The growth toward adulthood occurs mainly through one or several intermediate stages or larval forms. Driven by environmental and internal state cues, all animals undergo one or more transitions during their life, which are accompanied by changes in morphology, physiology, and behavior. Larval settlement in the annelid Platynereis dumerilii is a prime example of such a life-phase transition. In PNAS, Conzelmann et al. (1) show that larval settlement is under the control of a myoinhibitory neuropeptide (MIP) produced by neurosecretory cells in the animal’s brain.
Platynereis is an emerging model organism for the study of animal development and behavior. This marine annelid worm belongs to the Lophotrochozoa, the third major branch of bilaterian animals next to Ecdysozoa (insects and nematode worms) and Chordata (lancelets, tunicates, and vertebrates). Genome investigation revealed that the genes of Platynereis are more closely related to those of vertebrates than to those of insects or nematodes. The urbilaterian ancestor of protostomes and deuterostomes likely had complex, intron-rich genes that are still present in Platynereis and human, but have been lost in insect and nematode genomes (2). The CNS of Platynereis shares a complex molecular architecture with that of vertebrates, implying that the urbilaterian ancestor also possessed a well-developed CNS (3). Platynereis has retained ancient sensory-motor circuits with low levels of integration, as well as combined sensory-neurosecretory cell types that are also found in the vertebrate forebrain (4, 5). In addition, the sensory-associative centers in the annelid brain, known as the mushroom bodies, show deep homology with the vertebrate cortex, which indicates that the origin of higher brain centers also dates back to prebilaterian times (6).
Platynereis larvae swim for several days as plankton, change shape twice, and then turn into large segmented worms. Larval settlement is the process by which the planktonic larva stops swimming, explores, attaches to a substrate, and begins its benthic life (7). The transition to a next life-cycle stage is a dynamic event. Larvae can reject one site and select another for settlement. Such active site selection is often mediated by environmental cues. Although a variety of chemicals, including proteins, free fatty acids, polysaccharides, inorganic ions, and neurotransmitters, have been proposed as inducers of larval settlement in marine annelid worms, no internal regulator had so far been identified.
The most diverse class of signaling molecules in the CNS are neuropeptides, short sequences of amino acids that act through binding and activation of G protein-coupled receptors (8). By operating as hormones, neurotransmitters, or neuromodulators, neuropeptides are involved in many biological processes, such as reproduction, metabolism, feeding, circadian rhythms, sensorimotor integration, adaptive behaviors, and cognition. It has become clear that many neuropeptidergic systems have been conserved throughout the animal kingdom (9).
Like the larvae of most marine invertebrates, Platynereis larvae swim with the help of a band of cilia. The ciliated cells are innervated by axons from neuropeptide-containing neurons in the CNS. Conzelmann et al. exposed swimming larvae to various synthetic neuropeptides and found that they influenced ciliary swimming (10). The evolutionarily conserved MIP neuropeptide is of particular interest because it triggered the arrest of ciliary beating and induced exploratory crawling behavior on the substrate, two features typical for larval settlement behavior. Exposure to MIP did not induce larval settlement of swimming larvae in which the MIP receptor was knocked down by a specific morpholino oligonucleotide, which sterically blocks mRNA translation upon binding to MIP receptor transcripts. Conzelmann et al. (1) conclude that exposure to MIP bypasses the chemical environmental cues responsible for the timing of larval settlement, and that MIP triggers a behavioral program for larval settlement by functionally activating its G protein-coupled receptor expressed in cells adjacent to MIP-containing cells.
MIP neuropeptides are characterized by a W-X6–8-Wamide motif (11). In Platynereis, the last but not the first tryptophan (W) residue is crucial for MIP receptor activation and hence induction of larval settlement. The first MIP neuropeptide was identified in 1991 in the locust Locusta migratoria and was found to inhibit muscle contractions in vitro (12). MIPs (also named allatostatin B and prothoracicostatin) belong to the Wamide neuropeptide superfamily that contains (G)LWamides and many other Wamide-type peptides, sharing an amidated tryptophan residue preceded by a small aliphatic residue. Peptides of the Wamide family occur in all eumetazoan animals investigated. Interestingly, in cnidarians (polyps, jellyfishes, corals, and sea anemones), LWamide neuropeptides are called metamorphosins and have been shown to induce both larval settlement (13, 14), as well as metamorphosis of planula larvae into adult polyps (15). Knockout of LWamides in polyp larvae results in the inability to metamorphose. Although no neuropeptides have been identified in ametazoans to date, exposure to cnidarian LWamide neuropeptides triggers larval settlement in sponges, indicating that chemosensory communication also plays a role in the ability of sponge larvae to identify a suitable habitat (16). In insects, MIP-containing neurons are responsible for the initiation and execution of ecdysis behavior, a form of life-phase transition. Here, the MIP neuropeptide was proposed to silence neurons that are not required during the ecdysis program (17). Insect MIPs have also been shown to inhibit juvenile hormone synthesis in vitro (18), which is indicative of a promoting role in the transition to adult life. All these studies denote that the MIP/Wamide neuropeptidergic control of life-cycle changes seems to have been evolutionarily conserved since its inheritance from the last common ancestor of all animals.
Using serial sectioning transmission electron microscopy of 664 ultrathin sections, Conzelmann et al. show that the MIP-expressing neurons located in the most anterior part of the Platynereis brain are both sensory and neurosecretory (1). Such cell types with dual sensory-neurosecretory properties were probably the starting point for the evolution and diversification of neurosecretory brain centers in bilaterian animals (5). The Platynereis MIP cells also express the transcription factors dimm and orthopedia, which respectively are known to direct the differentiation of neuro-endocrine peptidergic MIP cells in Drosophila (19) and of hypothalamic neuropeptidergic neurons in mammals (20). Interestingly, the Wamide family of peptides is related to the bilaterian adipokinetic hormone/gonadotropin-releasing hormone (GnRH) neuropeptide family (21). Amino acid alignments show a common overall architecture (signal peptide, active peptide, associated peptide), with a similar α-helix random-coil secondary structure (22). In mammals, including humans, the activation of the hypothalamic GnRH neurons from a state of relative quiescence is critical
The report of Conzelmann et al. represents a major leap forward in our understanding of the molecular mechanisms underlying life-cycle transitions.
for initiating puberty, a life-history transition from juvenile to adult in mammals (23).
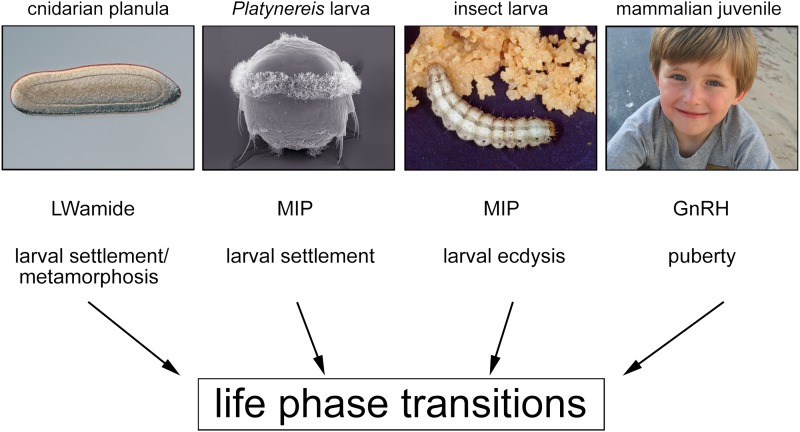
Behavioral responses are generated by the integration and processing of informationthrough specific neuronal circuits. Neuropeptides can change the flow of information along these cellular circuits, thereby adapting the behavioral output according to varying environmental and internal state cues (24). In this way, life-phase transitions can be fine-tuned to occur under optimal conditions. The basic neuronal circuit that generates larval settlement in Platynereis is modulated by MIP signaling. The MIP-controlled regulation of life-stage transition in Platynereis is comparable to the LWamide-controlled settlement in cnidarians, the MIP-controlled ecdysis behavior in insects, and the GnRH-controlled initiation of puberty in mammals (Fig. 1). This neuropeptidergic MIP/Wamide-mediated switch of lifestyle therefore seems to have deep evolutionary roots, and dates back to ancestral eumetazoan organisms that lived in the sea several hundred million years ago. Throughout evolution, diverse behavioral repertoires of neuropeptide-controlled life-phase switches could have been acquired by diversification of sensory modalities and by the spatiotemporal regulation of neuropeptide receptor expression. The report of Conzelmann et al. (1) represents a major leap forward in our understanding of the molecular mechanisms underlying life-cycle transitions. Unraveling neuropeptidergic control of complex behaviors at the cellular and molecular level has only just begun, and research on various model organisms is needed to understand the mechanisms and roots of neuronal communication in our own brain.
Fig. 1.
The MIP-controlled regulation of life-stage transition in Platynereis is comparable to the LWamide-controlled settlement in cnidarians, the MIP-controlled ecdysis behavior in insects, and the GnRH-controlled initiation of puberty in mammals.
Footnotes
The authors declare no conflict of interest.
See companion article on page 8224.
References
- 1.Conzelmann M, et al. Conserved MIP receptor–ligand pair regulates Platynereis larval settlement. Proc Natl Acad Sci USA. 2013;110:8224–8229. doi: 10.1073/pnas.1220285110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raible F, et al. Vertebrate-type intron-rich genes in the marine annelid Platynereis dumerilii. Science. 2005;310(5752):1325–1326. doi: 10.1126/science.1119089. [DOI] [PubMed] [Google Scholar]
- 3.Denes AS, et al. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell. 2007;129(2):277–288. doi: 10.1016/j.cell.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 4.Hartenstein V. The neuroendocrine system of invertebrates: A developmental and evolutionary perspective. J Endocrinol. 2006;190(3):555–570. doi: 10.1677/joe.1.06964. [DOI] [PubMed] [Google Scholar]
- 5.Tessmar-Raible K, et al. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: Insights into hypothalamus evolution. Cell. 2007;129(7):1389–1400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 6.Tomer R, Denes AS, Tessmar-Raible K, Arendt D. Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell. 2010;142(5):800–809. doi: 10.1016/j.cell.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 7.Qian P-Y. Larval settlement of polychaetes. Hydrobiologia. 1999;402(1):239–253. [Google Scholar]
- 8.Caers J, et al. More than two decades of research on insect neuropeptide GPCRs: An overview. Front Endocrinol (Lausanne) 2012;3:151. doi: 10.3389/fendo.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimmelikhuijzen CJ, Hauser F. Mini-review: The evolution of neuropeptide signaling. Regul Pept. 2012;177(Suppl):S6–S9. doi: 10.1016/j.regpep.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Conzelmann M, et al. Neuropeptides regulate swimming depth of Platynereis larvae. Proc Natl Acad Sci USA. 2011;108(46):E1174–E1183. doi: 10.1073/pnas.1109085108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poels J, et al. Myoinhibiting peptides are the ancestral ligands of the promiscuous Drosophila sex peptide receptor. Cell Mol Life Sci. 2010;67(20):3511–3522. doi: 10.1007/s00018-010-0393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoofs L, Holman GM, Hayes TK, Nachman RJ, De Loof A. Isolation, identification and synthesis of locustamyoinhibiting peptide (LOM-MIP), a novel biologically active neuropeptide from Locusta migratoria. Regul Pept. 1991;36(1):111–119. doi: 10.1016/0167-0115(91)90199-q. [DOI] [PubMed] [Google Scholar]
- 13.Katsukura Y, Ando H, David CN, Grimmelikhuijzen CJ, Sugiyama T. Control of planula migration by LWamide and RFamide neuropeptides in Hydractinia echinata. J Exp Biol. 2004;207(Pt 11):1803–1810. doi: 10.1242/jeb.00974. [DOI] [PubMed] [Google Scholar]
- 14.Grasso LC, et al. The biology of coral metamorphosis: Molecular responses of larvae to inducers of settlement and metamorphosis. Dev Biol. 2011;353(2):411–419. doi: 10.1016/j.ydbio.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T, Hatta M. The importance of GLWamide neuropeptides in cnidarian development and physiology. J Amino Acids. 2011;2011:424501. doi: 10.4061/2011/424501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whalan S, Webster NS, Negri AP. Crustose coralline algae and a cnidarian neuropeptide trigger larval settlement in two coral reef sponges. PLoS ONE. 2012;7(1):e30386. doi: 10.1371/journal.pone.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr Biol. 2006;16(14):1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz MW, Kellner R, Hoffmann KH. A family of neuropeptides that inhibit juvenile hormone biosynthesis in the cricket, Gryllus bimaculatus. J Biol Chem. 1995;270(36):21103–21108. doi: 10.1074/jbc.270.36.21103. [DOI] [PubMed] [Google Scholar]
- 19.Park D, et al. Molecular organization of Drosophila neuroendocrine cells by Dimmed. Curr Biol. 2011;21(18):1515–1524. doi: 10.1016/j.cub.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acampora D, et al. Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 1999;13(21):2787–2800. doi: 10.1101/gad.13.21.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-Pérez F, Becerra A, Valdés J, Zinker S, Aréchiga H. A possible molecular ancestor for mollusk APGWamide, insect adipokinetic hormone, and crustacean red pigment concentrating hormone. J Mol Evol. 2002;54(6):703–714. doi: 10.1007/s00239-001-0036-7. [DOI] [PubMed] [Google Scholar]
- 22.Lindemans M, et al. Adipokinetic hormone signaling through the gonadotropin-releasing hormone receptor modulates egg-laying in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2009;106(5):1642–1647. doi: 10.1073/pnas.0809881106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellison PT, et al. Puberty as a life history transition. Ann Hum Biol. 2012;39(5):352–360. doi: 10.3109/03014460.2012.693199. [DOI] [PubMed] [Google Scholar]
- 24.Bargmann CI. Beyond the connectome: How neuromodulators shape neural circuits. Bioessays. 2012;34(6):458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]