 and p38MAPK specific inhibitor SB203580 in rat SVZ (left panel) and mouse N20.1 cells (right panel). PDGF acutely stimulates the phosphorylation of multiple kinases e.g. ERK1, JNK1 and Jun. In contrast, PDGF fails to reverse the inhibitory effect of Tβ4 on phosphorylation of ERK1, JNK1 and Jun. Inhibition of p38MAPK with SB203580 elevates P-ERK1, P-JNK1, and P-c-Jun levels after 72 h.
Fig. 11. Effect of Tβ4 in ERK1 signaling pathways in rat SVZ and mouse N20.1 cells.
Western blot analyses show p38MAPK phosphorylation after treatment with PDGF, Tβ4, ERK1 specific inhibitor and their combination in rat SVZ (left panel) and mouse N20.1 cells (right panel). PDGF upregulates the phosphorylation of multiple kinases e.g. ERK1, JNK1 and Jun only in control rat SVZ and mouse N20.1 cells, but not in Tβ4 treated cells. Inhibition of ERK1 phosphorylation promotes p38 phosphorylation both in control and Tβ4 treated rat SVZ and mouse N20.1 cells.
Fig. 12. Effect of Tβ4 in JNK1 signaling pathways in rat SVZ and mouse N20.1 cells.
Western blot analyses indicate JNK1 phosphorylation after treatment with PDGF, however no phosphorylation of JNK1 or c-Jun is observed with Tβ4 treatment. JNK1 specific inhibitor demonstrates no phosphorylation of JNK1 or c-Jun in either PDGF or Tβ4 treatment.
Discussion
The present study demonstrates that Tβ4 enhanced generation of mature OLs in cultured primary SVZ neural progenitor cells and an OPC line and that activation of p38MAPK with a subsequent decrease in phosphorylation of ERK1, JNK1, and c-Jun by Tβ4 contributed to Tβ4-increased OLs. These data provide new insights into the signaling pathways that mediate Tβ4-enhanced OL differentiation. In adult rodent brain, SVZ neural progenitor cells and parenchymal OPCs in white matter contribute to oligodendrogenesis (Nicolay et al. 2007; Sim et al. 2002; Tanaka et al. 2003). We previously demonstrated that both cell populations contribute to oligodendrogenesis after brain injury and that Tβ4 promotes oligodendrogenesis (Morris et al. 2010; Xiong et al. 2010; Zhang et al. 2009). We therefore employed SVZ neural progenitor cells and an OPC line to investigate molecular mechanisms underlying Tβ4 enhanced oligodendrogenesis. Consistent with earlier observations of increased numbers of OPCs and OLs in damaged brain tissue in animal models of neurological injury (Morris et al. 2010; Xiong et al. 2010; Zhang et al. 2009), Tβ4 treatment increased protein levels of MBP and CNPase in both cultured cells, as well as augmented CNPase positive cells suggesting that Tβ4 promotes OPC differentiation. More importantly, Tβ4 treatment induced activation of p38MAPK, whereas blockage of p38MAPK with a pharmacological inhibitor suppressed Tβ4-elevated MBP and CNPase. In addition, attenuation of endogenous Tβ4 by siRNA downregulated p38MAPK levels. The p38MAPK is involved in a plethora of cellular functions, most notably, cell migration, proliferation and differentiation (Cargnello and Roux 2011; Cuadrado and Nebreda 2010). Baron et al first demonstrated that p38MAPK is necessary for OL differentiation (Baron et al. 2000). Subsequently, p38MAPK was observed to be involved in myelination of cultured Schwann cells and OPCs (Fragoso et al. 2007; Haines et al. 2008) and was colocalized with CNPase in mouse myelin-sheath (Maruyama et al.). The cause-effect of p38MAPK in mediating OL differentiation has been recently demonstrated (Chew et al. 2010). Together with our data, the present study indicates that the p38MAPK pathway regulates oligodendrogenesis induced by either exogenous or endogenous Tβ4.
Adult SVZ neural progenitor cells in the rodent differentiate into OPCs, neuroblasts and astrocytes (Hermann et al. 2009; Menn et al. 2006; Sanai et al. 2005). Our data indicate that Tβ4 specifically promotes differentiation of SVZ neural progenitor cells into OL because Tβ4 did not significantly alter populations of neuroblasts and GFAP positive astrocytes in the neural progenitor cells. Consistent with our results showing that 80% of Tβ4 treated cells were CNPase positive, Cavaliere et al demonstrated a similar observation of glutamate induced OL differentiation of rat SVZ cells showing 72% O4 positive SVZ cells (Cavaliere et al. 2012).
Tβ4 reduces apoptosis in cardiac and cornea injury models (Bock-Marquette et al. 2004; Smart et al. 2007; Sosne et al. 2007). Consistent with these observations, our data showed that Tβ4 treatment induced cell survival by inhibiting apoptosis in N20.1 and SVZ cells. Although Tβ4 treatment reduced apoptosis, the numbers of total viable cells in control and Tβ4 treated cells remained similar. Apoptosis in control cells may be balanced by proliferation resulting in statistically similar number of viable cells. However, the total number of non-viable (dead) cells/mm2 was significantly reduced after Tβ4 treatment supporting the observation of reduced apoptosis. As a result, our data show statistically similar numbers of total viable cells in control and Tβ4 treated cells supporting the primary effect of Tβ4 treatment on OL differentiation in cell culture model system for N20.1 and SVZ cells.
Exogenously administered Tβ4 internalizes into the cells and protects corneal epithelial cells against both apoptotic extrinsic and intrinsic death-signaling pathways (Ho et al. 2007). However, the mechanism of internalization of Tβ4 into the cells is not known, e.g., whether it is passive diffusion or receptor mediated. Ku80 and ATP-responsive purinergic receptor P2X4 are reported as possible receptors for Tβ4, but are not involved in internalization of Tβ4 into the cells (Bednarek et al. 2008; Freeman et al. 2011; Ho et al. 2007). Ku80 induces intracellular activity of Tβ4 while the ATP-responsive purinergic receptor P2X4 mediates Tβ4-induced HUVEC migration (Freeman et al. 2011). One dominant mechanism through which Tβ4 induces survival of human circulating endothelial progenitor cells is p38MAPK indicating a relevant link between Tβ4 and p38MAPK (Freeman et al. 2011; Zhao et al. 2011). These data together with our results, suggest that Tβ4 exerts its effects from extra-cellular to intracellular via p38MAPK signaling pathways.
The robust activation of p38MAPK in these cell culture models by Tβ4 demonstrates an important signaling mechanism that is observed in cell survival and differentiation. Activation of p38MAPK is commonly observed when cells are placed in a stressful environment (Cuadrado and Nebreda 2010). Cells can rapidly respond to stress using p38MAPK signaling as the process of activation through phosphorylation and dephosphorylation, can elicit a quick response to the stressor. This system was first observed in the immune system (Lee et al. 1994) and this present study suggests that treatment with Tβ4 may enable progenitor cells in the nervous system to quickly respond to neurological injury. There are five subfamilies of MAPKs e.g. ERK1/2 (ERK1 and ERK2), JNKs, p38 kinases, ERK3/4 (ERK3 and ERK4) and ERK5 in mammals (Roux and Blenis 2004). Each MAPK pathway contains a three tiered kinase cascade comprising a MAPKKK/MAPKK/MAPK (Davis 2000; Kyriakis and Avruch 2001) MAPKKs are specific for activation of p38 kinases, JNKs and ERKs. MAPKK3/6 phosphorylate p38 kinases and MAPKKs (MAPKK4/7) phosphorylate JNKs (Davis 2000; Kyriakis and Avruch 2001). In contrast, ERK1 and ERK2 are activated by MAPKK1/2. These activated kinases are translocated into the nucleus which in turn activate their specific transcription factors with subsequent trans-activation of their target genes (Davis 2000; Kyriakis and Avruch 2001) One of the targets is driven by MAPKKKs/MAPKK3/6/p38 is MBP, indicating the involvement of p38 in OL differentiation, as previously reported by Chew et al. (Chew et al. 2010). In contrast, phosphorylated JNK upon activation induces c-Jun and AP-1 trans-activation function leading to proliferation in normal and cancer cells (Hui et al. 2007). Tβ4 induced activation of p38MAPK, therefore, provides a basic mechanism of oligodengenesis providing a foundation of knowledge at both the biochemical and pre-clinical level.
Our experiments show that Tβ4 treatment induced p38MAPK, suppressing ERK1 and JNK activity and preventing accumulation of phosphorylated c-Jun which negatively regulates myelin gene promoter activity in OPCs. As a result, we suggest that the myelin gene promoters of MBP and CNPase are activated and transcribed. Our data are also consistent with other studies which suggest that myelination is inhibited after upregulation and activation of JNK1 and subsequent of phosphorylation of c-jun (Jessen and Mirsky 2008; Parkinson et al. 2008). Moreover, our data showed that Tβ4 treatment inhibited the activity of ERK1 which may suppress myelin synthesis (Jessen and Mirsky 2008). The inhibitors of p38MAPK down-regulate the dual specific MAPK phosphatase- MKP3/DUSP3, which dephosphorylates ERK1 (Kim et al. 2003). As a result, ERK1 is activated after p38MAPK inhibition. Specific inhibition of p38MAPK with SB203580 had no effect on phosphorylation of ERK1, JNK and c-Jun after Tβ4 treatment. Alternatively, the addition of PDGF, which stimulates phosphorylation of multiple kinases, activated the phosphorylation of ERK1, p38MAPK, JNK and c-Jun. However, addition of Tβ4 to the PDGF treated cells demonstrated an enormous expression of p38 MAPK with simultaneous inhibition of the phosphorylation effect of PDGF on ERK1, JNK1 and c-Jun. Therefore, this observation suggests that the increase of expression of p38 is due totally to Tβ4 and not a synergistic effect with PDGF. It is important to mention that PDGF signaling may also affect many other non-p38 pathways.
The PDGFRα has pleiotropic effects in OLs (Andrae et al. 2008). PDGFRα mediates both Src and PI3K as critical signaling mediators for OPC proliferation (Klinghoffer et al. 2002). In addition to proliferation of OPCs, PDGF also induces OPC survival via JAK/STAT signaling pathway (Dell'Albani et al. 1998). Regardless of the specific pathway involved, upregulation of MBP and CNPase after Tβ4 treatment in rat SVZ neural progenitor cells and mouse N20.1 cells suggests that Tβ4 is involved in OL differentiation. These findings are consistent with earlier observations of increased numbers of OPCs and OL in damaged brain tissue in animal models of neurological injury (Morris et al. 2010; Xiong et al. 2010; Zhang et al. 2009).
The composition of the protein complex that binds to myelin gene promoter is unknown. The consensus sequence (TGACTTA) of AP1-like region of myelin gene promoter where the protein complex binds, overlaps with TRE site (TGACTCA) specific for binding of Jun-Fos protein complex (Chew et al. 2010). As a result, an unknown protein complex may compete with Jun-Fos protein complex for binding to AP1-like region of myelin gene promoter. When phosphorylated c-Jun is down-regulated after Tβ4 treatment, we speculate that the unknown protein complex may bind to AP1-like region of myelin gene promoter and may induce myelin gene transcription in rat SVZ and mouse N20.1 cells. In addition, Ras mitogenic signaling is required for activation of Fos and Jun via JNK1 and ERK1 signaling pathways (Clark et al. 1997; Westwick et al. 1994). However, Ras activity is inhibited by activation of p38MAPK (Bulavin et al. 2004; Qi et al. 2004). Therefore, we speculate that activation of p38 MAPK inhibits Ras with subsequent inhibition of expression and activation of ERK1 and JNK1 after Tβ4 treatment. As a result, myelin gene promoter becomes assessable and transcribes myelin gene after Tβ4 treatment. Further investigation is required to find this unknown protein complex that may bind to AP1-like region of myelin gene promoter.
Tβ4 is ubiquitously expressed and naturally present in many tissues (Crockford 2007). Blood platelets, neutrophils, macrophages, and other lymphoid tissues express Tβ4 which is released after injury to protect cells and tissues from further damage, reduce apoptosis and inflammation (Crockford 2007; Goldstein et al. 2012). Recently, Tβ4 showed promise as a potential therapeutic approach to neural repair by demonstrating functional recovery and neurorestoration in animal models of multiple sclerosis, embolic stroke and traumatic brain injury (Morris et al. 2010; Xiong et al. 2010; Zhang et al. 2009). Although Tβ4 is expressed in the adult brain, its endogenous levels are low relative to the predictive increased concentrations of Tβ4 needed to evoke a neurorestorative or repair process (Mora et al. 1997; Zhang and Chopp 2009) Therefore, exogenous administration is needed to treat the damaged tissue. Injured neurological tissues have limited capacity to regenerate and by the addition of a regenerative molecule such as Tβ4, may indeed be the treatment necessary to improve patient outcomes.
In summary, Tβ4 mediated induction of p38MAPK activity inactivated ERK1, JNK1 and c-Jun leading to expression of MBP and CNPase. Tβ4 treatment suppresses accumulation of phosphorylated c-Jun by activating p38MAPK and inactivation of PDGFRα. In conjunction with our previous studies on animal models of neurological injury, these data support the concept of Tβ4 mediated oligodendrogenesis and treatment of demyelination with Tβ4 may, be a potential therapeutic option.
Acknowledgments
This research was supported in part by NINDS and NIA grants: PO1 NS23393, RO1 NS062832, R01 NS075156 and R01 AG038648. Tβ4 was supplied by RegeneRx Biopharmaceuticals Inc, Rockville, MD under a Material Transfer Agreement. A US Provisional Patent 61/163,556 has been filed for use of Tβ4 in neurological disease.
References
- Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. 2003;63(7):1684–95. [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron W, Metz B, Bansal R, Hoekstra D, de Vries H. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci. 2000;15(3):314–29. doi: 10.1006/mcne.1999.0827. [DOI] [PubMed] [Google Scholar]
- Bednarek R, Boncela J, Smolarczyk K, Cierniewska-Cieslak A, Wyroba E, Cierniewski CS. Ku80 as a novel receptor for thymosin beta4 that mediates its intracellular activity different from G-actin sequestering. J Biol Chem. 2008;283(3):1534–44. doi: 10.1074/jbc.M707539200. [DOI] [PubMed] [Google Scholar]
- Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432(7016):466–72. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CW, Appella E, Fornace AJ., Jr Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat Genet. 2004;36(4):343–50. doi: 10.1038/ng1317. [DOI] [PubMed] [Google Scholar]
- Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere F, Urra O, Alberdi E, Matute C. Oligodendrocyte differentiation from adult multipotent stem cells is modulated by glutamate. Cell Death Dis. 2012;3:e268. doi: 10.1038/cddis.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao JI, Yang JL. Opposite roles of ERK and p38 mitogen-activated protein kinases in cadmium-induced genotoxicity and mitotic arrest. Chem Res Toxicol. 2001;14(9):1193–202. doi: 10.1021/tx010041o. [DOI] [PubMed] [Google Scholar]
- Chew LJ, Coley W, Cheng Y, Gallo V. Mechanisms of regulation of oligodendrocyte development by p38 mitogen-activated protein kinase. J Neurosci. 2010;30(33):11011–27. doi: 10.1523/JNEUROSCI.2546-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GJ, Westwick JK, Der CJ. p120 GAP modulates Ras activation of Jun kinases and transformation. J Biol Chem. 1997;272(3):1677–81. doi: 10.1074/jbc.272.3.1677. [DOI] [PubMed] [Google Scholar]
- Crockford D. Development of thymosin beta4 for treatment of patients with ischemic heart disease. Ann N Y Acad Sci. 2007;1112:385–95. doi: 10.1196/annals.1415.051. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–17. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103(2):239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Dell'Albani P, Kahn MA, Cole R, Condorelli DF, Giuffrida-Stella AM, de Vellis J. Oligodendroglial survival factors, PDGF-AA and CNTF, activate similar JAK/STAT signaling pathways. J Neurosci Res. 1998;54(2):191–205. doi: 10.1002/(SICI)1097-4547(19981015)54:2<191::AID-JNR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Fragoso G, Haines JD, Roberston J, Pedraza L, Mushynski WE, Almazan G. p38 mitogen-activated protein kinase is required for central nervous system myelination. Glia. 2007;55(15):1531–41. doi: 10.1002/glia.20567. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3(9):705–14. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Freeman KW, Bowman BR, Zetter BR. Regenerative protein thymosin beta-4 is a novel regulator of purinergic signaling. FASEB J. 2011;25(3):907–15. doi: 10.1096/fj.10-169417. [DOI] [PubMed] [Google Scholar]
- Goldstein AL, Hannappel E, Kleinman HK. Thymosin beta4: actin-sequestering protein moonlights to repair injured tissues. Trends Mol Med. 2005;11(9):421–9. doi: 10.1016/j.molmed.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Goldstein AL, Hannappel E, Sosne G, Kleinman HK. Thymosin beta4: a multi-functional regenerative peptide. Basic properties and clinical applications. Expert Opin Biol Ther. 2012;12(1):37–51. doi: 10.1517/14712598.2012.634793. [DOI] [PubMed] [Google Scholar]
- Haines JD, Fragoso G, Hossain S, Mushynski WE, Almazan G. p38 Mitogen-activated protein kinase regulates myelination. J Mol Neurosci. 2008;35(1):23–33. doi: 10.1007/s12031-007-9011-0. [DOI] [PubMed] [Google Scholar]
- Hermann A, Suess C, Fauser M, Kanzler S, Witt M, Fabel K, Schwarz J, Hoglinger GU, Storch A. Rostro-caudal gradual loss of cellular diversity within the periventricular regions of the ventricular system. Stem Cells. 2009;27(4):928–41. doi: 10.1002/stem.21. [DOI] [PubMed] [Google Scholar]
- Ho JH, Chuang CH, Ho CY, Shih YR, Lee OK, Su Y. Internalization is essential for the antiapoptotic effects of exogenous thymosin beta-4 on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48(1):27–33. doi: 10.1167/iovs.06-0826. [DOI] [PubMed] [Google Scholar]
- Huff T, Rosorius O, Otto AM, Muller CS, Ballweber E, Hannappel E, Mannherz HG. Nuclear localisation of the G-actin sequestering peptide thymosin beta4. J Cell Sci. 2004;117(Pt 22):5333–41. doi: 10.1242/jcs.01404. [DOI] [PubMed] [Google Scholar]
- Hui L, Bakiri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H, Wagner EF. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet. 2007;39(6):741–9. doi: 10.1038/ng2033. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56(14):1552–65. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- Kim Y, Rice AE, Denu JM. Intramolecular dephosphorylation of ERK by MKP3. Biochemistry. 2003;42(51):15197–207. doi: 10.1021/bi035346b. [DOI] [PubMed] [Google Scholar]
- Klinghoffer RA, Hamilton TG, Hoch R, Soriano P. An allelic series at the PDGFalphaR locus indicates unequal contributions of distinct signaling pathways during development. Dev Cell. 2002;2(1):103–13. doi: 10.1016/s1534-5807(01)00103-4. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81(2):807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372(6508):739–46. doi: 10.1038/372739a0. others. [DOI] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Zhang RL, Hozeska-Solgot A, Gregg SC, Buller B, Lu M, Zhang ZG. Angiopoietin 2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J Biol Chem. 2009;284(34):22680–9. doi: 10.1074/jbc.M109.006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Malinda KM, Sidhu GS, Mani H, Banaudha K, Maheshwari RK, Goldstein AL, Kleinman HK. Thymosin beta4 accelerates wound healing. J Invest Dermatol. 1999;113(3):364–8. doi: 10.1046/j.1523-1747.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Sudo T, Kasuya Y, Shiga T, Hu B, Osada H. Immunolocalization of p38 MAP kinase in mouse brain. Brain Res. 2000;887(2):350–8. doi: 10.1016/s0006-8993(00)03063-8. [DOI] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26(30):7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora CA, Baumann CA, Paino JE, Goldstein AL, Badamchian M. Biodistribution of synthetic thymosin beta 4 in the serum, urine, and major organs of mice. Int J Immunopharmacol. 1997;19(1):1–8. doi: 10.1016/s0192-0561(97)00005-2. [DOI] [PubMed] [Google Scholar]
- Morris DC, Chopp M, Zhang L, Lu M, Zhang ZG. Thymosin beta4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience. 2010;169(2):674–82. doi: 10.1016/j.neuroscience.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Picard-Riera N, Kerninon C, Baron-Van Evercooren A. The role of SVZ-derived neural precursors in demyelinating diseases: from animal models to multiple sclerosis. J Neurol Sci. 2008;265(1-2):26–31. doi: 10.1016/j.jns.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Nicolay DJ, Doucette JR, Nazarali AJ. Transcriptional control of oligodendrogenesis. Glia. 2007;55(13):1287–99. doi: 10.1002/glia.20540. [DOI] [PubMed] [Google Scholar]
- Paez PM, Garcia CI, Davio C, Campagnoni AT, Soto EF, Pasquini JM. Apotransferrin promotes the differentiation of two oligodendroglial cell lines. Glia. 2004;46(2):207–17. doi: 10.1002/glia.20001. [DOI] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181(4):625–37. doi: 10.1083/jcb.200803013. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Tang J, Pramanik R, Schultz RM, Shirasawa S, Sasazuki T, Han J, Chen G. p38 MAPK activation selectively induces cell death in K-ras-mutated human colon cancer cells through regulation of vitamin D receptor. J Biol Chem. 2004;279(21):22138–44. doi: 10.1074/jbc.M313964200. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68(2):320–44. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer D, Elzinga M, Nachmias VT. Thymosin beta 4 and Fx, an actin-sequestering peptide, are indistinguishable. J Biol Chem. 1991;266(7):4029–32. [PubMed] [Google Scholar]
- Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353(8):811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- Sanders MC, Goldstein AL, Wang YL. Thymosin beta 4 (Fx peptide) is a potent regulator of actin polymerization in living cells. Proc Natl Acad Sci U S A. 1992;89(10):4678–82. doi: 10.1073/pnas.89.10.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra M, Katakowski M, Zhang RL, Zhang ZG, Meng H, Jiang F, Chopp M. Protection of adult mouse progenitor cells and human glioma cells by de novo decorin expression in an oxygen- and glucose-deprived cell culture model system. J Cereb Blood Flow Metab. 2006a;26(10):1311–22. doi: 10.1038/sj.jcbfm.9600285. [DOI] [PubMed] [Google Scholar]
- Santra M, Liu XS, Santra S, Zhang J, Zhang RL, Zhang ZG, Chopp M. Ectopic expression of doublecortin protects adult rat progenitor cells and human glioma cells from severe oxygen and glucose deprivation. Neuroscience. 2006b;142(3):739–52. doi: 10.1016/j.neuroscience.2006.06.065. [DOI] [PubMed] [Google Scholar]
- Santra M, Zhang X, Santra S, Jiang F, Chopp M. Ectopic doublecortin gene expression suppresses the malignant phenotype in glioblastoma cells. Cancer Res. 2006c;66(24):11726–35. doi: 10.1158/0008-5472.CAN-06-1978. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22(7):2451–9. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445(7124):177–82. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- Sosne G, Qiu P, Kurpakus-Wheater M. Thymosin beta-4 and the eye: I can see clearly now the pain is gone. Ann N Y Acad Sci. 2007;1112:114–22. doi: 10.1196/annals.1415.004. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003;989(2):172–9. doi: 10.1016/s0006-8993(03)03317-1. [DOI] [PubMed] [Google Scholar]
- Westwick JK, Cox AD, Der CJ, Cobb MH, Hibi M, Karin M, Brenner DA. Oncogenic Ras activates c-Jun via a separate pathway from the activation of extracellular signal-regulated kinases. Proc Natl Acad Sci U S A. 1994;91(13):6030–4. doi: 10.1073/pnas.91.13.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Meng Y, Zhang Y, Zhang ZG, Morris DC, Chopp M. Treatment of traumatic brain injury with thymosin beta(4) in rats. J Neurosurg. 2010 doi: 10.3171/2010.4.JNS10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang ZG, Morris D, Li Y, Roberts C, Elias SB, Chopp M. Neurological functional recovery after thymosin beta4 treatment in mice with experimental auto encephalomyelitis. Neuroscience. 2009;164(4):1887–93. doi: 10.1016/j.neuroscience.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24(4):441–8. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8(5):491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Qiu F, Xu S, Yu L, Fu G. Thymosin beta4 activates integrin-linked kinase and decreases endothelial progenitor cells apoptosis under serum deprivation. J Cell Physiol. 2011;226(11):2798–806. doi: 10.1002/jcp.22624. [DOI] [PubMed] [Google Scholar]