Abstract
Sustained fast neurotransmission requires the rapid replenishment of release-ready synaptic vesicles (SVs) at presynaptic active zones. Although the machineries for exocytic fusion and for subsequent endocytic membrane retrieval have been well characterized, little is known about the mechanisms underlying the rapid recruitment of SVs to release sites. Here we show that the Down syndrome-associated endocytic scaffold protein intersectin 1 is a crucial factor for the recruitment of release-ready SVs. Genetic deletion of intersectin 1 expression or acute interference with intersectin function inhibited the replenishment of release-ready vesicles, resulting in short-term depression, without significantly affecting the rate of endocytic membrane retrieval. Acute perturbation experiments suggest that intersectin-mediated vesicle replenishment involves the association of intersectin with the fissioning enzyme dynamin and with the actin regulatory GTPase CDC42. Our data indicate a role for the endocytic scaffold intersectin in fast neurotransmitter release, which may be of prime importance for information processing in the brain.
Keywords: endocytosis, synaptic transmission, synaptic vesicle recruitment
Neurotransmission depends on the exocytosis of synaptic vesicles (SVs) at active zones (AZs) and the subsequent retrieval of SV membranes by endocytosis. Sustained fast neurotransmission requires rapid replenishment of release-ready SVs composing the readily releasable pool (RRP) (1–3). Whereas the machinery for SV fusion is well understood, the mechanisms controlling the number of SVs within the RRP and the rate of replenishment remain enigmatic (4, 5). It has been postulated that the rate of replenishment of release-ready SVs in addition to molecular priming reactions is regulated by the availability of SV release sites (6–8). The reuse of release sites appears to depend on components of the endocytic machinery, including dynamin (9–12). However, considering that the rate of endocytosis is ∼10–100 times slower than that of replenishment of release-ready SVs, the question arises as to how endocytic proteins affect rapid neurotransmitter release.
Data from dynamin mutants can be interpreted as a deficit in SV recycling (e.g., loss of the SV reserve pool; ref. 13). Alternatively, endocytic proteins may regulate exocytosis more directly, independent of membrane retrieval. If so, interference with select endocytic factors might impair replenishment of release-ready SVs without affecting the rate of SV membrane retrieval.
A possible candidate for such regulation is the early-acting multidomain endocytic protein intersectin (14–18), a protein overexpressed in Down syndrome, which also associates with components of the exocytic machinery [i.e., synaptosomal-associated protein 25 (SNAP-25)] (19). Although a role for intersectin in endocytosis has been established in Drosophila (16, 17), the precise function of intersectin 1 in mammalian central synapses has not yet been analyzed extensively. Optical measurements of endocytosis in cultured neurons suggest mild effects on endocytosis as a consequence of intersectin 1 KO (20). These results indicate that intersectin might not be mandatory for SV membrane retrieval or might regulate other steps in endocytosis that are not rate-limiting for the process per se (21).
Using simultaneous recordings from presynaptic and postsynaptic compartments at the calyx of Held (22, 23), we have shown that intersectin 1 is a crucial factor in the replenishment of release-ready SVs within the RRP. We found that acute perturbation or genetic deletion of intersectin 1 inhibited the recruitment of release-ready SVs without affecting the rate of endocytic membrane retrieval under the same conditions. These data indicate an important role of the endocytic scaffold intersectin in fast neurotransmitter release.
Results
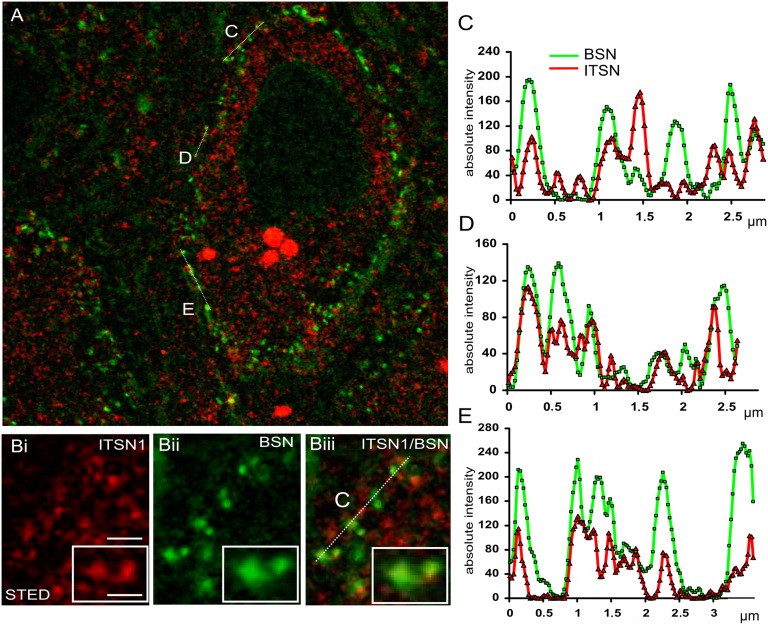
To test whether early-acting endocytic proteins such as intersectin may regulate fast neurotransmission, we first explored the subsynaptic distribution of intersectin by confocal and superresolution stimulated emission depletion (STED) microscopy in situ by preparing thin sections from the mature mouse calyx of Held, a giant terminal in the auditory brainstem (22, 23) (Fig. 1). Immunostaining for the AZ marker bassoon (24) revealed punctuate signals representing sites for rapid neurotransmitter release along the cup-like calyx terminal and at small conventional boutons (Fig. 1A). Intersectin was found at the calyx terminal and within postsynaptic neurons (red fluorescence in Fig. 1A). High-magnification STED imaging revealed a subset of intersectin puncta that overlapped with bassoon-containing AZs (Fig. 1B), as evident on intensity line profiles along the terminal, whereas a large fraction of intersectin puncta was also located peripheral to the bassoon hot spots (Fig. 1 C–E). The partial but clearly nonrandom colocalization of bassoon and intersectin clusters was corroborated by Pearson correlation analysis (Fig. S1).
Fig. 1.
Intersectin 1 is localized at and around AZs. (A) STED microscopy image of a mature mouse calyx of Held synapse (age P50–P70) immunostained for endogenous bassoon (BSN; green) and intersectin 1 (ITSN1; red). The postsynaptic neuron is surrounded by the calyx terminal immunopositive for the AZ marker bassoon. Note that bassoon staining is also detectable at noncalycial terminals. (Inset) Confocal image of bassoon immunopositive puncta. For the scale bar, see the line scan profiles in C–E. (B) Magnified images of the data shown in A (from line C). (B, i) ITSN1; (B, ii) BSN; (B, iii) merged image. (Insets) Intersectin-immunopositive puncta confined to the active zones. (Scale bars: 750 nm; 350 nm in Insets.) (C–E) Spatial intensity profiles of bassoon (green) and intersectin 1 (red). The regions of interest (lines C, D, and E in A) were selected where the presynaptic compartment showed a finger-like structure.
To study the role of intersectin 1 in fast neurotransmission, we examined synaptic responses in intersectin 1 KO mice (20), in which expression of all isoforms of intersectin 1 is abrogated. Loss of intersectin 1 did not cause any overt differences in the morphological architecture or composition of the calyx or of the medial nucleus of the trapezoid body (MNTB) area in the brainstem. Specifically, calyces from KO mice displayed a WT-like intensity and distribution of the abundant SV protein vesicular glutamate transporter 1 (VGLUT1), suggesting a normal number of SVs, at the level of light microscopy. Calyces from KO also showed no alterations in the localization or the amount of major endocytic proteins, such as clathrin and adaptor protein complex-2 (AP-2) (Fig. S2), arguing against major alterations or compensatory changes with respect to the endocytic machinery (20).
Consistent with the fact that heterozygous animals exhibit no phenotypic changes (20), we detected no differences in synaptic responses between calyces from WT or heterozygous mice; thus, these data were pooled to serve as a control. Simultaneous presynaptic and postsynaptic recordings were conducted in WT (n =4), heterozygous (n =4), and KO (n =8) calyces of Held at postnatal days 8–11 (P8–11) using a pulse protocol that allows for separation of two components of transmitter release within the RRP, a fast-releasing pool (FRP) and a slowly releasing pool (SRP) (Fig. 2A, dotted traces) (25), as described in Materials and Methods. Presynaptic terminals were depolarized from −80 mV to +70 mV for 2 ms, followed by repolarization to 0 mV for 50 ms. During the 0-mV period, a constant calcium (Ca) influx at the presynaptic side was observed. Similar Ca currents were elicited between control (1,301 ± 105 pA) and KO (1,150 ± 66 pA). The excitatory postsynaptic current (EPSC) decayed during the pulse protocol, indicating depletion of the RRP of SVs.
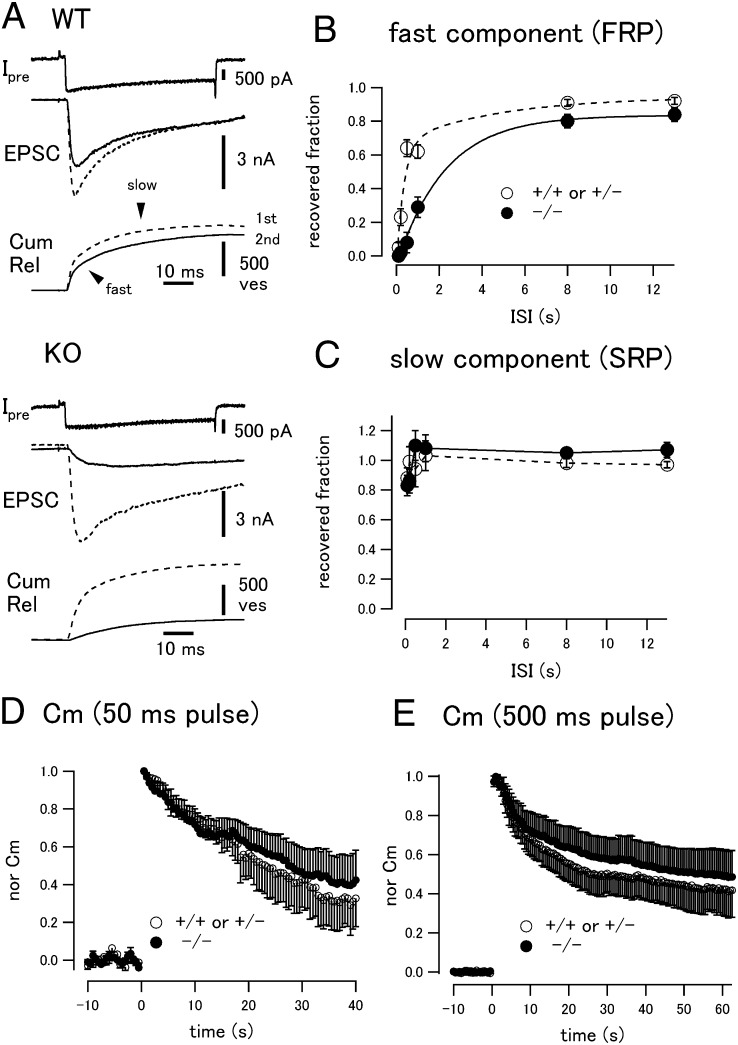
Fig. 2.
SV replenishment in intersectin 1 KO mice. (A) Simultaneous recordings of the presynaptic and postsynaptic compartments at the calyx of Held synapse. A pair of depolarizing pulses (0 mV for 50 ms, after a prepulse to +70 mV for 2 ms) was applied to the presynaptic terminal with an interval of 500 ms. During a 0-mV period, presynaptic Ca current was elicited. Evoked postsynaptic EPSCs (dotted line, first stimulation; solid line, second stimulation with an interval of 500 ms) and the cumulative release are shown. Cumulative release in response to the first pulse showed a double-exponential time course. (Upper) WT. (Lower) Intersectin 1 KO littermates. (B and C) Recovery of the fast (FRP; B) and the slow (SRP; C) components of release plotted against the stimulation interval. Dotted and solid circles represent data from control (four WT cells and four +/− cells) and KO (eight cells) mice, respectively. (D) The capacitance trace in response to a 50-ms pulse was normalized to the peak value and averaged among cells. Open and filled circles represent data from control and KO mice, respectively (n = 6 cells each). (E) The pulse duration increased to 500 ms. Note that for the open circle data, only downward error bars are displayed. Upward error bars are shown for the closed circle data for clarity.
Two components of transmitter release were observed during the pulse. The FRP had a time constant of a few ms [control: 1.8 ± 0.4 ms (50% ± 3.4% of total release); KO: 3.1 ± 0.4 ms (49% ± 3.3% of total release)], reflecting fusion of SVs, which can be released synchronously in response to action potentials (26). The SRP had a time constant of 20–30 ms (control: 21.7 ± 2.4 ms vs. KO: 18.6 ± 1.1 ms) and requires more accumulation of residual Ca. During a 50-ms pulse, cumulative release reached a plateau level, indicating fusion of all readily releasable SVs. Importantly, similar release kinetics were seen in control and KO, indicating that loss of intersectin 1 does not impair SV fusion per se. This finding is also in agreement with our biochemical and morphological analysis noted earlier (Fig. S2).
When the same stimulation was repeated after a resting interval of 500 ms, the presynaptic Ca current amplitude was almost identical. The EPSC recovered to ∼60% of its initial value in WT (Fig. 2A, solid traces), whereas the recovery of the synaptic response during the second pulse was significantly reduced in KO (Fig. 2A, Lower). We then changed the interval of the two consecutive pulses to monitor SV replenishment following depletion of the RRP. When the recovered amount of the FRP was plotted against the interstimulus interval (ISI) between the two pulses, the control data were fitted with a double-exponential function with time constants of a few hundreds of ms and several seconds (Fig. 2B) (25). Recovery of the FRP was markedly slowed in the intersectin 1 KO and could be fitted by a single exponential function with a time constant of several seconds (Fig. 2B). Importantly, the FRP recovered completely when given a sufficient time period for recovery, unlike manipulations causing defective SV recycling (13, 27). In contrast, recovery of the SRP remained unchanged (Fig. 2C). These data show that intersectin 1 specifically regulates the replenishment of release-ready SVs.
To further analyze whether intersectin 1-mediated SV replenishment reflects a role for intersectin 1 in endocytosis, we conducted presynaptic capacitance measurements (27–29) after pulses of 50 ms (Fig. 2D) or 500 ms (to elicit rapid endocytosis; Fig. 2E) in WT and KO calyces. Capacitance jumps in response to the 50-ms pulse were similar in control [280 ± 40 femtofarads (fF)] and KO (224 ± 28 fF). In response to the 500-ms pulse, the jumps were larger in WT (969 ± 86 fF) than in KO (689 ± 80 fF), presumably reflecting slow SV replenishment after vesicle pool depletion in KO. Strikingly, in both stimulation protocols, the time course of membrane retrieval was similar in control and KO terminals (Fig. S3 shows fast measurements). Of importance, given that membrane retrieval in KO terminals was affected only mildly, our observations here differ from kiss-and-run type exocytosis (30). In addition, WT and KO terminals responded similarly to perturbation of AP2 by dialysis of a Synaptotagmin 2 (Syt2) 2-derived AP2-binding peptide (9), resulting in nearly complete elimination of membrane retrieval and of SV replenishment in both genotypes (Fig. S4). This phenotype is clearly distinct from that induced by loss of intersectin 1 and argues against major changes in the mode of endocytosis in intersectin 1 KO mice. We conclude that deletion of intersectin 1 selectively perturbs SV replenishment without significantly affecting endocytic membrane retrieval under the same conditions, revealing a hitherto unknown function for intersectin 1 in regulating fast neurotransmitter release.
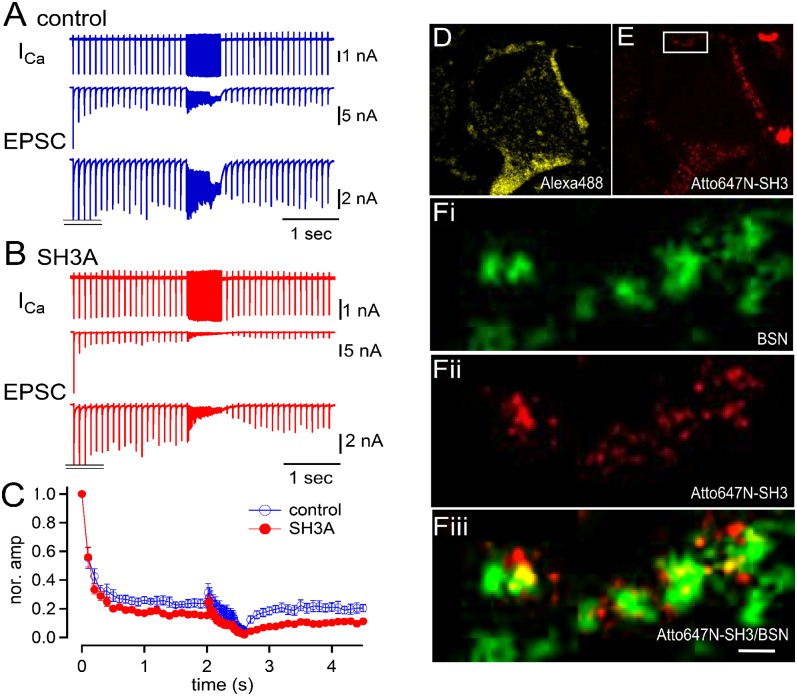
To unravel the molecular mechanism of the exocytic function of intersectin 1, we turned to acute perturbation experiments to selectively interfere with intersectin-based protein–protein interactions. The major neuronal isoform of intersectin 1 (intersectin 1L) contains five SH3 domains that associate among other proteins with the membrane fissioning GTPase dynamin. Intersectin 1L also harbors pleckstrin homology (PH) and disabled (Dbl) homology (DH) domains, which facilitate guanine nucleotide exchange on CDC42 (14–18). Given the postulated role of dynamin in regulating the reuse of release sites (9–12), we first focused on the dynamin-binding SH3A domain of intersectin 1. To this aim, we analyzed whether acute perturbation of the intersectin 1 SH3A domain function (5 μM) affected synaptic depression and recovery at the rat calyx of Held. A train of AP-like stimuli was applied 20 times at 10 Hz. Subsequently, the stimulation frequency was switched to 50 Hz, to 100 Hz, and then back to 10 Hz. As shown in Fig. 3 A and C, the 10-Hz stimulation caused a depression of postsynaptic responses. In the presence of the SH3A domain (Fig. 3B), the depression deepened and led to smaller steady-state EPSC amplitudes (P < 0.05). At higher frequency, such differences became less overt, presumably because of a more pronounced overall synaptic depression. When the stimulation frequency was switched back from 100 Hz to 10 Hz, recovery from synaptic depression was profoundly slowed by the presence of SH3A (P < 0.01). These data suggest that perturbation of intersectin 1 SH3A domain function affects synaptic depression and recovery. Consistent with this interpretation, ATTO647N-labeled SH3A domain (Fig. 3E) coinjected together with Alexa Fluor 488 (to identify injected calyces; Fig. 3D) was detectable as small puncta in close apposition to and partly colocalizing with bassoon-containing AZs in dual-color STED images (Fig. 3F). These results are consistent with the localization of endogenous intersectin 1 (Fig. 1 A–E and Fig. S1) and dynamin 1 (Fig. S5) detected by confocal and two-color STED microscopy.
Fig. 3.
Acute perturbation of intersectin 1 SH3A domain function causes short-term synaptic depression. Simultaneous recordings of the presynaptic and postsynaptic compartments at the calyx of Held synapse (rats, P8–P11). A train of AP-like stimuli (depolarization to +40 mV for 1.5 ms) was applied (20 pulses), at 10, 50, and 100 Hz, and then at 10 Hz. Presynaptic Ca currents (Top), EPSCs (Middle), and magnified EPSCs (with initial EPSC peaks truncated; Bottom) are shown. (A and B) Data under control conditions (A) and in the presence of SH3A domain (5 μM) (B). (C) Normalized time course of the EPSC amplitudes plotted over time. Red and blue symbols indicate the data under control and in the presence of the SH3A domain, respectively. (D and E) Confocal images of a calyx terminal (rats, P8–P11) preloaded with Alexa Fluor 488 (200 μM; D) and the Atto647N-conjugated SH3A domain of intersectin (10 μM; E). (F) STED images of magnified data outlined by the box in E, illustrating the localization of the AZ markers bassoon (F, i; green), and Atto-SH3A (F, ii; red). (F, iii) Composite image, with colocalizing puncta in yellow. (Scale bar: 500 nm.)
To further corroborate these findings, we measured the rates of SV recruitment to the FRP and SRP. We introduced the SH3A domain of intersectin 1 via the presynaptic patch pipette, thereby interfering with the recruitment of SH3A domain binding partners, such as dynamin 1 (Fig. S6 C and D), to endogenous intersectin. Presynaptic Ca currents were comparable with control conditions, as was the synaptic response during the first pulse (Table S1), whereas recovery of the synaptic response (FRP) during the second pulse was reduced significantly (Fig. 4 A and B), similar to the phenotype observed in intersectin 1 KO calyces (compare Fig. 2). A mutant SH3A domain unable to associate with proline-rich ligands, including dynamin (Fig. S6 C and D), did not block recovery (Fig. 4D), nor did the related SH3 domain of endophilin A1 (recovery of 60% ± 6%, n = 3 cells; Table S1), indicating that the effect is specific to the SH3A domain of intersectin 1.
Fig. 4.
Acute perturbation of the intersectin 1 SH3A domain slows SV replenishment. A pair of depolarizing pulses (0 mV for 50 ms after a prepulse to +70 mV for 2 ms) was applied to the presynaptic terminal with an interval of 500 ms. (A–C) EPSCs (dotted line, first stimulation; solid line, second stimulation, with an interval of 500 ms) and cumulative release (cum rel) are shown. (A) Control conditions. (B and C) Terminals dialyzed with SH3A domain (5 μM) (B) or antibodies directed against intersectin 1 SH3A (2,030 μg/mL) (C). (D) Similar experiments as in A–C, but with the stimulus interval of the two pulses varied. Recovery of the FRP (Left) and the SRP (Right) after RRP depletion was plotted against the ISI. Data from control conditions (open circles) and obtained in the presence of SH3A (filled circles), mutant SH3A (filled squares), or antibodies against the intersectin 1 SH3A domain (filled triangles) are shown. (E) Recovery of the fast-releasing component is plotted against ISI. The proline-rich domain of dynamin (filled triangles), a dynamin 1-derived proline-rich peptide (filled circles), or the proline-rich domain of N-WASP (filled squares) were introduced into the terminal (ISI = 0.5 and 1 s). Open circles, control condition. (F) Same as in E, except that the SH3E domain of intersectin 1 was introduced into the terminal (filled circles). Open circles, control condition.
Given that dominant-negative interference with protein function may cause side effects through sequestration of endogenous components, we sought to corroborate our results using other reagents. To this aim, we dialyzed an antibody that specifically recognizes the SH3A domain of intersectin 1 (31) into the terminal, thereby occluding the binding site on SH3A for proline-rich ligands including dynamin. Slowed recovery from vesicle pool depletion similar to that seen for the injected SH3A domain was seen after anti-SH3A antibody injection (20–30 μg/mL; Fig. 4C). Analysis of SV replenishment after depletion of the RRP by two consecutive pulses applied at changing ISIs in calyces injected with the SH3A domain or anti-SH3A antibodies confirmed a selective role of intersectin 1 in the recovery of the FRP (Fig. 4D, Left) without perturbing the SRP (Fig. 4D, Right). If the effect of intersectin 1 SH3A on replenishment of the FRP were mediated by its association with dynamin, then dialysis of a dynamin-derived proline-rich SH3A ligand should result in a phenocopy. Indeed, application of the dynamin proline-rich domain (Dyn1 509–864; 3 μM) [but not the proline-rich domain from neuronal Wiskott Aldrich syndrome protein (N-WASP)] or of a SH3 domain-binding peptide (Dyn1 773–794; 1 mg/mL) that competes with endogenous dynamin for the same site on intersectin 1 SH3A (Fig. S6 A and B) slowed recovery of the FRP (Fig. 4E). Inhibition of FRP refilling was also seen in the dynamin-binding SH3E domain of intersectin 1 (Fig. 4F), whereas SRP refilling was unaffected (Fig. S7). These findings suggest that the proline-rich domain of dynamin 1 is involved in the recruitment of SVs to the FRP (9).
Consistent with the KO data, endocytic membrane retrieval assayed by capacitance measurements proceeded relatively unperturbed in the presence of either the SH3A domain or anti-intersectin 1 SH3A-directed antibodies under different stimulation conditions (Fig. S8). Moreover, no differences between microinjected calyces and control conditions were observed when capacitance traces were monitored at fast time resolution or after a strong depolarizing pulse of 500 ms (Fig. S8). The findings from these acute perturbation experiments are consistent with our results for intersectin 1 KO mice and establish a function of intersectin 1 and its SH3 domains in fast neurotransmission via regulation of recovery from SV pool depletion independent of alterations in endocytic membrane retrieval.
How can intersectin 1 regulate recruitment of release-ready SVs? The long neuronal isoform (L) of intersectin 1 serves as a molecular scaffold that, along with its ability to associate with endocytic proteins such as dynamin, also contains a protein module that regulates actin polymerization. This module comprises DH and PH domains that together serve as a guanine nucleotide exchange factor (GEF) for the Rho family small GTPase CDC42, a crucial regulator of actin cytoskeleton reorganization (14–18). This structure, together with our finding that loss of dynamin function is associated with a profound increase in cortical actin polymerization, led us to speculate that CDC42 may serve as another downstream target of intersectin 1 in FRP replenishment. We explored this possibility by introducing the DH-PH domain of intersectin (5 μM) into the presynaptic terminal to interfere with intersectin 1–CDC42 complex formation. We found that recovery of the fast component of release was significantly slowed in DH-PH domain-injected terminals (Fig. 5A). To corroborate these data, we probed the function of Rho family small G proteins, including CDC42, directly. We examined the effect of introducing the enzymatic domains of toxin B from Clostridum botulinum (1 μM), a well-established inhibitor of Rho family small G proteins, including Rho, CDC42, and Rac (32). Application of toxin B inhibited recovery of the FRP of release (Fig. 5B). A similar effect was observed for secramine A, a recently developed specific blocker of CDC42 (33) (Fig. 5C). Refilling of the SRP was not affected by any of these reagents (Fig. S7). Taken together, these results are consistent with a model in which intersectin 1L-mediated activation of CDC42 regulates replenishment of release-ready SVs at the calyx of Held.
Fig. 5.
Blocking the activity of the intersectin binding partner CDC42 impairs recruitment of release-ready SVs. (A) Simultaneous recordings of the pre- and postsynaptic compartments at the calyx of Held synapse (as depicted in Figs. 2 and 4). The DH-PH domain of intersectin 1 was introduced into the terminal. (Left) panel is similar to Fig 4 and the traces from one cell pair are shown (dotted: first stimulation, solid: second stimulation with an interval of 500 ms). Right) Plot of recovery of the fast component (FRP) against the stimulus interval. Filled circles, Dbl homology-pleckstrin homology (DH-PH) domain; open circles, control condition. (B) Same as in A, except with 1 μM toxin B introduced into the presynaptic terminal. Toxin B potently inhibits Rho family small G proteins. Open and filled circles represent the data from controls and in the presence of toxin B, respectively. (C) Same as in A, except that 20 μM secramine A (filled circles), a specific small-molecule inhibitor of CDC42, was applied to the terminal.
Discussion
The data reported herein suggest an unexpected function for the endocytic scaffold intersectin 1 in fast neurotransmission at the calyx of Held through regulation of replenishment of a pool of fast-releasing SVs at or near AZs that is independent of endocytic membrane retrieval. This phenotype is distinct from that of other endocytic proteins; for example, dynamin 1 KO mice exhibit normal endocytosis in response to weak stimulation, but their endocytic capacity is readily saturated in response to strong stimulation (34). Nonetheless, our results do not exclude the possibility that intersectin 1 may be involved in endocytosis under certain stimulation conditions distinct from those used in the present study or at different synapses. In addition, our data were obtained in young animals and at room temperature, and endocytic mechanisms may undergo developmental changes that could involve intersectin 1. Indeed, a role for intersectin (including other isoforms) in endocytic SV recycling has been reported at other synapses and under different physiological conditions (14–18). Finally, intersectin 1 may regulate discrete steps within the endocytic limb of the SV cycle (e.g., sorting of SV cargo) that do not result in altered membrane retrieval and thus would have escaped detection by capacitance measurements. Irrespective of these considerations, our data clearly show that loss of intersectin 1 at the calyx of Held (P8–P12) impairs replenishment of release-ready SVs with no apparent change in capacitative membrane retrieval assayed under the same conditions, revealing an unexpected function for intersectin 1 in fast neurotransmission.
How might an endocytic protein affect replenishment of fast-releasing SVs? One possibility is that release sites may be mutually occupied by exocytic or endocytic protein complexes. In this scenario, removal of an endocytic protein complex by intersectin would be necessary for priming of SVs. This idea is consistent with a possible interaction between the SNARE complex (syntaxin/SNAP-25) and dynamin (12, 19), and also with our preliminary observation that the distribution of dynamin at and around AZs is altered in intersectin 1 KO mice. A second possibility may relate to a potential function of intersectin 1 in SV priming, although to our knowledge, no data exist to support this idea. A third possibility is that released SV proteins or dead-end cis-SNARE complexes may jam the release site at AZ membranes. Such jammed release sites may be unavailable for the recruitment of new release-ready SVs until the material clogging the fusion zone has been removed. This removal could involve lateral translocation of membrane proteins, including cis-SNARE complexes, within the plane of the membrane, for example, by intersectin 1-regulated actin-driven movement out of the AZ. Intersectin 1 consistently binds to SNAP-25 (19), and SNAP-25 mutants in Drosophila melanogaster suffer from short-term depression (35).
Based on our data, the function of intersectin 1 in replenishment of release-ready vesicles also necessitates its association with dynamin (Fig. 4), perhaps reflecting a requirement for membrane remodeling in release site clearance. Irrespective of the precise mechanisms involved, the results reported here support a crucial regulatory role for the endocytic scaffold intersectin 1 in fast neurotransmission and short-term plasticity independent of membrane retrieval, suggesting that the machineries for exocytosis and endocytosis may be coupled more tightly than previously thought (6, 9, 12). Such coupling is likely of crucial physiological importance for fast neurotransmission and for information processing in the brain, particularly in sensory systems that fire at hundreds of Hz.
Materials and Methods
Electrophysiology.
Transverse brainstem slices (200 μm thick) were prepared from 8- to 12- day-old Wistar rats (22, 23) or from intersectin 1 KO mice, along with corresponding WT or heterozygous littermates used as controls (20). Experiments have been approved by the review board at Doshisha University, Max Planck institute for biophysical chemistry and Leibniz Institut für Molekulare Pharmakologie, Berlin. The extracellular solution contained 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 25 mM glucose, 25 mM NaHCO3, 1.25 mM NaH2PO4, 0.4 mM ascorbic acid, 3 mM myoinositol, and 2 mM Na-pyruvate [pH 7.4; bubbled with 95% (vol/vol) O2 and 5% (vol/vol) CO2]. Experiments were performed at room temperature. During the recordings, 1 μM TTX and 10 mM tetraethylammonium chloride (TEA-Cl) were included in the external solution to block Na+ and K+ channels. In paired recordings, 50 μM D-(-)-2-amino-5-phosphonopentanoic acid (D-AP5), 100 µM cyclothiazide (CTZ), and 1 mM or 2 mM (for the experiments in mice) kynurenic acid (Kyn) were added to isolate postsynaptic AMPA receptor- mediated EPSCs and to block desensitization and possible saturation of AMPA receptors (36). A calyx of Held and its postsynaptic MNTB principal neuron were whole-cell voltage clamped at –70 mV (for the experiments in mice) or at −80 mV using an EPC 10/2 amplifier (HEKA). The presynaptic patch pipette (3–5 MΩ) solution contained 135 mM Cs-gluconate, 20 mM TEA-Cl, 10 mM Hepes, 5 mM Na2-phosphocreatine, 4 mM MgATP, 0.3 mM GTP, and either 0.05 or 0.5 mM EGTA (pH 7.2). The presynaptic series resistance (5–20 MΩ) was compensated for by 30–70%. The postsynaptic pipette (2–3.5 MΩ) contained the same solution as the presynaptic pipette, except that the EGTA concentration was increased to 5 mM. The postsynaptic series resistance (3–8 MΩ) was compensated for by the amplifier, so that the uncompensated resistance was below 3 MΩ. The remaining resistance was further compensated for offline.
Presynaptic capacitance measurements were carried out using an EPC10/2 amplifier in the sine + DC configuration. A sine wave (30 mV amplitude, 1,000 Hz) was superimposed on a holding potential of −80 mV. Measurements were included in the dataset if the presynaptic series resistance was below 20 MΩ. Occasionally, baseline capacitance traces exhibited a slow shift that was corrected for analysis.
CTZ, d-AP5, and Kyn were obtained from Tocris. SH3 domains, antibodies. and peptides were dialyzed to the presynaptic solution.
Quantal release rates were estimated by the deconvolution method, adapted for the calyx of Held (36). Quantal release rates, determined by deconvolution, were integrated to obtain the cumulative release. Cumulative release was fitted by a double-exponential function after correction for SV replenishment. In the paired-pulse protocol, the time constants for the second pulse were derived in the same manner as those for the first pulse, because a small fraction of the fast-releasing component might have been missed by using a single exponential with free parameters, which fit the data equally well in some cases. Data are shown as mean ± SEM.
Peptides and Protein Domains.
The SH3 domain binding dynamin 1-derived proline-rich peptide (amino acid sequence: WT peptide, 773RSPTSSPTPQRRAPAVPPARPG794; mutant peptide, 773RSPTSSPTPQRRAAAVAPARPG794) was obtained by chemical synthesis. The following hexahistidine-tagged recombinant proteins were expressed and purified from overexpressing Escherichia coli (BL21) using nickel nitrilo-triacetic acid (Ni-NTA) affinity chromatography according to the manufacturer's (Sigma-Aldrich Inc., St. Louis) instructions: human intersectin 1 SH3A domain (residues 738–803), intersectin 1L-SH3A mutant (residues 738–803 with G794R/ P797L mutations), human intersectin 1 SH3E domain (residues 1152–1214), human intersectin 1 DH-PH domain (residues 1229–1581), and mouse endophilin A1 (residues 283–352). The affinity purification experiments shown in Fig. S6 were carried out essentially as described previously (31).
Supplementary Material
Acknowledgments
We thank the Leibniz Institute for Neurobiology, Magdeburg, Special Laboratory Electron- and Laserscanning Microscopy Center for instrument use and scientific and technical assistance. We also thank Dr. Melanie Pritchard (Monash University) for providing intersectin 1 KO mice, Dr. Tanja Martizen for help with the experiments using KO mice, and Dr. T. Kirchhausen (Harvard Medical School) for kindly providing Secramine A. The study was supported by the Japan Society for the Promotion of Science (KAKENHI Grants 24300144 and 24650218 and Core-to-Core Program A, to T.S.), the Toray Science Foundation (T.S.), the Uehara Foundation (T.S.), the Swedish Research Council (A.P. and O.S.), and the German Research Foundation DFG (Grants EXC 257 and SFB958/A01 to V.H. and SFB958/Z02 to J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Y.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219234110/-/DCSupplemental.
References
- 1.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 2.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59(6):861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Stevens CF. Neurotransmitter release at central synapses. Neuron. 2003;40(2):381–388. doi: 10.1016/s0896-6273(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 4.Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 5.Rosenmund C, Rettig J, Brose N. Molecular mechanisms of active zone function. Curr Opin Neurobiol. 2003;13(5):509–519. doi: 10.1016/j.conb.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Haucke V, Neher E, Sigrist SJ. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci. 2011;12(3):127–138. doi: 10.1038/nrn2948. [DOI] [PubMed] [Google Scholar]
- 7.Dittman JS, Kreitzer AC, Regehr WG. Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J Neurosci. 2000;20(4):1374–1385. doi: 10.1523/JNEUROSCI.20-04-01374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan B, Zucker RS. A general model of synaptic transmission and short-term plasticity. Neuron. 2009;62(4):539–554. doi: 10.1016/j.neuron.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosoi N, Holt M, Sakaba T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63(2):216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Wu XS, et al. Ca(2+) and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci. 2009;12(8):1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki F, Hazen M, Ordway RW. Fast synaptic fatigue in shibire mutants reveals a rapid requirement for dynamin in synaptic vesicle membrane trafficking. Nat Neurosci. 2000;3(9):859–860. doi: 10.1038/78753. [DOI] [PubMed] [Google Scholar]
- 12.Peters C, Baars TL, Bühler S, Mayer A. Mutual control of membrane fission and fusion proteins. Cell. 2004;119(5):667–678. doi: 10.1016/j.cell.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Dickman DK, Horne JA, Meinertzhagen IA, Schwarz TL. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell. 2005;123(3):521–533. doi: 10.1016/j.cell.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Pechstein A, Shupliakov O, Haucke V. Intersectin 1: A versatile actor in the synaptic vesicle cycle. Biochem Soc Trans. 2010;38(Pt 1):181–186. doi: 10.1042/BST0380181. [DOI] [PubMed] [Google Scholar]
- 15.Henne WM, et al. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328(5983):1281–1284. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43(2):193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Marie B, et al. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43(2):207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 18.O’Bryan JP. Intersecting pathways in cell biology. Sci Signal. 2010;3(152):re10. doi: 10.1126/scisignal.3152re10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto M, Schoch S, Südhof TC. EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25: A protein connection between exocytosis and endocytosis? J Biol Chem. 1999;274(26):18446–18454. doi: 10.1074/jbc.274.26.18446. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y, et al. Mice deficient for the chromosome 21 ortholog Itsn1 exhibit vesicle-trafficking abnormalities. Hum Mol Genet. 2008;17(21):3281–3290. doi: 10.1093/hmg/ddn224. [DOI] [PubMed] [Google Scholar]
- 21.Ramaswami M, Krishnan KS, Kelly RB. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994;13(2):363–375. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 22.Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J Physiol. 1994;479(Pt 3):381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383(6599):431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- 24.Gundelfinger ED, Kessels MM, Qualmann B. Temporal and spatial coordination of exocytosis and endocytosis. Nat Rev Mol Cell Biol. 2003;4(2):127–139. doi: 10.1038/nrm1016. [DOI] [PubMed] [Google Scholar]
- 25.Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32(6):1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- 26.Sakaba T. Roles of the fast-releasing and the slowly releasing vesicles in synaptic transmission at the calyx of held. J Neurosci. 2006;26(22):5863–5871. doi: 10.1523/JNEUROSCI.0182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita T, Hige T, Takahashi T. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 2005;307(5706):124–127. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- 28.Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417(6888):555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- 29.Renden R, von Gersdorff H. Synaptic vesicle endocytosis at a CNS nerve terminal: Faster kinetics at physiological temperatures and increased endocytotic capacity during maturation. J Neurophysiol. 2007;98(6):3349–3359. doi: 10.1152/jn.00898.2007. [DOI] [PubMed] [Google Scholar]
- 30.Park H, Li Y, Tsien RW. Influence of synaptic vesicle position on release probability and exocytotic fusion mode. Science. 2012;335(6074):1362–1366. doi: 10.1126/science.1216937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pechstein A, et al. Regulation of synaptic vesicle recycling by complex formation between intersectin 1 and the clathrin adaptor complex AP2. Proc Natl Acad Sci USA. 2010;107(9):4206–4211. doi: 10.1073/pnas.0911073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schirmer J, Aktories K. Large clostridial cytotoxins: Cellular biology of Rho/Ras-glucosylating toxins. Biochim Biophys Acta. 2004;1673(1-2):66–74. doi: 10.1016/j.bbagen.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Pelish HE, et al. Secramine inhibits Cdc42-dependent functions in cells and Cdc42 activation in vitro. Nat Chem Biol. 2006;2(1):39–46. doi: 10.1038/nchembio751. [DOI] [PubMed] [Google Scholar]
- 34.Lou X, Paradise S, Ferguson SM, De Camilli P. Selective saturation of slow endocytosis at a giant glutamatergic central synapse lacking dynamin 1. Proc Natl Acad Sci USA. 2008;105(45):17555–17560. doi: 10.1073/pnas.0809621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawasaki F, Ordway RW. Molecular mechanisms determining conserved properties of short-term synaptic depression revealed in NSF and SNAP-25 conditional mutants. Proc Natl Acad Sci USA. 2009;106(34):14658–14663. doi: 10.1073/pnas.0907144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neher E, Sakaba T. Combining deconvolution and noise analysis for the estimation of transmitter release rates at the calyx of held. J Neurosci. 2001;21(2):444–461. doi: 10.1523/JNEUROSCI.21-02-00444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.