Abstract
The tumor protein p63 (p63), and more specifically the NH2-terminal truncated (ΔN) p63 isoform, is a marker of basal epithelial cells and is required for normal development of several epithelial tissues, including the bladder and prostate glands. Although p63-expressing cells are proposed to be the stem cells of the developing prostate epithelium and bladder urothelium, cell lineages in these endoderm-derived epithelia remain highly controversial, and rigorous lineage tracing studies are warranted. Here, we generated knock-in mice expressing Cre recombinase (Cre) under the control of the endogenous ΔNp63 promoter. Heterozygote ΔNp63+/Cre mice were phenotypically normal and fertile. Cre-mediated recombination in ΔNp63+/Cre;ROSA26EYFP reporter mice faithfully recapitulated the pattern of ΔNp63 expression and were useful for genetic lineage tracing of ΔNp63-expressing cells of the caudal endoderm in vivo. We found that ΔNp63-positive cells of the urogenital sinus generated all epithelial lineages of the prostate and bladder, indicating that these cells represent the stem/progenitor cells of those epithelia during development. We also observed ΔNp63 expression in caudal gut endoderm and the contribution of ΔNp63-positive cells to the stem/progenitor compartment of adult colorectal epithelium. Because p63 is a master regulator of stratified epithelial development, this finding provides a unique developmental insight into the cell of origin of squamous cell metaplasia and squamous cell carcinoma of the colon.
Keywords: large intestine, hindgut, genitourinary tract
The identification of stem cells and their derivative lineages in epithelial organs such as the bladder and prostate glands can shed light on mechanisms that underlie a wide range of genitourinary diseases, including congenital defects and cancer. The glandular epithelium of the adult male prostate contains two principal cell types: luminal/secretory cells that produce seminal fluid and basal cells located at the interface of luminal cells and the basement membrane. A third cell type, neuroendocrine cells, is rare and of uncertain function (1). The multilayered epithelium of the bladder (urothelium) consists of a luminal layer of umbrella cells involved in urothelial permeability, overlying layers of intermediate and basal cells (2).
Cellular hierarchies in development and maintenance of the prostate epithelium are relevant to the origins of cancer and have been debated for decades; the relationship between basal and secretory cells remains controversial. Ex vivo studies reveal that subsets of mouse basal cells isolated from adult prostate glands are multipotent and can self-renew in vitro and in prostate reconstitution assays (3, 4). Similarly, a subset of adult human prostate basal cells is able to reconstitute prostatic glands when engrafted with mouse urogenital mesenchyme under the renal capsule of murine hosts (5). However, recent in vivo lineage studies in the adult prostate gave conflicting results regarding basal cells’ ability to give rise to luminal cells (6–9). Knowledge about urothelial cell lineages is also uncertain and controversial (2, 10).
The gene encoding the tumor protein p63 (p63) is a member of the p53 family and, like other family members, contains two different promoters that generate two classes of p63 proteins, the transactivating (TA) p63 and the NH2-terminal truncated (ΔN) p63. TAp63 contains an NH2-terminal transactivation domain that is absent in ΔNp63. Both TAp63 and ΔNp63 can be alternatively spliced at the 3′ terminus to produce α, β, and γ isoforms (11). ∆Np63 isoforms are selectively expressed at high levels in basal cell compartments of stratified and glandular epithelia, including in the bladder and prostate (12–14).
p63 plays an important role in embryogenesis. Heterozygous p63 mutations underlie various human syndromes of ectodermal dysplasia, orofacial clefting, and limb malformation (15), and p63 KO mice show defects in limb, craniofacial, and epithelial development. These mice lack all stratified epithelia and their derivatives (i.e., mammary, lachrymal, and salivary glands), die at birth from dehydration, and have markedly abnormal prostate and bladder epithelia (12, 13, 16, 17). Specific KO mice for the TA and the ΔNp63 isoforms reveal that these anomalies result from ΔNp63 absence (18, 19). Phenotypes in p63 KO or mutant mice result, among other reasons, from apparent defects in stem and progenitors cells’ capacity to proliferate or survive (19–24).
One-day-old p63-deficient mice show defects in prostate bud formation, suggesting that p63-expressing cells may represent developing prostatic stem cells. Moreover, p63−/− blastocyst complementation experiments suggested that p63-positive basal cells are required for development of p63-negative prostate luminal cells. By contrast, transplants of p63−/− urogenital sinus (UGS) revealed that luminal cells can form and regenerate in the absence of basal cells, hinting that the two cell types might represent independent cell lineages during development (12, 16, 25). Similarly, p63-deficient mouse urothelium contains umbrella-like cells in the absence of p63-positive basal/intermediate cells, suggesting that the cells are not related hierarchically (13, 16, 17). Because epithelial cell lineages in the developing bladder and prostate glands need to be further clarified, we generated knock-in mice expressing Cre recombinase (Cre) under control of the endogenous ΔNp63 promoter and performed a rigorous genetic lineage tracing analysis of ΔNp63-expressing cells in the developing caudal endoderm that gives rise to the prostate, bladder, and colorectal epithelia.
Results
Selective Cre-Mediated Recombination in ∆Np63-Expressing Cells.
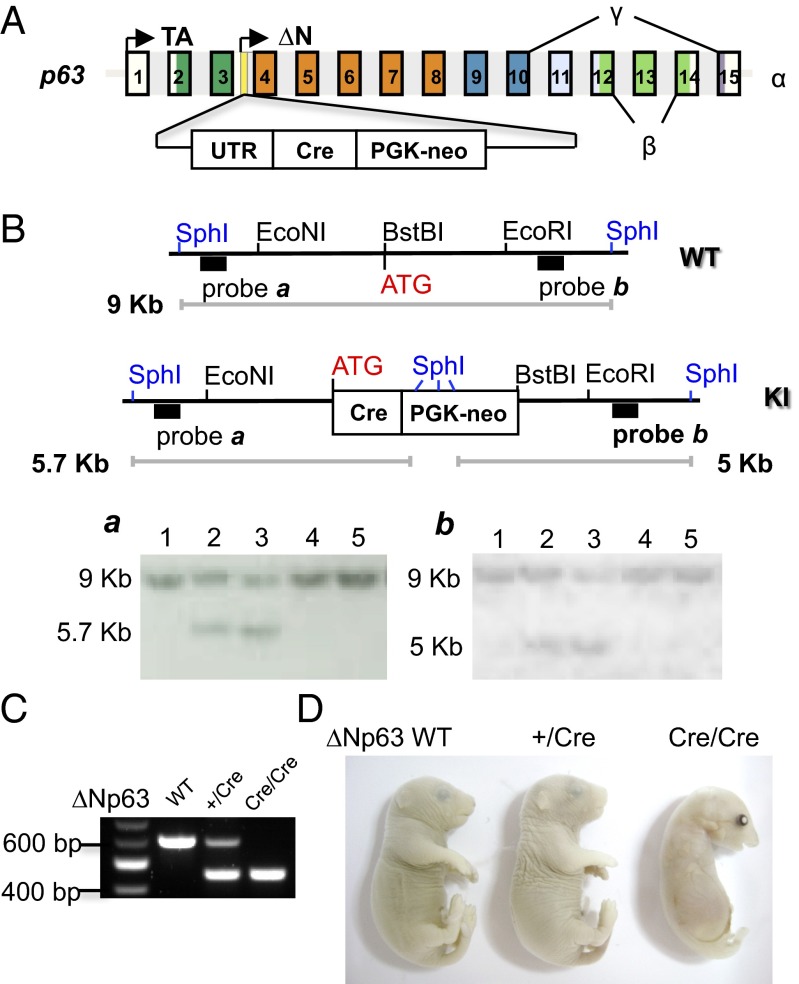
To engineer mice that selectively express Cre in ∆Np63-positive cells, we inserted a Cre-phosphoglycerate kinase (PGK)-neomycin resistant gene (Neo) cassette immediately downstream of the endogenous ∆Np63 promoter (Fig. 1A). Southern blot analysis, using unique probes and restriction sites (SphI) that lie outside the regions of homology, identified correctly recombined, neomycin-resistant ES cell clones (Fig. 1B). ES cells bearing this p63 allele, with insertion of Cre in intron 3, were used to generate ∆Np63+/Cre mice. In keeping with the normal phenotype of p63+/− mice, ∆Np63+/Cre mice also showed no gross or microscopic defects and were fertile. As predicted, mice homozygous for the mutation (∆Np63Cre/Cre) exhibited phenotypic alterations identical to p63-null mice (Fig. 1D and Fig. S1), further confirming specific targeting of the p63 locus (26, 27).
Fig. 1.
Generation of ∆Np63+/Cre knock-in (KI) mice. (A) Schematic representation of the strategy used to generate mice that express Cre under the ∆Np63 promoter. Cre recombinase followed the PGK-Neo selection cassette was inserted in p63 intron 3 located on chromosome 16 so that the ATG of Cre replaces the ATG of ∆Np63. (B) Southern blot analysis of ES cells electroporated with ∆Np63-Cre targeting vectors. DNA was digested with SphI and, after blotting, hybridized with probe a for 5′ arm homologous recombination (HR) screening. The membrane was then stripped and rehybridized with probe b for 3′ arm HR screening. The 9-kb band (a and b) represents the wt allele, the 5.7-kb band (a) represents the 5′ arm targeting event, and the 5-kb band (b) represents the 3′ targeting event. Lanes 2 and 3 in a and b show the expected bands, indicating successful HR. (C) PCR-based assay for mouse genotyping. (D) Macroscopic features of ∆Np63+/Cre and ∆Np63Cre/Cre P0-1 mice.
Because accurate lineage tracing using the Cre-loxP system depends on cell-specific Cre activity, we first used ∆Np63+/Cre;ROSA26EYFP embryos to test if Cre-mediated recombination faithfully recapitulates temporal and spatial ∆Np63 expression.
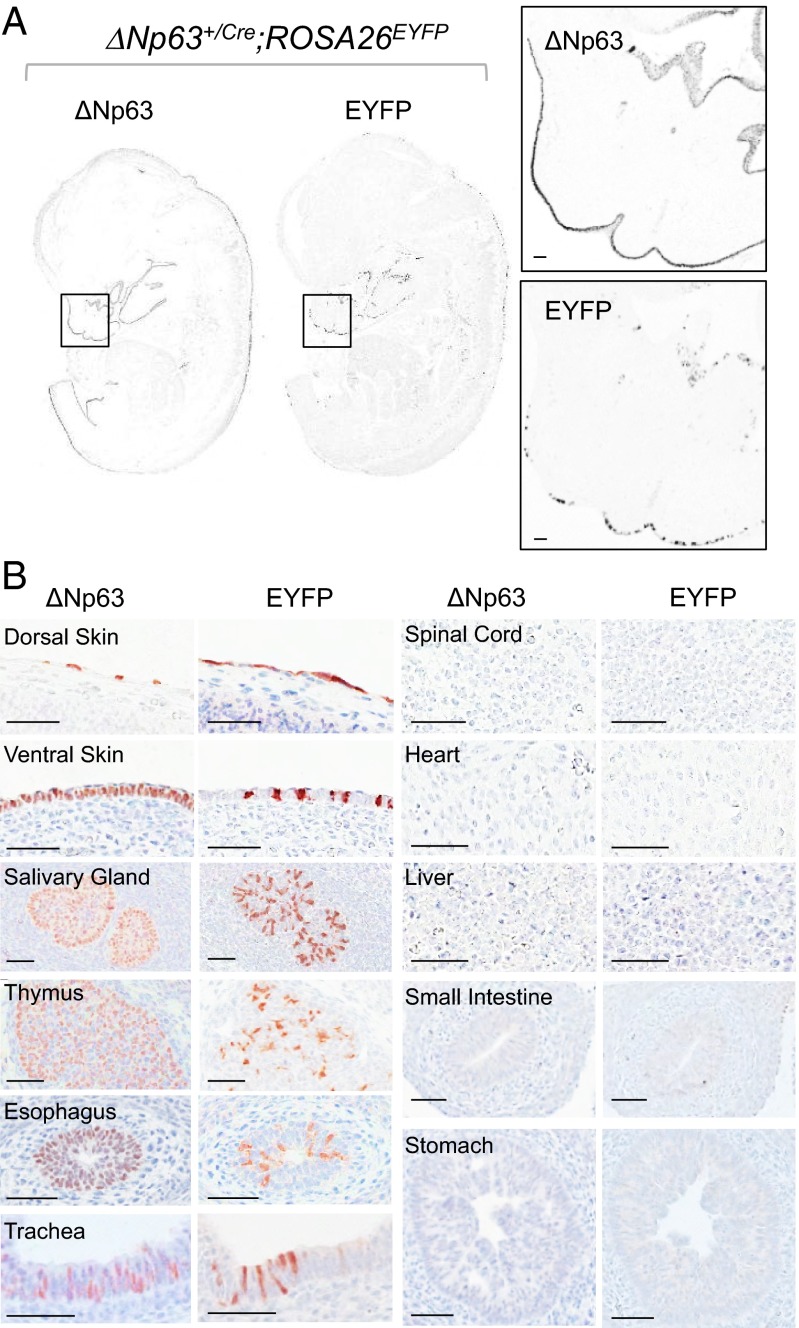
∆Np63 and the enhanced yellow fluorescent protein (EYFP) were coexpressed as early as 9.5 days postcoitum (dpc) in the primitive skin of ∆Np63+/Cre;ROSA26EYFP embryos (Fig. S2A). Importantly, 100% of EYFP-positive cells also expressed p63 (Fig. S2B), although Cre-mediated recombination and consequent expression of the reporter occurred only in a subset of cells, ranging from 12.2% to 25.4% (mean ± SD = 18.8 ± 9.3%). At 13.5 dpc, EYFP expression remained restricted to tissues expressing ∆Np63 (Fig. 2 A and B). Nuclear and cytoplasmic EYFP was detected in all organs expressing ∆Np63, including skin, thymus, salivary gland, esophagus, and trachea. Organs lacking ∆Np63 expression, such as fetal liver, spinal cord, heart, stomach, and small intestine, consistently lacked EYFP expression. Organs that were ∆Np63-negative in embryos continued to lack EYFP expression in adult ∆Np63+/Cre;ROSA26EYFP animals (Fig. S2C). No EYFP was detected in control ROSA26EYFP embryos (Fig. S2D), and ovarian germ cells, which express TAp63 but not ∆Np63 (18, 28), also did not express EYFP in ∆Np63+/Cre;ROSA26EYFP mice (Fig. S3). These results demonstrate that Cre-mediated recombination in ∆Np63+/Cre mice occurs selectively in cells expressing ∆Np63.
Fig. 2.
Cre-mediated recombination mirrors the expression pattern of ∆Np63 in ∆Np63+/Cre;ROSA26EYFP 13.5 dpc embryos. IHC analyses of ΔNp63 and EYFP expression in 13.5 dpc ∆Np63+/Cre;ROSA26EYFP embryos show that EYFP is expressed selectively in ΔNp63-positive tissues. (A) Representative images of serial sagittal sections of whole embryos. Higher magnifications of the selected areas are also shown. (B) Representative images of serial sections from various organs. (Scale bar: 50 μm.)
Basal, Intermediate, and Umbrella Cells of the Adult Urothelium Originate from ∆Np63-Expressing Stem Cells.
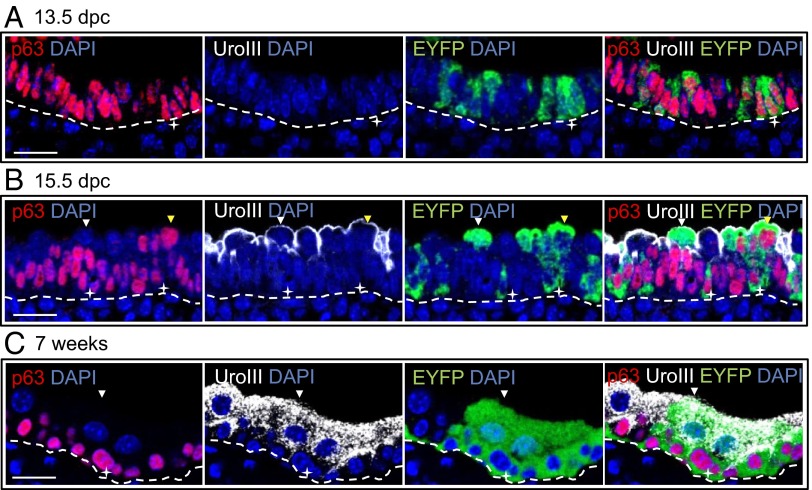
During development, the caudal endoderm subdivides in two along the dorsoventral axis. The urorectal septum separates the cloacal cavity dorsally into the last portion of the large intestine and ventrally into the primitive UGS. After further growth, the primitive ventral UGS subdivides into the bladder and the definitive UGS, which gives rise to the urethra and later to the prostate gland. To define the role of p63-positive cells in urothelial development, we examined ∆Np63 and EYFP expression by immunohistochemistry (IHC) and/or immunofluorescence in the bladder of ∆Np63+/Cre;ROSA26EYFP embryos and adult mice. At 13.5 dpc, when the bladder is anatomically distinct from the definitive UGS, the primitive urothelium consisted of a bistratified epithelium. ∆Np63 (but not TAp63) expression was detected in the vast majority of urothelial cells, whereas basal cell cytokeratin 5 (CK5) and the umbrella cell marker uroplakin III (29) were not yet expressed (Fig. 3A and Fig. S4 A and B). In ∆Np63+/Cre;ROSA26EYFP embryos, a variable fraction of ∆Np63-positive cells already expressed EYFP (mean ± SD = 19.2 ± 10.2%) (Fig. 3A).
Fig. 3.
∆Np63-negative umbrella cells of ∆Np63+/Cre;ROSA26EYFP mice express EYFP, demonstrating that they form from ∆Np63-positive stem cells of the primitive bladder. (A–C) Representative images of sagittal sections of the bladder from ∆Np63+/Cre;ROSA26EYFP mice at 13.5 dpc (A), 15.5 dpc (B), and 7 wk of age (C) triple-stained for p63/EYFP/uroplakin III (UroIII). White stars indicate p63+ EYFP+ uroplakin III− cells. Yellow arrowheads point to p63+ uroplakin III+ EYFP+ superficial cells and white arrowheads show p63− uroplakin III+ EYFP+ umbrella cells. Dotted lines represent basal membrane. (Scale bar: 20 μm.)
At 15.5 dpc, the urothelium appeared multistratified. The basal and suprabasal layers consisted of ∆Np63-positive cells that also expressed CK5 but not TAp63 (Fig. S4 A and B). The superficial layer contained cells uniformly expressing uroplakin III at the cell surface. Although most superficial cells were ∆Np63-negative, ∆Np63 was expressed in a subset of uroplakin III–positive superficial cells (mean ± SD = 33.8 ± 7.4%) (Fig. 3B). In ∆Np63+/Cre;ROSA26EYFP embryos, we observed EYFP expression not only in ∆Np63-positive cells, but also in a fraction of ∆Np63-negative/uroplakin III–positive cells within the superficial cell layer (mean ± SD = 17.5 ± 7%) (Fig. 3B). This result indicated that superficial cells originate from ∆Np63-expressing cells and that ∆Np63 levels are down-regulated upon differentiation.
In the stratified transitional epithelium of the adult bladder (7-wk-old mice), ∆Np63 expression was restricted to the basal and suprabasal (i.e., intermediate) compartments. As reported by our group and others, fully differentiated umbrella cells were consistently ∆Np63-negative and expressed uroplakin III (Fig. 3C and Fig. S4A). In keeping with findings in 15.5 dpc embryos, in adult ∆Np63+/Cre;ROSA26EYFP mice, EYFP was detected not only in ∆Np63-positive basal and intermediate cells, but also inumbrella cells (at an average rate of 21.2 ± 4.5%) (Fig. 3C). Overall, these results demonstrate that all cell types of the adult bladder urothelium (i.e., basal, intermediate, and umbrella cells) can derive from ∆Np63-positive progenitor/stem cells of the primitive bladder.
All Epithelial Lineages of the Adult Prostate Originate from ∆Np63-Expressing Stem Cells.
The prostate starts forming shortly after 17.5 dpc as solid epithelial buds that emerge from the UGS. Extensive epithelial branching starts at postnatal day 0 (P0). Nascent prostatic ducts elongate and branch to form an extensive network while, at the same time, the buds canalize in a proximal to distal direction along the developing ducts (30). To study the contribution of p63-positive cells toward other lineages, we investigated ∆Np63 expression in parallel with EYFP in the developing and adult prostate glands of ∆Np63+/Cre;ROSA26EYFP mice.
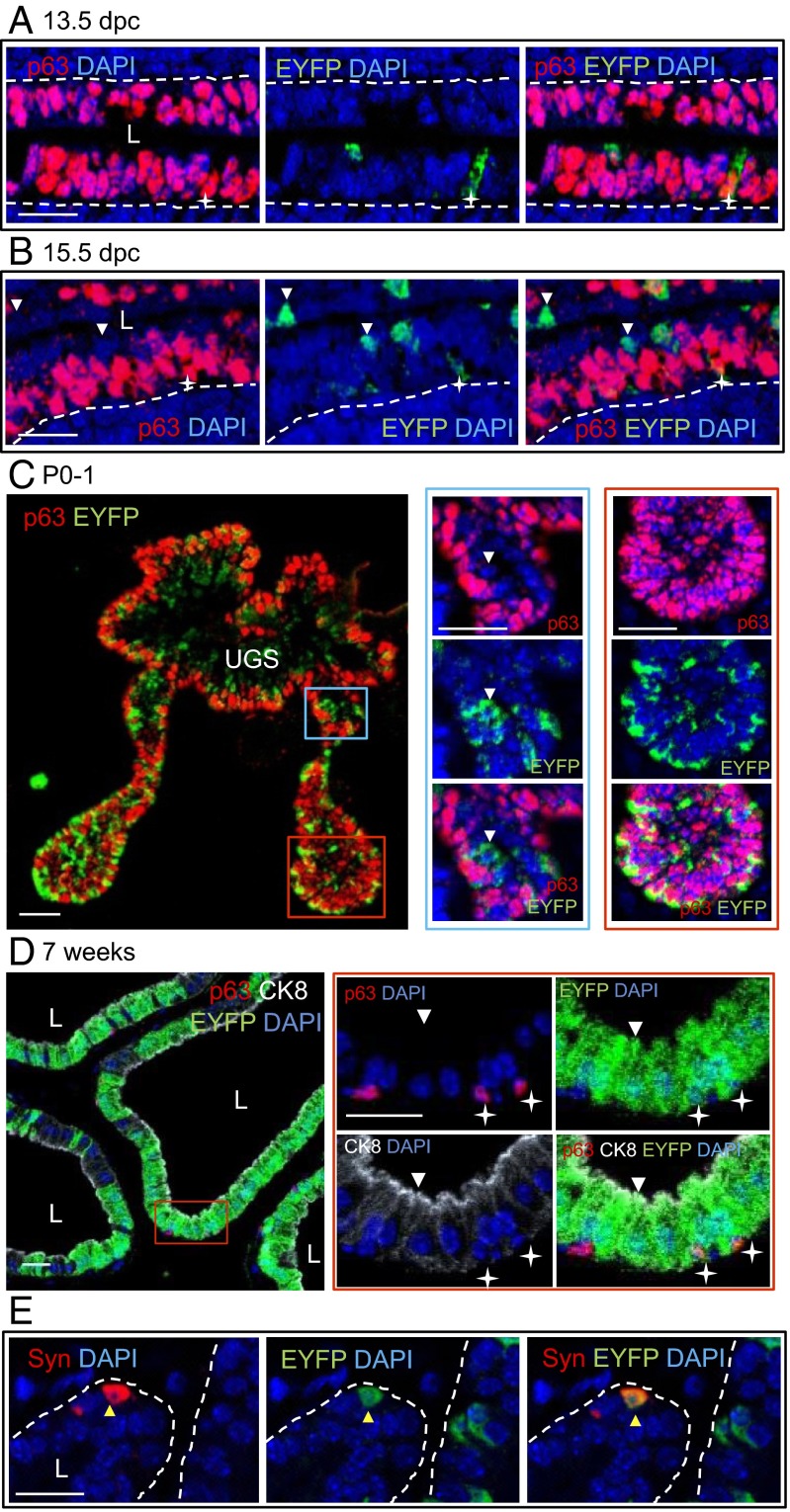
In the pseudostratified epithelium of the early UGS (13.5 dpc), most cells coexpressed ∆Np63 and the low-molecular weight CK8; CK5 was only coexpressed in dorsal UGS epithelium (Figs. S5 and S6A). In ∆Np63+/Cre;ROSA26EYFP embryos, a fraction of ∆Np63-positive cells already expressed EYFP (mean ± SD = 29.3 ± 13.8%) (Fig. 4A). At 15.5 dpc, the entire UGS epithelium continued to express CK8, whereas ∆Np63 was largely restricted to the CK5-expressing basal and suprabasal cell layers. Most epithelial cells lining the UGS lumen either lacked both ∆Np63 and CK5 or expressed CK5 but not ∆Np63 (Fig. 4B and Fig. S6B). In ∆Np63+/Cre;ROSA26EYFP embryos, EYFP was expressed in both ∆Np63-positive and -negative cells (Fig. 4B).
Fig. 4.
∆Np63-negative prostate luminal and neuroendocrine cells of ∆Np63+/Cre;ROSA26EYFP mice express EYFP, demonstrating that they derive from ∆Np63-positive stem cells. Representative images of sagittal UGS sections from 13.5 dpc (A) and 15.5 dpc (B) ∆Np63+/Cre;ROSA26EYFP embryos double-stained for EYFP/p63. Stars indicate p63+ EYFP+ cells. White arrowheads point to p63− EYFP+ cells. (C) Representative images of transversal sections of prostatic buds from P0-1 ∆Np63+/Cre;ROSA26EYFP mice double-stained for EYFP/p63. Higher magnifications of the selected areas are also shown. The blue box highlights the proximal region; the red box highlights the distal region of the buds. The white arrowhead points to a p63− EYFP+ cell. (D) Representative images of the prostate from 7-wk-old ∆Np63+/Cre;ROSA26EYFP mice stained for p63/CK8/EYFP. Higher magnifications of the area selected by the red box are shown. White stars indicate p63+ EYFP+ cells. The white arrowhead points to a p63− CK8+ EYFP+ luminal cell. (E) Representative images of the prostate from 7-wk-old ∆Np63+/Cre;ROSA26EYFP mice stained for synaptophysin (Syn)/EYFP. The yellow arrowhead shows a Syn+ EYFP+ neuroendocrine cell. L indicates the lumen; dotted lines represent basal membrane. (Scale bar: 20 μm.)
In the developing prostate of neonatal mice, we observed a proximal-to-distal gradient of ∆Np63 expression and absence of TAp63. All cells within the distal portion of the prostate buds expressed ∆Np63 and CK8, with a few cells also expressing CK5. In contrast, in the proximal buds, ∆Np63 and CK5 were expressed at high levels in basal cells, whereas suprabasal cells were low or negative for both ∆Np63 and CK5 and expressed high CK8 levels (Fig. 4C and Fig. S6C). These findings are consistent with the knowledge that prostate epithelium differentiates in a proximal-to-distal wave (1, 31). In ∆Np63+/Cre;ROSA26EYFP neonates, EYFP was detected in ∆Np63-positive cells throughout the length of the prostatic buds. Some p63-negative cells of the proximal prostate buds also expressed EYFP (Fig. 4C), implying that p63 is down-regulated during luminal differentiation. In the prostate of 7-wk-old adult mice, ∆Np63 was strictly confined to the basal cell compartment of prostatic glands (Fig. S5). Analysis of ∆Np63+/Cre;ROSA26EYFP mice showed EYFP in both ∆Np63-positive CK8-negative basal cells and ∆Np63-negative CK8-positive luminal cells (Fig. 4D). EYFP was also detected in cells expressing synaptophysin, a marker of neuroendocrine differentiation, at an average rate of 25.6 ± 14% (Fig. 4E). To verify these lineage tracing results, we crossed ∆Np63+/Cre mice with ROSA26mTomato/mEGFP reporter mice, which express a membranous form of the enhanced green fluorescent protein (mEGFP) upon Cre-mediated deletion of the gene encoding a membranous form of the Tomato reporter (mTomato). Expression of membranous EGFP in developing and adult ∆Np63+/Cre;ROSA26mTomato/mEGFP prostate glands paralleled EYFP expression in ∆Np63+/Cre;ROSA26EYFP mice (Fig. S7). In sum, these data demonstrate that p63-expressing cells of the UGS and developing prostate are able to generate all epithelial lineages of the adult prostate (i.e., basal, luminal, and neuroendocrine cells) and thus represent prostate progenitor/stem cells.
Adult Colorectal Epithelium also Originates from ∆Np63-Expressing Stem Cells of the Caudal Endoderm.
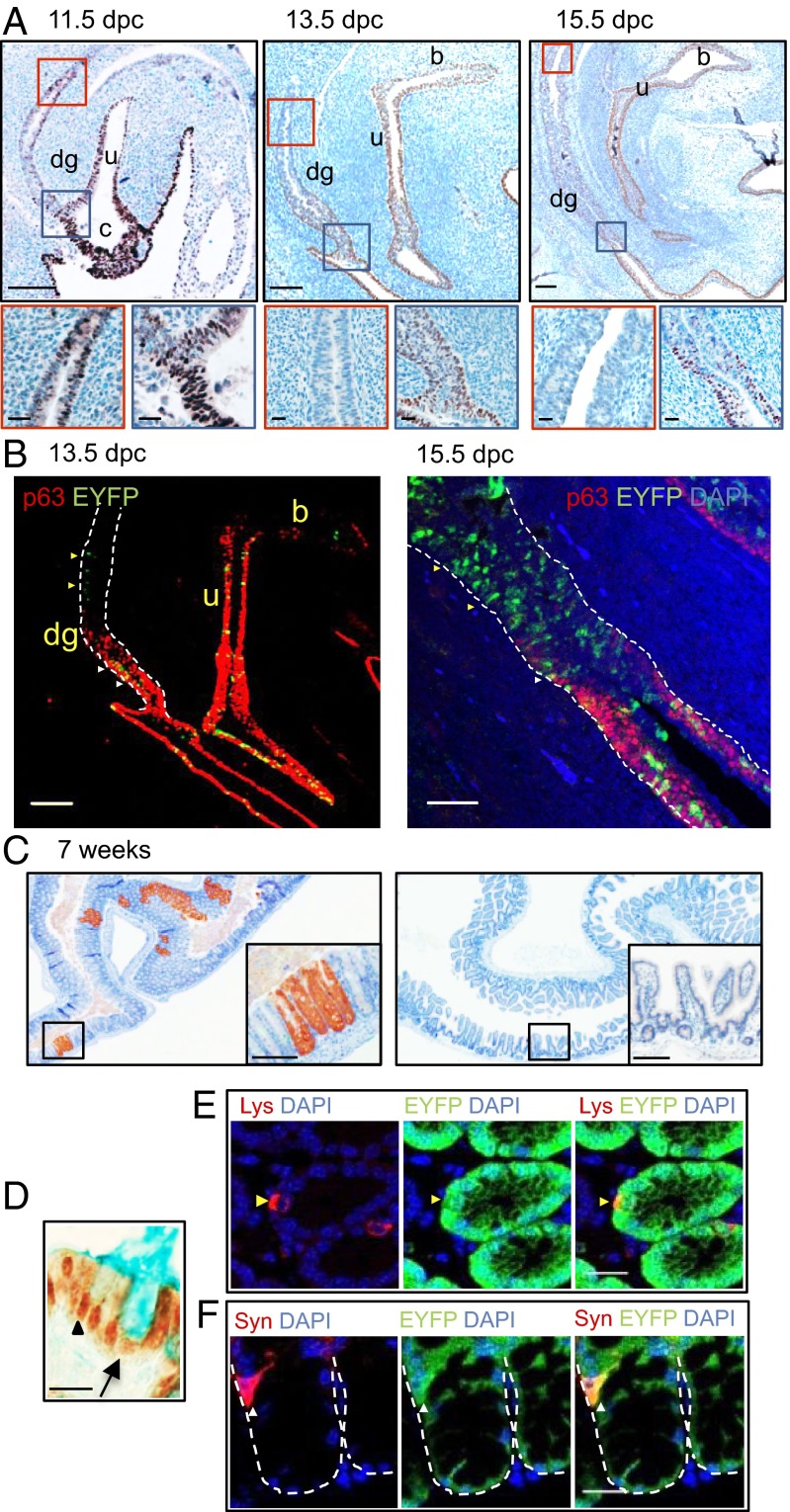
While examining ΔNp63 expression in the developing UGS, we observed expression in the caudal gut. To further assess its role in the developing colon, we studied its expression before and after septation of the cloaca into the definitive UGS and the last portion of the large intestine. IHC analysis of the caudal endoderm at 11.5 dpc revealed ∆Np63 expression throughout the primitive UGS, cloaca, and distal hindgut, where TAp63 was absent. However, the proportion of ∆Np63-positive cells diminished over time in a proximal to distal direction, and by 15.5 dpc ∆Np63 expression was confined to the most distal portion of the hindgut (Fig. 5A and Fig. S8A). Whereas ∆Np63 expression at earlier time points (i.e., 13.5 dpc) was coupled with CK8 but not CK5 expression, by 15.5 dpc some ∆Np63-positive cells coexpressed both cytokeratins. In line with previous reports, ∆Np63 was not expressed in the fully differentiated intestinal epithelium of adult mice but was detected in the squamous mucosa of the anal canal (Fig. S8B).
Fig. 5.
∆Np63-negative cells of the large intestine of ∆Np63+/Cre;ROSA26EYFP mice express EYFP, demonstrating that they derive from ∆Np63-positive stem cells of the caudal endoderm. (A) Representative images of sagittal sections of the urorectal region of ∆Np63+/Cre;ROSA26EYFP mice at 11.5 dpc (Left), 13.5 dpc (Center), and 15.5 dpc (Right) immunostained for ∆Np63. Higher magnifications of the selected areas are shown. The red box highlights the proximal region; the blue box highlights the distal region of the developing gut. (Scale bar: Upper, 100 μm; Lower, 20 μm.) (B) Representative images of sagittal sections of the urorectal region at 13.5 dpc (Left) and distal hindgut at 15.5 dpc (Right) double-stained for EYFP/p63. White arrowheads indicate p63+ EYFP+ cells; yellow arrowheads point to p63− EYFP+ cells. (Scale bar: Left, 100 μm; Right, 50 μm.) In both A and B, c indicates the cloaca, dg indicates the developing gut, u indicates the UGS, and b indicates the bladder. (C) Representative images of tissue sections of the large (Left) and small (Right) intestine from 7-wk-old ∆Np63+/Cre;ROSA26EYFP mice immunostained for EYFP. (Scale bar: 100 μm.) (D) Representative images of the adult colonic epithelium double-stained for EYFP/Alcian Blue. The black arrow indicates an EYFP+ Alcian Blue+ goblet cell; the black arrowhead points to an EYFP+ Alcian Blue− enterocyte. (Scale bar: 10 μm.) (E) Representative images of the adult colonic epithelium double stained for EYFP/lysozyme. The yellow arrowhead points to an EYFP+ lysozyme+ Paneth cell. Scale bar, 20 μm. (F) Representative images of the adult colonic epithelium double stained for EYFP/Syn. The white arrowhead indicates a synaptophysin+ EYFP+ enteroendocrine cell. (Scale bar: 20 μm.) The dotted line represents the basal membrane.
The finding that ∆Np63 was expressed prominently but transiently in developing colonic endoderm suggested, by analogy to our observations in the embryonic bladder and prostate, that it might also mark intestinal progenitor/stem cells that reduce ∆Np63 expression before differentiating into colorectal epithelium. To test this hypothesis, we followed the fate of ∆Np63-positive cells in the primitive colon of ∆Np63+/Cre;ROSA26EYFP mice. At 13.5 dpc, we detected EYFP in the distal portion of the hindgut, which still expressed ∆Np63 as well as in more proximal, ∆Np63-negative caudal gut endoderm (Fig. 5B). Similar results were obtained at 15.5 dpc, when ∆Np63 expression was confined to the distal tip of the developing colon at the ano-rectal junction. Notably, adult ∆Np63+/Cre;ROSA26EYFP mice consistently expressed EYFP in the large intestine (Fig. 5C) in regions stretching from the cecum to the anus. Histochemistry and IHC revealed EYFP expression in mature colonic lineages, including enterocytes, Alcian blue-avid goblet cells (Fig. 5D), lysozyme-positive Paneth cells in the cecum (Fig. 5E), and synaptophysin-positive neuroendocrine cells (Fig. 5F). In contrast, EYFP was never expressed in the small intestine (Fig. 5B). Because the adult colonic epithelium turns over fully every few days, all mature cells in 7-wk-old mice must have originated recently in self-renewing stem cells. EYFP expression in these mature cells thus indicates that some stem cells in the p63-negative adult colorectal epithelium clearly derive from embryonic progenitor/stem cells that once expressed ∆Np63 in the endodermal lining of the primitive distal gut.
Discussion
This in vivo cell lineage analysis investigates the stem cell capabilities of p63-expressing cells in the caudal endoderm. We provide unequivocal evidence that all cell types of the adult prostate, including luminal, basal, and neuroendocrine cells, derive from multipotent, ΔNp63-expressing cells of the developing prostatic buds. How do these data reconcile with previous reports showing that the prostate luminal cell compartment can form and expand in the complete absence of ΔNp63-positive cells? In other tissues, p63 (and specifically ΔNp63) is required to maintain progenitor/stem cells but is dispensable for cell differentiation (19–24). For instance, the epidermis of p63−/− embryos at 17 dpc contains clusters of cells expressing the differentiation markers loricrin, filaggrin, and involucrin, indicating that p63-null ectoderm can produce differentiated epidermal cells in the absence of functional progenitors (19, 27). Along these lines, it is therefore plausible that endodermal cells of the p63−/− UGS can differentiate into luminal cells in the absence of progenitor basal cells (16, 17, 25). One major difference between the two epithelial tissues, however, is that, whereas terminally differentiated epidermal cells are unable to proliferate and undergo apoptosis, prostate luminal cells are long-lived and able to divide (32). Of note, recent lineage tracing studies in the adult prostate suggest that basal and luminal cells are largely self-sustaining cell lineages (6, 8, 9). Our data support a model in which during prostate development, luminal cells form from multipotent, ΔNp63-expressing stem/progenitor cells; subsequently, ΔNp63-positive basal cells (or a least some of them) transition to unipotency and luminal cells acquire self-renewal capacities. The role of basal cells as progenitors during development is further supported by recent demonstration that CK14/CK5-positive basal cells are multipotent soon after birth, when prostate glands undergo substantial elongation and branching (33).
One central question that needs further clarification is whether a subset of ΔNp63-positive prostate basal cells retains stem cell activities and plays a significant role in prostate epithelium renewal in adulthood. In the two adult basal cell lineage tracing studies reported to date, Cre-mediated recombination was achieved in only a relatively small fraction of prostate basal cells, raising the possibility that relatively rare basal stem/progenitor cells might have eluded targeting (8, 9). Furthermore, the presence of the stem cell niche located in the proximal tubule of the prostate and enriched for basal cells with stem cell properties was not considered in these analyses. Indeed, ex vivo prostate reconstitution assays demonstrate unequivocally that stem cell antigen 1-positive and CD49f-positive or CD49f-positive and tumor-associated calcium signal transducer 2-highly positive basal cells enriched in the proximal ducts are multipotent and can regenerate the entire prostate epithelium (3–5, 34). Additional in vivo studies might establish whether specific basal-cell subsets play a role in renewal of the adult prostate in various pathophysiological conditions.
In line with results reported by Cheng et al., here we show that ΔNp63 is the main p63 isoform expressed in the embryonic urothelium (13). We demonstrate that ∆Np63-negative umbrella cells lining the luminal surface of the urothelium originate from ∆Np63-expressing stem cells of the primitive bladder. The urothelium of p63−/− mice carries a simple layer of umbrella-like cells (13, 16, 17). Moreover, phenotypically normal mice produced from p63−/− blastocysts complemented with p63+/+ ES cells show the presence of p63−/− umbrella cells (16). Taken together, these data support the concept that p63 is not essential for urothelial differentiation but plays a major role in the proliferation/survival of progenitor cells during urothelial development.
Sonic hedgehog (SHH), a morphogen required for the development of several organs, controls proliferation of progenitors with stem-cell properties (35). SHH is a downstream transcriptional target of p63 and its expression is reduced in p63-null urothelium (13, 36). In keeping with our observations during development, SHH-expressing basal cells of the adult urothelium were recently shown to give rise to umbrella cells during normal homeostasis and after urothelial cell injury (37). Thus, in contrast to the prostate, current evidence suggests that the urothelial cell lineage does not change after birth and that basal cells represent urothelial progenitor/stem cells in both development and adulthood.
p63 expression is absent from both human and murine postnatal intestinal epithelium (16, 38). However, our lineage analysis of the caudal endoderm revealed that early in development (11.5 dpc), ∆Np63 is expressed in stem cells of the primitive UGS, cloaca, and caudal gut. ∆Np63 expression is then down-regulated in a proximal to distal direction in the developing gut but persists in the UGS. We previously observed that p63−/− UGS endoderm undergoes aberrant intestinal differentiation, documented by expression of the intestinal homeobox gene CDX2 (16). Therefore, ∆Np63 might function as a cell-fate switch in the caudal endoderm, with its continuous expression in the UGS impeding intestinal differentiation and timely down-regulation in the hindgut favoring intestinal differentiation. Future studies might prove that lack of ∆Np63 expression in the endoderm is not only sufficient but also necessary for intestinal cell differentiation.
Last, expression of ∆Np63 in early colorectal progenitors also provides developmental insight into the cell of origin of squamous cell metaplasia and squamous cell carcinoma, rare but distinct disease entities of colorectal epithelium (39–41). Because p63 is a master regulator of stratified epithelial development (42), either persistence of ∆Np63-positive progenitor cells or regression to a primitive, ∆Np63-positive state may reflect the colorectal epithelium’s latent potential to recall its developmental origins and differentiate aberrantly into the squamous cell lineage.
Materials and Methods
Mice.
∆Np63+/Cre knock-in mice were generated and genotyped as described in SI Materials and Methods. ∆Np63+/Cre mice were crossed with the reporter mice B6.129 × 1-Gt(ROSA)26Sortm1(EYFP)Cos/J (ROSA26EYFP) or B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (ROSA26mTomato/mEGFP) from Jackson Laboratories. Animal care and experiments were performed according to the guidelines of the Harvard Medical School Center for Animal Resources and Comparative Medicine Standing Committee (under approved Animal Protocol 04066).
Tissue Preparation.
Embryos and newborn mice were fixed in 10% (vol/vol) buffered formalin at room temperature for 24 and 48 h, respectively. Adult mice were euthanized and transcardially perfused with PBS, followed by 10% formalin. Adult organs were further fixed for 48 h in formalin before being processed and embedded in paraffin.
Immunostaining and Quantification.
Immunohistochemistry and immunofluorescence were performed as described in SI Materials and Methods. The following antibodies were used: mouse anti–pan-p63 (4A4, Santa Cruz), goat anti-∆Np63 (N-16, Santa Cruz), mouse anti-TAp63 (6E6) (18), rabbit anti-CK5 (Covance), rabbit polyclonal anti-GFP (Abcam), mouse anti-synaptophysin (SY38, Millipore), goat anti-lysozyme C (Santa Cruz), mouse anti-uroplakin III (AU1, Fitzgerald), and rat anti-CK8 (TROMA-1, Developmental Studies of Hybridoma Bank). Anti-∆Np63 antibody was validated as described in Fig. S9. For immunofluorescence, image acquisition was performed using a Two Photon Zeiss LSM 510 META upright confocal microscope. Cells were counted manually using confocal ×40 photomicrographs. When available, at least 500 cells per mouse from three different animals were counted with the exception of 9.5 dpc embryos for which only two animals were analyzed. For the expression of EYFP in neuroendocrine cells, only 40 cells per mouse were evaluated.
Supplementary Material
Acknowledgments
We thank L. Ding and D. Tom (Enhanced Neuroimaging Core, Harvard Neurodiscovery Center) for help with acquisition of the confocal images. This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants 1R21DK72152 and 1R01DK089975 and the Harvard Stem Cell Institute (S.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221216110/-/DCSupplemental.
References
- 1.Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253(2):165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 2.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol. 2009;297(6):F1477–F1501. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goto K, et al. Proximal prostatic stem cells are programmed to regenerate a proximal-distal ductal axis. Stem Cells. 2006;24(8):1859–1868. doi: 10.1634/stemcells.2005-0585. [DOI] [PubMed] [Google Scholar]
- 4.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA. 2007;104(1):181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329(5991):568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, et al. Regenerated luminal epithelial cells are derived from preexisting luminal epithelial cells in adult mouse prostate. Mol Endocrinol. 2011;25(11):1849–1857. doi: 10.1210/me.2011-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461(7263):495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi N, Zhang B, Zhang L, Ittmann M, Xin L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell. 2012;21(2):253–265. doi: 10.1016/j.ccr.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ZA, et al. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat Cell Biol. 2013;15(3):274–283. doi: 10.1038/ncb2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo-Martin M, Domingo-Domenech J, Karni-Schmidt O, Matos T, Cordon-Cardo C. Molecular pathways of urothelial development and bladder tumorigenesis. Urol Oncol. 2010;28(4):401–408. doi: 10.1016/j.urolonc.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Yang A, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2(3):305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 12.Signoretti S, et al. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157(6):1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng W, et al. DeltaNp63 plays an anti-apoptotic role in ventral bladder development. Development. 2006;133(23):4783–4792. doi: 10.1242/dev.02621. [DOI] [PubMed] [Google Scholar]
- 14.Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells. 2007;25(11):2760–2769. doi: 10.1634/stemcells.2007-0355. [DOI] [PubMed] [Google Scholar]
- 15.Vanbokhoven H, Melino G, Candi E, Declercq W. p63, a story of mice and men. J Invest Dermatol. 2011;131(6):1196–1207. doi: 10.1038/jid.2011.84. [DOI] [PubMed] [Google Scholar]
- 16.Signoretti S, et al. p63 regulates commitment to the prostate cell lineage. Proc Natl Acad Sci USA. 2005;102(32):11355–11360. doi: 10.1073/pnas.0500165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karni-Schmidt O, et al. Distinct expression profiles of p63 variants during urothelial development and bladder cancer progression. Am J Pathol. 2011;178(3):1350–1360. doi: 10.1016/j.ajpath.2010.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh EK, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444(7119):624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- 19.Romano RA, et al. ΔNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development. 2012;139(4):772–782. doi: 10.1242/dev.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129(3):523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 21.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452(7184):225–229. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su X, et al. Rescue of key features of the p63-null epithelial phenotype by inactivation of Ink4a and Arf. EMBO J. 2009;28(13):1904–1915. doi: 10.1038/emboj.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher RB, et al. p63 regulates olfactory stem cell self-renewal and differentiation. Neuron. 2011;72(5):748–759. doi: 10.1016/j.neuron.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferone G, et al. Mutant p63 causes defective expansion of ectodermal progenitor cells and impaired FGF signalling in AEC syndrome. EMBO Mol Med. 2012;4(3):192–205. doi: 10.1002/emmm.201100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development. 2004;131(20):4955–4964. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- 26.Mills AA, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398(6729):708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 27.Yang A, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398(6729):714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 28.Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT. Differential expression of p63 isoforms in female reproductive organs. Mech Dev. 2005;122(9):1043–1055. doi: 10.1016/j.mod.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Hu P, et al. Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol. 2000;151(5):961–972. doi: 10.1083/jcb.151.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34(5):961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- 31.Hayward SW, et al. Epithelial development in the rat ventral prostate, anterior prostate and seminal vesicle. Acta Anat (Basel) 1996;155(2):81–93. doi: 10.1159/000147793. [DOI] [PubMed] [Google Scholar]
- 32.Evans GS, Chandler JA. Cell proliferation studies in rat prostate. I. The proliferative role of basal and secretory epithelial cells during normal growth. Prostate. 1987;10(2):163–178. doi: 10.1002/pros.2990100208. [DOI] [PubMed] [Google Scholar]
- 33.Ousset M, et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat Cell Biol. 2012;14(11):1131–1138. doi: 10.1038/ncb2600. [DOI] [PubMed] [Google Scholar]
- 34.Tsujimura A, et al. Proximal location of mouse prostate epithelial stem cells: A model of prostatic homeostasis. J Cell Biol. 2002;157(7):1257–1265. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caserta TM, et al. p63 overexpression induces the expression of Sonic Hedgehog. Mol Cancer Res. 2006;4(10):759–768. doi: 10.1158/1541-7786.MCR-05-0149. [DOI] [PubMed] [Google Scholar]
- 37.Shin K, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472(7341):110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Como CJ, et al. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002;8(2):494–501. [PubMed] [Google Scholar]
- 39.Cheng H, Sitrin MD, Satchidanand SK, Novak JM. Colonic squamous cell carcinoma in ulcerative colitis: Report of a case and review of the literature. Can J Gastroenterol. 2007;21(1):47–50. doi: 10.1155/2007/904081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sameer AS, Syeed N, Chowdri NA, Parray FQ, Siddiqi MA. Squamous cell carcinoma of rectum presenting in a man: A case report. J Med Case Reports. 2010;4:392. doi: 10.1186/1752-1947-4-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodesch G, et al. An unusual presentation of a cystic duplication of the sigmoid colon entirely lined with squamous epithelium. J Pediatr Surg. 2009;44(9):1831–1834. doi: 10.1016/j.jpedsurg.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 42.Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18(2):126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.