Abstract
A consolidated memory can be transiently destabilized by memory retrieval, after which memories are reconsolidated within a few hours; however, the molecular substrates underlying this destabilization process remain essentially unknown. Here we show that at lateral amygdala synapses, fear memory consolidation correlates with increased surface expression of calcium-impermeable AMPA receptors (CI-AMPARs), which are known to be more stable at the synapse, whereas memory retrieval induces an abrupt exchange of CI-AMPARs to calcium-permeable AMPARs (CP-AMPARs), which are known to be less stable at the synapse. We found that blockade of either CI-AMPAR endocytosis or NMDA receptor activity during memory retrieval, both of which blocked the exchange to CP-AMPARs, prevented memory destabilization, indicating that this transient exchange of AMPARs may underlie the transformation of a stable memory into an unstable memory. These newly inserted CP-AMPARs gradually exchanged back to CI-AMPARs within hours, which coincided with the course of reconsolidation. Furthermore, blocking the activity of these newly inserted CP-AMPARs after retrieval impaired reconsolidation, suggesting that they serve as synaptic “tags” that support synapse-specific reconsolidation. Taken together, our results reveal unexpected physiological roles of CI-AMPARs and CP-AMPARs in transforming a consolidated memory into an unstable memory and subsequently guiding reconsolidation.
Keywords: fear conditioning, memory reactivation, transient memory deconsolidation, labile memory
New memories initially exist in an unstable state and are then stabilized/consolidated over a period of hours into long-term memories that can persist for even a lifetime (1). The learning and consolidation of long-term memory require stable synaptic modifications in neural circuits (2). One such modification is the postsynaptic potentiation of excitatory synaptic transmission, which has been shown to underlie associative memory in the amygdala and hippocampus (3–5). This potentiation involves an increase in postsynaptic AMPA receptors (AMPARs) (6), tetrameric glutamate receptors that display distinct physical characteristics according to their subunit composition (7, 8). Calcium-permeable AMPARs (CP-AMPARs) mostly consist of GluA2-lacking AMPA receptors and support acute synaptic potentiation, but are less stable at the synapse, whereas calcium-impermeable AMPARs (CI-AMPARs) make up for most of the basal synaptic transmission and are more stable at the synapse because of their interaction with synaptic molecules through the GluA2 subunit (9, 10). Accordingly, long-term potentiation (LTP), a cellular model for long-term memory consolidation, involves early increases in GluA1-containing AMPARs, which are gradually replaced by GluA2-containing AMPARs (11).
It is now known that even consolidated memories can become destabilized again with retrieval (reactivation), after which memories must undergo a de novo protein synthesis-dependent reconsolidation process to persist (12, 13). Although memory retrieval and subsequent destabilization/reconsolidation have been a major focus of memory research for the last decade, the substrate of this lability at the synaptic receptor level remains largely unknown. Recently, two groups have demonstrated the involvement of CI-AMPARs or CP-AMPARs in “reconsolidation-update” (14), a specific variation of extinction in which conditioned fear memory is retrieved 1 h before extinction training (15, 16). Their studies focused primarily on the relationship between CI-AMPARs or CP-AMPARs and the memory-erasing effect of reconsolidation-update, and were not directed at the molecular mechanisms underlying memory destabilization on retrieval per se. We reasoned that finding manipulations that prevent the amnesic effect of combining memory retrieval with protein synthesis blockade would be vital in defining the mechanisms underlying this destabilization (12, 17). Here we show that memory retrieval induces an abrupt exchange of CI-AMPARs to CP-AMPARs that reverts back along the course of reconsolidation and is critical for memory destabilization. This molecular exchange requires both NMDA receptor (NMDAR) activation and the endocytosis of GluA2-containing CI-AMPARs.
Results
Memory Retrieval Causes Transient Replacement of CI-AMPARs by CP-AMPARs.
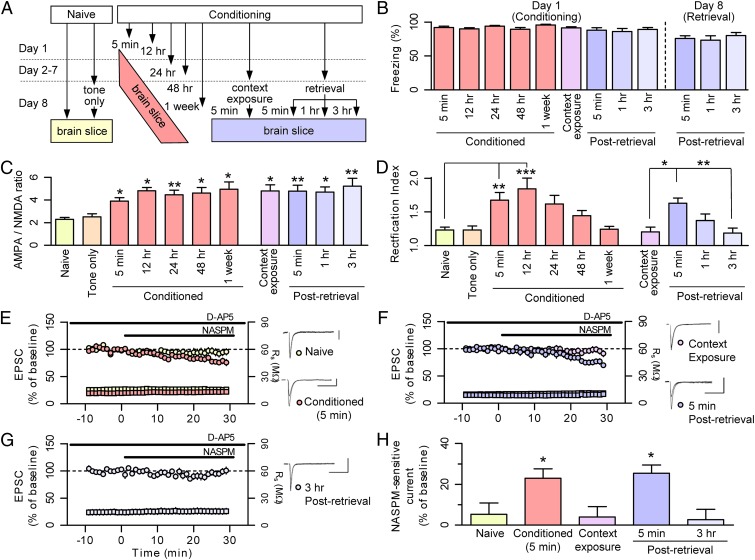
Auditory-cued fear memory is believed to be stored in the form of synaptic potentiation at the thalamic input synapses to the lateral amygdala (LA), known as T-LA synapses (4, 6). We characterized the changes at these synapses induced by fear learning and subsequent memory retrieval (Fig. 1 A and B). We identified the synaptic changes in brain slices prepared at various time points after fear conditioning (5 min, 12 h, 24 h, 48 h, and 1 wk); we used two control groups for the conditioned rats: naïve controls and the tone-only group, in which a single tone was presented to naïve rats. For fear memory retrieval, conditioned rats were tested with a single conditioned stimulus (CS) in a distinct context on day 8, and slices were prepared at 5 min, 1 h, or 3 h after retrieval. As a control, conditioned rats were exposed to the memory retrieval context for the same duration as the three postretrieval groups but without tone presentation (context exposure group).
Fig. 1.
Memory retrieval abruptly induces synaptic CI-AMPAR–to–CP-AMPAR exchange, without changing the synaptic strength at T-LA synapses. (A) Behavioral procedures. (B) Freezing levels in response to the last trial of fear conditioning in all conditioned groups shown in A (day 1). Memory retrieval on day 8 was tested in the three postretrieval groups (5 min postretrieval, 1 h postretrieval, and 3 h postretrieval). Naïve and tone-only control groups were omitted for a better display. (C) AMPA EPSC/NMDA EPSC ratios measured after fear conditioning and subsequent retrieval. All of the conditioned groups showed significant potentiation compared with naïve controls (naïve, 2.3 ± 0.2, n = 12; tone-only, 2.5 ± 0.3, n = 10; conditioned 5 min, 3.9 ± 0.3, n = 14; conditioned 12 h, 4.8 ± 0.3, n = 8; conditioned 24 h, 4.5 ± 0.4, n = 12; conditioned 48 h, 4.6 ± 0.5, n = 14; conditioned 1 wk, 5.0 ± 0.7, n = 8; context exposure, 4.8 ± 0.5, n = 15; 5 min postretrieval, 4.8 ± 0.5, n = 15; 1 h postretrieval, 4.7 ± 0.5, n = 10; 3 h postretrieval, 5.2 ± 0.7, n = 10; F10,117 = 4.067, P < 0.0001, one-way ANOVA; *P < 0.05, **P < 0.01, Newman–Keuls post hoc test vs. naïve). (D) Rectification index of the synaptic AMPA receptor-mediated currents. Fear conditioning and retrieval induced an increase of rectification compared with naïve/tone-only controls and context-exposed controls, respectively (naïve, 1.23 ± 0.04, n = 16; conditioned 5 min, 1.67 ± 0.11, n = 15; conditioned 12 h, 1.84 ± 0.16, n = 9; conditioned 24 h, 1.62 ± 0.13, n = 11; conditioned 48 h, 1.44 ± 0.08, n = 11; conditioned 1 wk, 1.24 ± 0.04, n = 16; context exposure, 1.20 ± 0.07, n = 10; 5 min postretrieval, 1.63 ± 0.08, n = 21; 1 h postretrieval, 1.37 ± 0.10, n = 8; 3 h postretrieval, 1.19 ± 0.06, n = 12; F10,127 = 6.526, P < 0.0001, one-way ANOVA; *P < 0.05, **P < 0.01, Newman–Keuls post hoc test). (E–G) AMPAR-EPSC sensitivity to NASPM increased after fear conditioning (E) and memory retrieval (F), but diminished within 3 h after retrieval (G). Circles represent EPSC amplitudes, and squares indicate series resistance of whole-cell recordings. (Scale bars: 100 pA and 50 ms.) (H) Summarized results for E–G (naïve, 5.3 ± 5.6%, n = 11; conditioned 5 min, 23.0 ± 4.6%, n = 11; context exposure, 4.0 ± 5.1, n = 11%; 5 min postretrieval, 15.5 ± 4.1%, n = 9; 3 h postretrieval, 2.7 ± 5.1%, n = 8; F4,45 = 4.839, P < 0.005, one-way ANOVA; *P < 0.05, Newman–Keuls post hoc test). All data points represent group means ± SEM.
We first assessed synaptic function based on the ratio of AMPAR-mediated currents to NMDAR-mediated currents. The AMPA/NMDA ratio sharply increased after fear conditioning and remained significantly greater than the baseline value for 1 week (Fig. 1C and Fig. S1), consistent with a previous report (15). Intriguingly, memory retrieval had no effect on the AMPA/NMDA ratio, and neither did context exposure alone. These results, considered along with our previous observations (18), show that conditioning-induced potentiation at T-LA synapses remains largely unchanged after memory retrieval (19, 20).
We next determined the relative contribution of CI-AMPARs and CP-AMPARs to AMPAR-mediated synaptic transmission at T-LA synapses (SI Materials and Methods). For this, we measured both AMPA excitatory postsynaptic current (EPSC) rectification and sensitivity to polyamine derivatives. CI-AMPARs generally exhibit linear current-voltage (I-V) relationships, whereas CP-AMPARs inwardly rectify (21) and thereby display a rectification index significantly greater than unity. High CP-AMPAR content is also indicated by responsiveness of synaptic responses to 1-naphthylacetylsperimine (NASPM), a polyamine derivative that use-dependently blocks CP-AMPAR activity (7). We first confirmed Clem and Huganir’s finding that CP-AMPARs are transiently inserted after conditioning (15). Fear conditioning produced a transient increase in the rectification index, and the enhanced rectification indices steadily decreased after 12 h and subsided to basal levels within 1 wk (Fig. 1D and Fig. S2). Consistent with this process, AMPAR-mediated EPSCs became more responsive to NASPM inhibition after fear conditioning (residual amplitude: naïve, 94.7 ± 5.6%, n = 11; conditioned for 5 min, 77.0 ± 4.6%, n = 11) (Fig. 1E).
We next examined whether fear memory retrieval alters surface expression of CP-AMPARs. Immediately after fear memory retrieval, rectification at T-LA synapses showed a transient increase which dissipated within hours, whereas context exposure alone had no effect (Fig. 1D and Fig. S2). Consistent with the increased rectification, fear memory retrieval rendered AMPAR-mediated EPSCs more sensitive to NASPM inhibition in slices prepared at 5 min after retrieval, whereas context exposure alone did not (5 min postretrieval, 74.5 ± 4.1%, n = 9; context exposure, 96.0 ± 5.1%, n = 11) (Fig. 1F). NASPM inhibition was no longer evident in slices prepared at 3 h after retrieval (3 h postretrieval, 97.3 ± 5.1%, n = 8) (Fig. 1G). Thus, sensitivity to NASPM inhibition was transiently enhanced after fear memory retrieval (P < 0.05 for designated pairs, Newman–Keuls post hoc test) (Fig. 1H), but then dissipated.
These results show that fear memory retrieval leads to the rapid insertion of CP-AMPARs into LA synapses (SI Materials and Methods). Because synaptic strength is maintained despite this increase in CP-AMPARs on retrieval, this suggests that retrieval induces the synaptic removal of CI-AMPARs, which is known to occur through endocytosis involving the C-terminal tail of GluA2 (22). Collectively, these findings suggest that LA synapses undergo an abrupt exchange from CI-AMPARs to CP-AMPARs.
Blocking of CI-AMPAR Endocytosis Prevents Transformation of a Consolidated Memory to an Unstable Memory.
Given that GluA2 stabilizes AMPARs at synapses through interaction with synaptic proteins (11, 23–27) and also stabilizes dendritic spines (26, 28), supporting memory storage (29–31), CI-AMPAR endocytosis may destabilize the potentiated synaptic strength underlying consolidated memory. We tested whether the memory retrieval-induced decrease in CI-AMPARs is critical for rendering the memory unstable using GluA23Y. This synthetic peptide mimicks the C-terminal tail of GluA2 and thus prevents GluA2-dependent endocytosis of AMPAR (32). GluA23Y was made cell membrane-permeable by fusing it to the cell membrane transduction domain of the HIV-1 transactivator of transcription (Tat) protein (32).
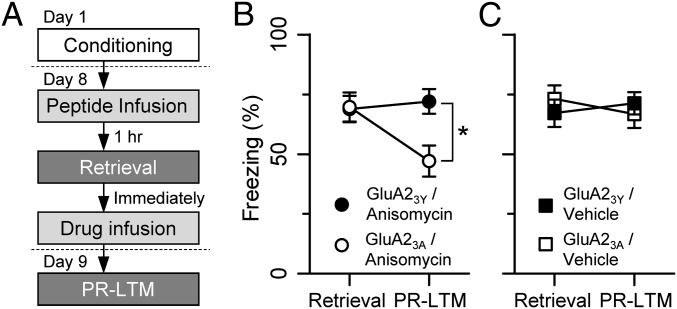
Memory retrieval and subsequent anisomycin treatment are known to block reconsolidation, resulting in amnesia (12). Rats were conditioned on day 1 and presented with a single CS in a distinct context on day 8, followed by anisomycin microinfusion. The animals were then tested for amnesia 24 h later with a protocol for postretrieval long-term memory (PR-LTM) (Fig. 2A). Microinfusion of GluA23Y into the LA before retrieval protected the memory from the amnesic effect of anisomycin (GluA23Y/anisomycin retrieval, 68.9 ± 5.5%; GluA23Y/anisomycin PR-LTM, 72.0 ± 5.1%, n = 9; P > 0.05, Bonferroni post hoc test) (Fig. 2B), whereas the control peptide GluA23A spared the amnesic effect (GluA23A/anisomycin retrieval, 69.7 ± 6.13%; GluA23A/anisomycin PR-LTM, 47.2 ± 6.5%, n = 4; P < 0.05, Bonferroni post hoc test). Two-way ANOVA revealed a significant session × peptide interaction (F1,11 = 10.89, P < 0.01) and a significant effect of behavioral session (retrieval vs. PR-LTM, F1,11 = 6.25, P < 0.05), but no effect of peptide (GluA23Y vs. GluA23A, F1,11 = 2.13, P > 0.05). Bonferroni post hoc tests indicated significantly different freezing levels between the GluA23Y/anisomycin and GluA23A/anisomycin groups at PR-LTM (P < 0.05), but not at retrieval (P > 0.05). These results suggest that the endocytosis of GluA2-containing CI-AMPARs is required for the transformation of consolidated memories into unstable memories by retrieval.
Fig. 2.
Blockade of GluA2-containing AMPAR endocytosis prevents memory destabilization on retrieval. (A) Behavioral procedure. (B) Microinfusion of GluA23Y at 1 h before retrieval into the LA rendered the memory insensitive to the amnesic effect of anisomycin, whereas the control peptide GluA23A did not block this amnesic effect. (C) When vehicle was microinfused immediately after retrieval instead of anisomycin, GluA23Y (filled square) had no effect on either retrieval or PR-LTM compared with GluA23A (open square). Diffusion of the GluA23Y peptide (1.5 nmol) was confined within the LA when measured at 1 h after the microinjection (Fig. S3A), and cannula tip placement was confirmed for the experiments shown in B and C (Fig. S3B).
This effect is not due to the effects of GluA23Y or GluA23A memory retention, given that these peptides alone, without anisomycin microinjection, did not affect either retrieval or PR-LTM (retrieval: GluA23Y/vehicle, 67.1 ± 5.8%; GluA23A/vehicle, 73.0 ± 5.7%, n = 8; P > 0.05, Bonferroni post hoc test; PR-LTM: GluA23Y/vehicle, 71.3 ± 4.7%; GluA23A/vehicle, 66.7 ± 5.8%, n = 8; P > 0.05, Bonferroni post hoc test). Two-way ANOVA revealed no significant interaction (F1,14 = 1.11, P > 0.05), effect of behavioral session (F1,14 = 0.01, P > 0.05), or effect of peptide (F1,14 = 0.05, P > 0.05) (Fig. 2C).
AMPAR Exchange on Memory Retrieval Requires CI-AMPAR Endocytosis and NMDAR Activity.
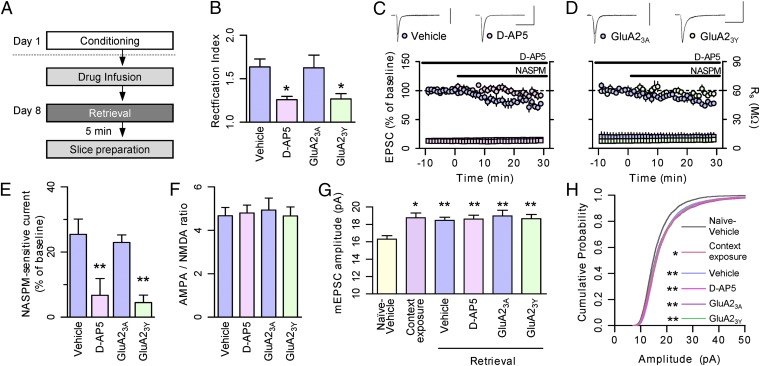
To test the consequences of blocking CI-AMPAR endocytosis on retrieval at the synaptic level, and to further characterize the molecular mechanisms of AMPAR exchange, we combined in vivo behavioral manipulation and drug microinfusion with ex vivo whole-cell recordings (Fig. S4 and SI Materials and Methods). Because the microinfusion of the NMDAR antagonist d-(-)-2-amino-5-phosphonopentanoic acid (d-AP5) into the amygdala is known to block retrieval-induced memory destabilization (17), we first tested whether CI-AMPAR–to–CP-AMPAR exchange would also be blocked at the same dose (2.5 μg/0.5 μL/side) (Fig. 3A). We found that d-AP5 microinfusion blocked the retrieval-induced increases in rectification (Fig. 3B and Fig. S5) and NASPM sensitivity (Fig. 3 C and E). In contrast, robust increases in both rectification and NASPM sensitivity were detected after vehicle injection. Despite this impairment of CP-AMPAR insertion by d-AP5 microinjection, we detected no change in synaptic strength in either the AMPA/NMDA ratio (Fig. 3F and Fig. S6) or AMPAR miniature EPSC (mEPSC) characteristics (Fig. 3 G and H and Fig. S7) compared with vehicle controls, indicating that CI-AMPAR removal was also blocked by inhibition of NMDARs. These results show that the CI-AMPAR–to–CP-AMPAR exchange on memory retrieval is triggered by NMDAR activation.
Fig. 3.
GluA2-AMPAR endocytosis and NMDAR activity are required for AMPAR exchange on retrieval. (A) Behavioral procedure. AMPAR-mediated transmission changes on retrieval were monitored ex vivo after drug (or peptide) microinjection and subsequent retrieval. (B) Rectification index of the synaptic AMPA receptor-mediated currents measured after drug (or peptide) infusion and subsequent retrieval. d-AP5 and GluA23Y microinfusion attenuated the increase of rectification on retrieval compared with vehicle and GluA23A controls, respectively (vehicle, 1.64 ± 0.09, n = 15; d-AP5, 1.26 ± 0.03, n = 10; GluA23A, 1.63 ± 0.14, n = 10; GluA23Y, 1.27 ± 0.06, n = 15; F3,46 = 5.537, P < 0.005, one-way ANOVA; *P < 0.05, Newman–Keuls post hoc test). (C and D) Enhanced sensitivity to NASPM after memory retrieval was blocked in slices prepared from d-AP5- (C) or GluA23Y-injected animals (D) compared with vehicle or GluA23A-injected animals, respectively. Circles represent EPSC amplitudes, and squares indicate series resistance of whole-cell recordings. (Scale bars: 100 pA and 50 ms.) (E) Summarized results for C and D (vehicle, 25.5 ± 4.6%, n = 6; d-AP5, 6.7 ± 5.2%, n = 6; GluA23A, 23.0 ± 2.3%, n = 5; GluA23Y, 4.5 ± 2.3%, n = 7; F3,20 = 8.039, P < 0.005, one-way ANOVA; **P < 0.01, Newman–Keuls post hoc test vs. designated control). (F) AMPA EPSC/NMDA EPSC ratios measured after drug (or peptide) infusion and subsequent retrieval. All four groups exhibited similarly potentiated ratios (vehicle, 4.7 ± 0.4, n = 16; d-AP5, 4.8 ± 0.4, n = 14; GluA23A, 4.9 ± 0.5, n = 12; GluA23Y, 4.9 ± 0.5, n = 12; F3,50 = 0.085, P > 0.05, one-way ANOVA; P > 0.05 for all pairs, Newman–Keuls post hoc test). (G and H) AMPAR-mediated mEPSCs measured after drug (or peptide) infusion and subsequent retrieval. All five of the conditioned groups (context exposure-vehicle, vehicle, d-AP5, GluA23A, and GluA23Y) displayed enhanced mEPSC amplitude compared with the naïve vehicle group irrespective of drug infusion or memory retrieval, but mEPSC amplitude was not significantly different among the five conditioned groups (naïve-vehicle, 16.3 ± 0.4 pA, n = 14; context exposure-vehicle, 18.8 ± 0.5 pA, n = 12; vehicle, 18.4 ± 0.4 pA, n = 15; d-AP5, 18.6 ± 0.4 pA, n = 14; GluA23A, 18.9 ± 0.7 pA, n = 17; GluA23Y, 18.7 ± 0.5 pA, n = 18; F5,84= 3.301, P < 0.001, one-way ANOVA; *P < 0.05; **P < 0.01, Newman–Keuls post hoc test vs. naïve-vehicle). Thus, AMPAR mEPSC amplitude was potentiated by fear conditioning, but neither amplitude nor frequency changed on retrieval compared with context exposure-vehicle controls irrespective of drug infusion (Fig. S7), consistent with the results of the AMPA/NMDA ratio measurements (Fig. 1C), as well as with our previous findings (18). Cannula tip placement was confirmed for the experiments shown in B–H (Fig. S4E).
We then tested whether the blockade of GluA2-containing CI-AMPAR endocytosis inhibits AMPAR exchange. In contrast to the GluA23A control peptide, the GluA23Y peptide used earlier also blocked the rise in both EPSC rectification (Fig. 3B) and NASPM sensitivity (Fig. 3 D and E) on retrieval. Moreover, the synaptic strength was unchanged by GluA23Y microinfusion and subsequent memory retrieval compared with GluA23A controls (Fig. 3 F, G, and H, Fig. S6, and Fig. S7), indicating that inhibition of GluA2-containing CI-AMPAR endocytosis also blocks the insertion of additional CP-AMPARs. This finding suggests that removal of CI-AMPAR is a prerequisite for additional CP-AMPAR insertion on retrieval. Collectively, these results demonstrate that memory lability and CI-AMPAR–to–CP-AMPAR exchange share strikingly similar molecular requirements.
Activity of CP-AMPARs Newly Inserted by Retrieval Is Required for Reconsolidation.
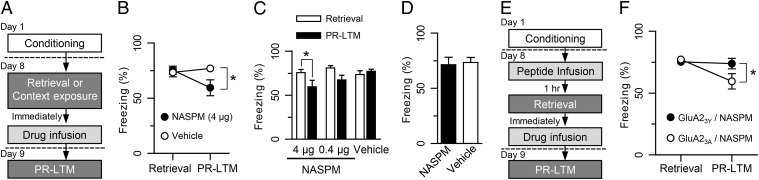
What is the role of these newly inserted CP-AMPARs? Because these receptors are inserted into synapses in an activity-dependent manner, they presumably accumulate at synapses that are strongly involved in the retrieved memory. The receptors and the local calcium influx that they allow at resting potentials (33, 34) would be poised to guide the synapse-specific molecular events required for reconsolidation (18, 19). Thus, we tested whether blocking CP-AMPARs immediately after retrieval would impair the reconsolidation of destabilized memory. Rats were conditioned on day 1 and presented with a single CS in a distinct context on day 8 (Fig. 4A). Immediately after retrieval, NASPM was microinfused into LA, and memory retention was tested for PR-LTM 24 h later. Two-way ANOVA revealed a significant session × drug interaction (F2,30 = 4.38, P < 0.05) and a significant effect of behavioral session (retrieval vs. PR-LTM, F1,30 = 7.83, P < 0.01), but no effect of drugs (high-dose NASPM vs. low-dose NASPM vs. vehicle, F2,30 = 1.37, P > 0.05). PR-LTM was selectively impaired in the NASPM-treated group (4 μg/0.5 μL/side) compared with the vehicle-treated group (high-dose NASPM: retrieval, 75.4 ± 3.7%; PR-LTM, 59.5 ± 7.8%, n = 11; vehicle: retrieval, 73.5 ± 4.2%; PR-LTM, 77.0 ± 2.3%, n = 13; high-dose NASPM vs. vehicle: retrieval, P > 0.05; PR-LTM, P < 0.05, Bonferroni post hoc test) (Fig. 4 B and C). This amnesic effect of NASPM was dose-dependent, as demonstrated by the lesser effect of the low-dose NASPM (0.4 μg/0.5 μL/side: retrieval, 80.9 ± 2.5%, PR-LTM, 67.2 ± 5.4%, n = 9; NASPM low-dose vs. vehicle: both retrieval and PR-LTM, P > 0.05, Bonferroni post hoc tests) (Fig. 4C). A subset of these rats were reconditioned, when both the vehicle-treated and high-dose NASPM-treated groups showed robust memory retention (NASPM, 70.2 ± 6.1%, n = 6; vehicle, 70.3 ± 4.8%, n = 6; P > 0.05, unpaired t test), suggesting that the amnesic effect was not due to permanent damage to the LA neurons.
Fig. 4.
Blockade of newly inserted CP-AMPARs on retrieval impairs memory reconsolidation. (A) Behavioral procedure for the experiments shown in B–D. (B and C) NASPM infusion immediately after retrieval dose-dependently impaired PR-LTM compared with vehicle controls. *P < 0.05. (D) Memory retention at 24 h after context exposure was similar in the NASPM- and vehicle-treated groups. (E) Behavioral procedure for the experiments shown in F. (F) GluA23Y peptide infusion prevented the memory impairment induced by NASPM compared with the GluA23A controls. Cannula tip placement was confirmed for the experiments shown in B, C, D, and F (Fig. S3 C–E).
This amnesic effect of NASPM required memory retrieval. When NASPM was microinfused into the LA of rats that received only context exposure without tone (CS) presentation, memory retention was similar in both groups with testing 24 h after the context exposure (NASPM, 71.5 ± 6.8%, n = 10; vehicle, 73.5 ± 4.6%, n = 14; P > 0.05, unpaired t test) (Fig. 4D).
We further reasoned that if CP-AMPAR insertion were prevented during retrieval, then blockade by NASPM would lose its effect on memory. We tested this idea by microinfusing either GluA23Y peptide or control peptide before memory retrieval, followed by NASPM immediately after retrieval (Fig. 4E). Consistent with our hypothesis, infusion of the GluA23Y peptide prevented the memory impairment induced by the NASPM treatment (GluA23Y/NASPM retrieval, 75.3 ± 2.6%; GluA23Y/NASPM PR-LTM, 73.9 ± 4.3%, n = 13; P > 0.05, Bonferroni post hoc test) (Fig. 4F), unlike the sparing of the NASPM effect seen with the control peptide (GluA23A/NASPM retrieval, 77.3 ± 2.5%; GluA23A/NASPM PR-LTM, 59.6 ± 6.2%, n = 10; P < 0.05, Bonferroni post hoc test). Infusion of peptide alone before retrieval demonstrated no effect on memory retrieval or retention (Fig. 2C). Two-way ANOVA revealed a significant session × drug interaction (F1,21 = 4.47, P < 0.05) and a significant effect of peptide (GluA23Y vs. GluA23A, F1,21 = 6.17, P < 0.05), but no effect of behavioral session (retrieval vs. PR-LTM, F1,21 = 6.17, P > 0.05). Bonferroni post hoc tests indicated significantly different freezing levels between the GluA23Y/NASPM and GluA23A/NASPM groups when tested for PR-LTM (P < 0.05), but not at retrieval (P > 0.05). These findings suggest that CP-AMPARs that are inserted into synapses during retrieval have a critical role in memory reconsolidation.
Discussion
Here we show that the exchange from CI-AMPARs to CP-AMPARs is the major synaptic change on memory retrieval. Importantly, blocking the synaptic removal of GluA2-containing CI-AMPARs during retrieval prevented this AMPAR composition exchange, and also protected the consolidated memory against becoming labile. Because GluA2 stabilizes dendritic spines (26, 28) and retains AMPARs at synapses through direct interaction with synaptic proteins (11, 23–27), the loss of CI-AMPARs may destabilize potentiated synaptic strength, and hence memory. Our results suggest that other manipulations leading to synaptic removal of CI-AMPARs may destabilize consolidated memory as well.
The CP-AMPARs inserted on memory retrieval appear to be labile at the synapse and to undergo gradual replacement by CI-AMPARs over the course of memory reconsolidation. This conversion from CP-AMPARs to CI-AMPARs also has been observed during the stabilization of LTP (11, 33, 35), in association with preservation of enlarged synapse and spine structures (7). Intriguingly, a recent study in cultured hippocampal neurons showed that the replacement of CP-AMPARs by CI-AMPARs after synaptic potentiation requires the ongoing activity of newly inserted CP-AMPARs (33), very much like the reconsolidation described here.
NASPM has multiple molecular targets, but is known to be more selective for CP-AMPARs (7). Accordingly, researchers have used NASPM in vivo as a specific CP-AMPAR antagonist (36). In our study, NASPM had no effects on learned fear when injected without retrieval (Fig. 4D), indicating that the in vivo NASPM effect requires retrieval-induced changes (e.g., CP-AMPAR insertion). Furthermore, microinjection of the GluA23Y peptide into the LA and subsequent retrieval blocked both CP-AMPAR insertion (Fig. 3) and the in vivo NASPM effect (Fig. 4F). Taken together, these findings indicate that NASPM may exert its amnesic effect by acting on the CP-AMPARs inserted after retrieval; however, we cannot rule out the possibility that other calcium-permeating ion channels recruited after retrieval are involved as well.
Memory retrieval is known to trigger the protein degradation that is crucial for destabilization (37). Because GluA2 is known to undergo activity-dependent ubiquitination (38), it is conceivable that memory retrieval may evoke the degradation of CI-AMPARs along with other synaptic proteins, thereby contributing to the lability of memory. Accordingly, GluA2 is a likely candidate for resynthesis and incorporation during reconsolidation, insofar as signaling pathways known to promote GluA2 expression are required for reconsolidation (39, 40). Together with previous studies, our results suggest that the abrupt CI-AMPAR–to–CP-AMPAR exchange and subsequent gradual reversion represent a coordinated molecular scheme underlying memory destabilization and reconsolidation.
How do CP-AMPARs contribute to reconsolidation after retrieval? CP-AMPARs are already thought to be inserted after LTP and support the consolidation of LTP (33, 35), a leading cellular model of long-term memory. Because they allow calcium permeation at resting membrane potentials, these channels may serve as synaptic “tags” (41) that support the consolidation of synapse-specific potentiation (19), and thus memory, during spontaneous activity or off-line neuronal “replay” (42). These receptors themselves and/or the Ca2+ microdomains that they induce (34) may guide the incorporation of newly synthesized synaptic molecules (33) in the synapse-specific manner required for proper memory update on reconsolidation. Intriguingly, CP-AMPARs accumulate at synapses during the daytime and decrease during sleep (43, 44). In light of the deep involvement of sleep in consolidation and reconsolidation (45), it is tempting to hypothesize that daytime learning and retrieval induces CP-AMPARs, which in turn guide consolidation during subsequent sleep.
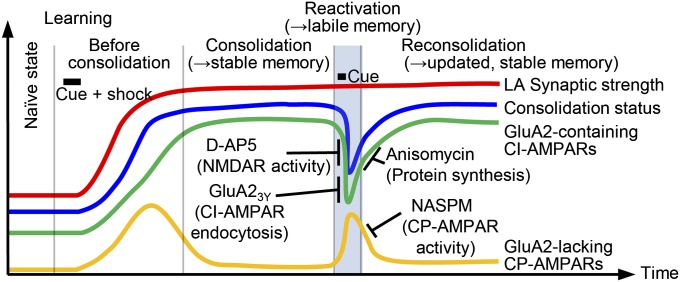
Is the AMPAR exchange characterized here a unique phenomenon occurring only on memory retrieval and reconsolidation or a general mechanism of synaptic destabilization and stabilization occurring also with memory acquisition and consolidation? Clem and Huganir (15) have shown that fear conditioning induces the synaptic insertion of CP-AMPARs, which are gradually replaced by CI-AMPARs. It would be interesting to pin down the precise role of CP-AMPARs and CI-AMPARs in memory consolidation as tested here with NASPM and reconsolidation after memory retrieval. Nonetheless, the findings of Clem and Huganir and our results dovetail well in yielding a global picture of the synaptic changes occurring on memory acquisition and retrieval (Fig. 5).
Fig. 5.
Hypothetical model of synaptic mechanisms involved in memory consolidation, retrieval, and reconsolidation. After learning, LA synaptic strength is persistently potentiated, first mostly by CP-AMPARs but later almost solely by CI-AMPARs. On memory retrieval, NMDAR activity induces CI-AMPAR endocytosis and subsequent CP-AMPAR insertion, causing an unstable state of synaptic potentiation. Blocking this NMDAR activity or CI-AMPAR endocytosis prevents memory lability, occluding subsequent effects of anisomycin. The newly inserted CP-AMPARs contribute to memory reconsolidation and update, but are in turn removed from synapses over the course of reconsolidation.
Our experiments indicate that as much as ∼30% of the potentiated LA synaptic transmission encoding fear memory undergoes an abrupt change from CI-AMPARs to CP-AMPARs. One might question how a minority fraction of receptors could play such a critical role in the stabilization of consolidated synapses and memories. One possibility is that a small but key fraction of CI-AMPARs at potentiated synapses are involved in stabilizing memory-storing dendritic spines, and that selective removal of these receptors thus exerts a strong effect on the stability of consolidated memory. Alternatively, or additionally, the temporary insertion of CP-AMPARs may allow a sufficient calcium influx to contribute to the gradual induction of synaptic depression or depotentiation (15), thereby causing a large decrement in synaptic strength. Whatever the downstream mechanism, our experiments identify CI-AMPAR–to–CP-AMPAR exchange as a key molecular event supporting transient memory destabilization. This reinforces the idea that the presence of CI-AMPARs at memory-encoding synapses is critical for the persistence of consolidated memory, while also providing fresh targets for possible clinical disruption of aberrant memory.
Materials and Methods
Male Sprague–Dawley rats were fear-conditioned to an auditory cue (3 pairings of cue and footshock; cf. ref. 46), and a single nonreinforced tone was used for subsequent memory retrieval. Brain slices were prepared for electrophysiological recordings as described previously (47). Rats were cannulated to enable microinfusion of drugs or peptides into the LA (18, 47). Details of this and all other experimental procedures are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to Beomjong Song for his assistance in electrophysiology and helpful discussions. We thank J. E. Le Doux and three expert reviewers for helpful comments. This research was supported by Grant 2012K001111 from the Brain Research Center of the 21st Century Frontier Research Program, funded by the Korean Ministry of Education, Science and Technology, and by National Institutes of Health Grant MH071739 (to R.W.T.). I.H. was supported by National Research Foundation of Korea (NRF) Fellowship 2012R1A6A3A01019438, and J.K. and J.K. were supported by Brain Korea 21 (BK21) Research Fellowships.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305235110/-/DCSupplemental.
References
- 1.McGaugh JL. Memory—a century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 3.Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390(6660):604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 4.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390(6660):607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 5.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 6.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308(5718):83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 7.Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54(6):859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 9.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8(2):101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 10.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 11.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105(3):331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 12.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 13.Lewis DJ. Psychobiology of active and inactive memory. Psychol Bull. 1979;86(5):1054–1083. [PubMed] [Google Scholar]
- 14.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: Key to persistent attenuation of fear memories. Science. 2009;324(5929):951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330(6007):1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao-Ruiz P, et al. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci. 2011;14(10):1302–1308. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- 17.Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9(10):1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, et al. Reactivation of fear memory renders consolidated amygdala synapses labile. J Neurosci. 2010;30(28):9631–9640. doi: 10.1523/JNEUROSCI.0940-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyère V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10(4):414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- 20.Boatman JA, Kim JJ. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. Eur J Neurosci. 2006;24(3):894–900. doi: 10.1111/j.1460-9568.2006.04965.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30(3):126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11(7):459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- 23.Song I, et al. Interaction of the N-ethylmaleimide–sensitive factor with AMPA receptors. Neuron. 1998;21(2):393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 24.Nishimune A, et al. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21(1):87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 25.Pozo K, et al. β3 integrin interacts directly with GluA2 AMPA receptor subunit and regulates AMPA receptor expression in hippocampal neurons. Proc Natl Acad Sci USA. 2012;109(4):1323–1328. doi: 10.1073/pnas.1113736109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saglietti L, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54(3):461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Dong H, et al. GRIP: A synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386(6622):279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 28.Passafaro M, Nakagawa T, Sala C, Sheng M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature. 2003;424(6949):677–681. doi: 10.1038/nature01781. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo N, Reijmers L, Mayford M. Spine-type–specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319(5866):1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamprecht R, Farb CR, Rodrigues SM, LeDoux JE. Fear conditioning drives profilin into amygdala dendritic spines. Nat Neurosci. 2006;9(4):481–483. doi: 10.1038/nn1672. [DOI] [PubMed] [Google Scholar]
- 31.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462(7275):920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brebner K, et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310(5752):1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- 33.Jaafari N, Henley JM, Hanley JG. PICK1 mediates transient synaptic expression of GluA2-lacking AMPA receptors during glycine-induced AMPA receptor trafficking. J Neurosci. 2012;32(34):11618–11630. doi: 10.1523/JNEUROSCI.5068-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg JH, Tamas G, Aronov D, Yuste R. Calcium microdomains in aspiny dendrites. Neuron. 2003;40(4):807–821. doi: 10.1016/s0896-6273(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 35.Plant K, et al. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9(5):602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- 36.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SH, et al. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319(5867):1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- 38.Lussier MP, Nasu-Nishimura Y, Roche KW. Activity-dependent ubiquitination of the AMPA receptor subunit GluA2. J Neurosci. 2011;31(8):3077–3081. doi: 10.1523/JNEUROSCI.5944-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gieros K, Sobczuk A, Salinska E. Differential involvement of mGluR1 and mGluR5 in memory reconsolidation and retrieval in a passive avoidance task in 1-day-old chicks. Neurobiol Learn Mem. 2012;97(1):165–172. doi: 10.1016/j.nlm.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Mameli M, Balland B, Luján R, Lüscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317(5837):530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 41.Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385(6616):533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 42.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265(5172):676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 43.Lanté F, Toledo-Salas JC, Ondrejcak T, Rowan MJ, Ulrich D. Removal of synaptic Ca²+-permeable AMPA receptors during sleep. J Neurosci. 2011;31(11):3953–3961. doi: 10.1523/JNEUROSCI.3210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11(2):200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 45.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44(1):121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, et al. Learning and reconsolidation implicate different synaptic mechanisms. Proc Natl Acad Sci USA. 2013;110(12):4798–4803. doi: 10.1073/pnas.1217878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, et al. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci USA. 2007;104(52):20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.