Abstract
Regulator of G protein signaling 4 (Rgs4) is a signal transduction protein that controls the function of monoamine, opiate, muscarinic, and other G protein-coupled receptors via interactions with Gα subunits. Rgs4 is expressed in several brain regions involved in mood, movement, cognition, and addiction and is regulated by psychotropic drugs, stress, and corticosteroids. In this study, we use genetic mouse models and viral-mediated gene transfer to examine the role of Rgs4 in the actions of antidepressant medications. We first analyzed human postmortem brain tissue and found robust up-regulation of RGS4 expression in the nucleus accumbens (NAc) of subjects receiving standard antidepressant medications that target monoamine systems. Behavioral studies of mice lacking Rgs4, including specific knockdowns in NAc, demonstrate that Rgs4 in this brain region acts as a positive modulator of the antidepressant-like and antiallodynic-like actions of several monoamine-directed antidepressant drugs, including tricyclic antidepressants, selective serotonin reuptake inhibitors, and norepinephrine reuptake inhibitors. Studies using viral-mediated increases in Rgs4 activity in NAc further support this hypothesis. Interestingly, in prefrontal cortex, Rgs4 acts as a negative modulator of the actions of nonmonoamine-directed drugs that are purported to act as antidepressants: the N-methyl-D-aspartate glutamate receptor antagonist ketamine and the delta opioid agonist (+)-4-[(αR)-α-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide. Together, these data reveal a unique modulatory role of Rgs4 in the brain region-specific actions of a wide range of antidepressant drugs and indicate that pharmacological interventions at the level of RGS4 activity may enhance the actions of such drugs used for the treatment of depression and neuropathic pain.
Keywords: conditional knockout mice, desipramine, adeno-associated viruses, mood disorders
Tricyclic antidepressants (TCAs), and the related serotonin selective reuptake inhibitors (SSRIs) and combined serotonin/norepinephrine reuptake inhibitors (SNRIs), are among the most widely prescribed medications worldwide, used commonly to treat not only depression but a variety of other chronic syndromes, in particular, neuropathic pain. Indeed, depression and chronic pain show high rates of comorbidity (1). At the same time, there is great interest in the development of novel antidepressant treatments that do not target the brain’s monoamine pathways. Unfortunately, we still know relatively little about the signal transduction mechanisms that underlie the therapeutic actions of different classes of antidepressant medications. Understanding such signal transduction events could lead to a better categorization of these drugs and to their more efficient prescription for a range of disorders.
Regulator of G protein signaling 4 (Rgs4) is a 28-kDa protein known to control postreceptor signaling cascades for numerous G protein-coupled receptors (GPCRs), including dopamine, serotonin, adrenergic, muscarinic, and mu and delta opioid receptors (2–9). Rgs4 is generally thought to be a negative modulator of Gα subunits by promoting their hydrolysis of GTP and by antagonizing the regulation of their effectors, although the actions of Rgs proteins are more complex, because they can either inhibit or facilitate several actions of their associated receptors (10, 11). Preclinical and clinical studies link Rgs4 dysfunction to hypertension, cardiac hypertrophy, schizophrenia, Parkinson disease, and drug addiction, among other disorders (11–15). In the brain, Rgs4 is expressed in high abundance in prefrontal cortex (PFC) and is also present at high levels in regions associated with stress and depression, including nucleus accumbens (NAc), locus coeruleus, hippocampus, and several other limbic brain regions (16, 17). Although earlier studies in rodents demonstrated dynamic transcriptional regulation of Rgs4 by stress and corticosteroids (17), the role of this protein in antidepressant drug actions remains unknown.
Based on the distribution pattern of Rgs4 in the CNS, and on the fact that Rgs4 modulates several types of monoamine receptors (2, 6–10), we hypothesized that this protein might modulate the actions of drugs used for the treatment of mood disorders. The lack of pharmacological compounds that directly target Rgs4 activity has been a major limitation for studies of the in vivo function of this protein. Using genetic mouse models and brain region-specific manipulations, we explore here the role of Rgs4 in the actions of a wide range of antidepressant drugs. Our studies on postmortem human brain demonstrate dramatic up-regulation of RGS4 expression in NAc in subjects treated with monoamine-directed antidepressants. Similar findings were observed in mouse NAc. Using constitutive and conditional knockout models and viral-mediated gene transfer, we demonstrate that Rgs4 in NAc increases responsiveness to antidepressants targeting monoamine systems in mouse models of both depression and neuropathic pain. In contrast, Rgs4 acts as a negative modulator of the antidepressant- and antiallodynic-like actions of two putative unique antidepressants with nonmonoamine mechanisms. Notably, these latter drugs down-regulate Rgs4 in mouse PFC without affecting levels of the protein in NAc. These findings thus provide insight into the cellular mechanisms underlying the actions of clinically used antidepressants and identify Rgs4 as a key modulator of treatment responsiveness.
Results
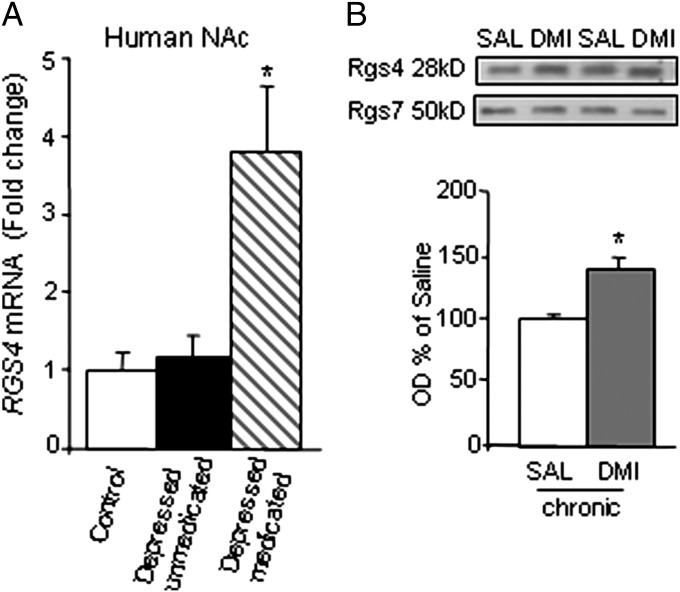
Our first set of studies examined RGS4 expression in NAc of human postmortem brains from control subjects and subjects suffering from depression who were either on or off a range of antidepressant medications at their time of death. Quantitative PCR (qPCR) analysis reveals that, although depression per se is not associated with altered RGS4 expression in NAc, treatment with monoamine-targeting antidepressants (Table S1) leads to a nearly fourfold up-regulation of RGS4 mRNA levels in this brain region (Fig. 1A). Consistent with these data from human NAc, chronic (but not acute) treatment with the TCA desipramine (DMI) leads to a significant up-regulation of Rgs4 protein levels in mouse NAc (Fig. 1B). DMI also up-regulates Rgs4 in mouse PFC (saline = 100 ± 13.9, DMI = 158 ± 6; P < 0.01, paired t test). Rgs4 is up-regulated in NAc after chronic treatment with the SSRI, fluoxetine (FLX, 15 mg/kg once a day for 30 d i.p.) (saline = 100 ± 15, FLX = 155 ± 19, n = 3 per group; P < 0.05, unpaired t test).
Fig. 1.
RGS4 is regulated by chronic antidepressant treatment. (A) Antidepressant treatment promotes the expression of RGS4 in human NAc. qPCR analysis of postmortem NAc tissue from control, depressed-nonmedicated (subjects who were not on antidepressant medication at time of death), and depressed-medicated subjects [subjects treated with antidepressants (Table S1) at time of death] reveals that although RGS4 expression is not affected by depression per se, there is a nearly fourfold increase in RGS4 mRNA levels in subjects treated with antidepressants. The average postmortem interval was 16 h. The different groups were matched as closely as possible for race, sex, age, pH, postmortem interval, and RNA integrity number (n = 8 per group; see Table S1 for further information). (B) Similarly, DMI treatment (10 mg/kg i.p. twice a day for 2 wk) significantly increased Rgs4 protein levels in NAc of C57BL/6 mice. Mice were analyzed 24 h after the last drug injection (n = 3 per group; *P < 0.01, t test). DMI, desipramine; OD, optical density; SAL, saline.
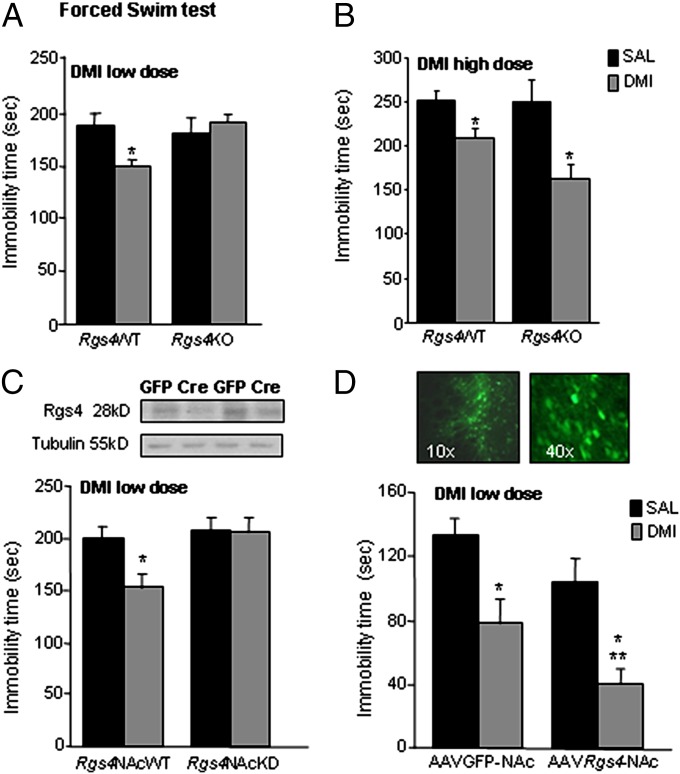
Based on these findings, we hypothesized that Rgs4 might modulate the actions of antidepressant drugs. To test this hypothesis, we first used genetically modified mice to examine the consequences of Rgs4 deletion in behavioral responses to DMI. Constitutive knockout of Rgs4 prevents the actions of a low DMI dose (10 mg/kg i.p.) in the forced swim test (FST), a rapid assay of acute antidepressant responses (Fig. 2A). As shown in Fig. 2B, higher doses of DMI produce similar responses in both genotypes, suggesting that Rgs4 plays a positive modulatory role in DMI responses in this assay. We next investigated the consequences of the selective knockdown of Rgs4 from NAc in DMI responses. Knockdown of Rgs4 in NAc (Rgs4NAcKD) was achieved by bilaterally injecting floxed Rgs4 mice with an AAV (adeno-associated virus) vector expressing Cre recombinase (18), which leads to a 67 ± 11% reduction in Rgs4 levels (P < 0.05, t test). As shown in Fig. 2C, loss of Rgs4 in NAc prevents the reduction in immobility time observed after DMI treatment in wild-type animals in the forced swim test. Notably, local or global inactivation of Rgs4 does not affect baseline responses (i.e., in the absence of DMI) in this test. Conversely, overexpression of Rgs4 in NAc of C57BL/6 mice, via stereotaxic infusion of an AAV-Rgs4 construct, enhances the actions of the low DMI dose in the forced swim test compared with control animals injected with an AAV-GFP vector (Fig. 2D).
Fig. 2.
Rgs4 modulates the actions of desipramine. (A) Rgs4KO mice show no change in the immobility time in the FST in response to a low DMI dose (10 mg/kg i.p. 24 h before FST, 10 mg/kg at 5 h and 20 mg/kg 1 h before FST, n = 5–7 per group) in contrast to Rgs4WT controls that show the expected reduction in immobility in response to DMI. (B) Rgs4WT and Rgs4KO mice show similar FST responses when a higher dose of DMI (20 mg/kg i.p. 24 h before FST, 20 mg/kg at 5 h and 40 mg/kg 1 h before FST, n = 5–7 per group) is applied. (C) Mice with a selective knockdown of Rgs4 in NAc (Rgs4NAcKD) show no response to a dose of DMI (as in A), which reduces immobility in the forced swim assay in their wild-type littermates (n = 8–12 per group). Local knockdown of Rgs4 from NAc of adult floxed Rgs4 mice was achieved with stereotaxic injection of AAV-Cre; Western blots show reduced Rgs4 levels in Cre-injected brains (see Fig. S1 for infection sites). (D) The opposite phenotype is observed when Rgs4 is overexpressed in NAc of adult C57BL/6 mice by using an AAV-Rgs4 construct (n = 5–8 per group; *P < 0.05 between treatments and **P < 0.05 between genotypes, DMI dose as in A). Image shows AAV-GFP distribution at the injection site (see also Fig. S1). For all behavioral studies, data are expressed as average ± SEM and analyzed by using two-way ANOVA followed by Bonferroni post hoc test. OD, optical density; SAL, saline.
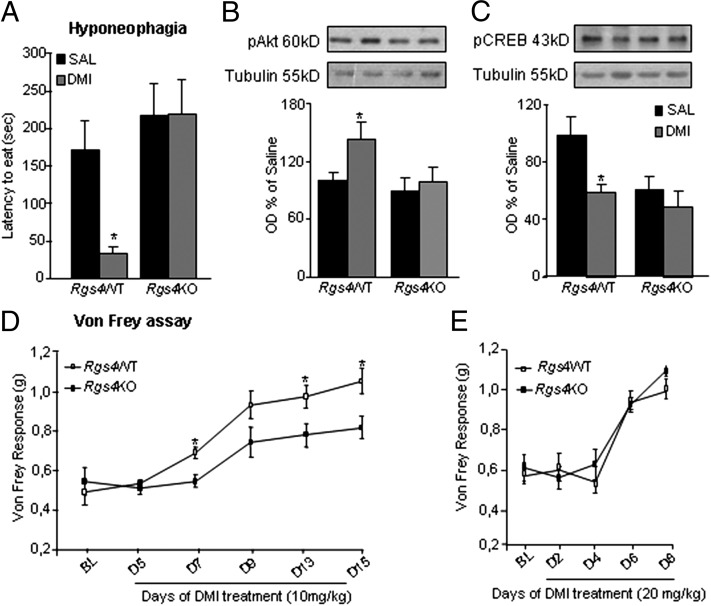
We next used the hyponeophagia paradigm to examine the influence of Rgs4 in chronic DMI actions. As shown in Fig. 3A, chronic DMI treatment significantly reduces the latency to eat food from the center of an open field in Rgs4WT mice, but has no effect in constitutive Rgs4KO mice. Using Western blot analysis, we examined differences in signal transduction events triggered by chronic DMI (10 mg/kg twice a day for 2 wk) in NAc of Rgs4WT and Rgs4KO mice used in the hyponeophagia test. Brains were dissected 2 h after the test and 24 h after the last drug injection. Chronic DMI increases Akt (a serine/threonine kinase also known as protein kinase B) phosphorylation at Ser473 (Fig. 3B), a site associated with increased Akt catalytic activity, and decreases cAMP response element binding protein (CREB) phosphorylation at Ser133 (Fig. 3C), a site associated with CREB activation, in NAc of Rgs4WT mice. Both of these DMI effects are lost completely in Rgs4KO mice. Notably, inactivation of the Rgs4 gene leads to a significant decrease in phospho-CREB levels in NAc of drug-naive animals, with no further regulation observed upon DMI administration (Fig. 3C). Total Akt and CREB levels were not affected by treatment or genotype (Fig. S2). Although the influence of Akt signaling in NAc on depression-related behaviors has not yet been investigated, reduced CREB activity in NAc exerts a robust antidepressant-like effect in a wide range of rodent models (19, 20). Thus, our findings that the ability of chronic DMI to decrease CREB phosphorylation/activity in NAc is abolished upon Rgs4 knockout provide further evidence that Rgs4 is required for normal therapeutic-like responses to this drug.
Fig. 3.
RGS4 is a positive modulator of the chronic actions of DMI. Rgs4WT and Rgs4KO mice were evaluated in the hyponeophagia paradigm before and after 2 wk of DMI treatment (10 mg/kg i.p. twice a day). (A) DMI reduces the latency to eat food from the center of an open field in Rgs4WT but not in Rgs4KO mice (n = 5–8 per group; *P < 0.01). (B) Using Western blot analysis, we examined the effect of Rgs4 on signal transduction events triggered by DMI. DMI treatment increases Akt phosphorylation (pAkt) at Ser473 in NAc of Rgs4WT animals used in the hyponeophagia test, whereas it has no effect on Rgs4KO mice (*P < 0.01, n = 5 per treatment) (C) DMI also decreases CREB phosphorylation (pCREB) at Ser133 in NAc of Rgs4WT mice. Interestingly, phospho-CREB levels are significantly lower in the NAc of drug-naïve Rgs4KO mice, whereas DMI has no further effect on CREB phosphorylation (*P < 0.05, **P < 0.01; n = 5 per group). (D) The antiallodynic actions of DMI in a mouse model of neuropathic pain are reduced in Rgs4KO compared with their Rgs4WT controls. The graph shows Von Frey responses of the ipsilateral hind paw in SNI mice treated with DMI (10 mg/kg i.p. for 2 wk, n = 9–11 per group; *P < 0.05 for genotype versus treatment) (E). At higher doses of DMI (20 mg/kg), both genotypes exhibit the same antiallodynic response (n = 12–13). Data are expressed as average ± SEM and analyzed by using two-way ANOVA followed by Bonferroni post hoc test. OD, optical density; SAL, saline.
As stated in the Introduction, TCAs are also used for the treatment of neuropathic pain symptoms, like mechanical allodynia (21). Using the unilateral spared nerve injury (SNI) paradigm of neuropathic pain, we evaluated the ability of DMI to suppress mechanical allodynia in Rgs4WT and Rgs4KO mice. Fig. 3D shows Von Frey mechanical allodynia responses in such mice during 15 d of DMI treatment. The antiallodynic actions of DMI are significantly lower in Rgs4KO mice compared with their wild-type controls throughout the 2-wk treatment period. When higher doses of DMI are used, both genotypes show similar antiallodynic responses (Fig. 3E).
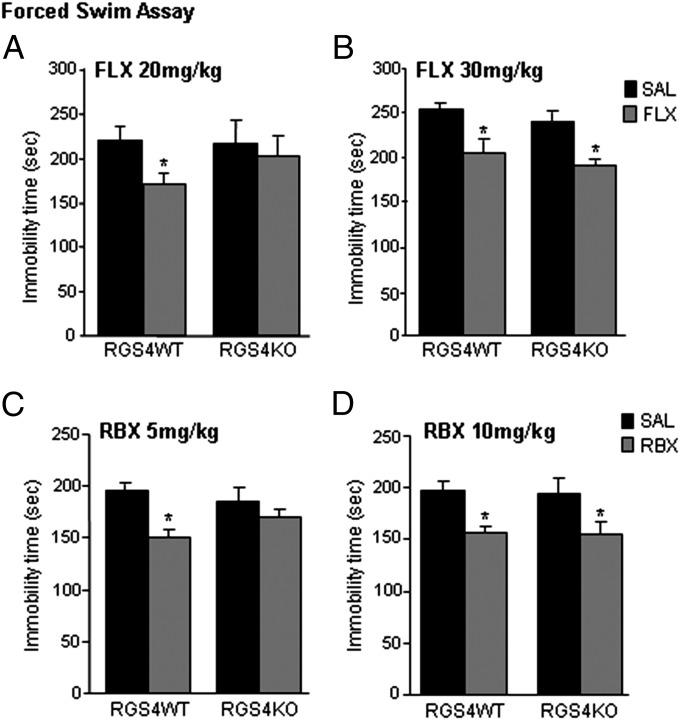
The next experiment used the forced swim assay to investigate the role of Rgs4 in responses to FLX. As shown in Fig. 4A, Rgs4KO mice do not respond to a low fluoxetine dose (20 mg/kg, i.p.), which reduces immobility time in their wild-type controls. A similar phenotype is observed when animals receive a norepinephrine reuptake inhibitor (NRI), reboxetine (RBX), at a 5 mg/kg dose (Fig. 4C). Both genotypes show similar responses when a higher dose of FLX (30 mg/kg) or RBX (10 mg/kg) is used (Fig. 4 B and D).
Fig. 4.
RGS4 is a positive modulator of SSRI and NRI responses. (A) Rgs4KO mice show no response to the SSRI, FLX, at a low dose (20 mg/kg) that reduces immobility time in Rgs4WT mice (n = 7–8 per group; *P < 0.05). (B) When higher doses of FLX are applied (30 mg/kg, n = 5–7 per group; *P < 0.05), both genotypes show similar immobility times. (C) Rgs4KO mice do not respond to a low dose (5 mg/kg) of the NRI, RBX (n = 13–18 per group; *P < 0.05), but both genotypes respond to a higher RBX dose (10 mg/kg, n = 8–10 per group; *P < 0.05) (D). Data are expressed as average ± SEM and analyzed by using two-way ANOVA followed by Bonferroni post hoc test. SAL, saline.
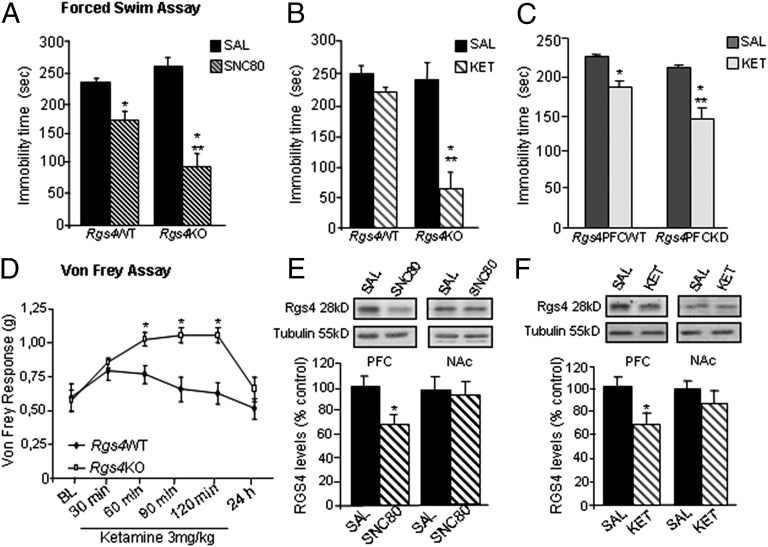
Our behavioral studies so far indicate that Rgs4 modulates the actions of several monoamine-targeting antidepressants. We next examined whether Rgs4 modulates the actions of purported antidepressants that act via distinct, nonmonoamine mechanisms. Specifically, we examined the influence of Rgs4 in the actions of the delta opioid receptor agonist (+)-4-[(αR)-α-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC80), a drug that has antidepressant-like actions in rodent models (22). In contrast with our observations using monoamine-targeting antidepressants, Rgs4KO mice respond better to SNC80 in the forced swim test than their wild-type controls (Fig. 5A). A separate group of animals received ketamine, a noncompetitive N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, which has been shown to produce rapid antidepressant responses in treatment-resistant depression in several clinical studies (23–27). Loss of Rgs4 leads to augmented responses to a low ketamine dose (3 mg/kg, i.p.) in the forced swim test (Fig. 5B). This effect in constitutive Rgs4KO mice was replicated in mice with a local viral-mediated knockdown of Rgs4 from PFC (Fig. 5C). Ketamine is also known to exert antinociceptive effects in humans (28). We thus examined the actions of a low ketamine dose (3 mg/kg) in the SNI neuropathic pain model and found antiallodynic responses only in Rgs4KO mice (Fig. 5D), again suggesting that Rgs4 acts as a negative modulator of ketamine actions. Western blot analysis demonstrates that, 1 h after acute SCN80 (5 mg/kg, i.p.) or 3 h after ketamine (5 mg/kg, i.p.) administration, there is a significant reduction in Rgs4 levels in PFC (Fig. 5 E and F). In contrast, acute SNC80 or acute ketamine treatment had no effect on Rgs4 levels in NAc (Fig. 5 E and F).
Fig. 5.
RGS4 is a negative modulator of SNC80 and ketamine responses. (A) Rgs4KO mice show a greater response to the delta opioid receptor agonist SNC80 (5 mg/kg, 1 h before test) than their Rgs4WT controls in the forced swim test (n = 5–8 per group, *P < 0.05 between treatments, **P < 0.05 for treatment versus genotype). (B) Similarly, Rgs4KO mice show a greater response to ketamine (KET, 3 mg/kg, 3 h before test) in the forced swim test compared with their Rgs4WT controls (n = 5–7 per group, *P < 0.01 for treatment effect and **P < 0.01 for treatment versus genotype). (C) Conditional knockdown of Rgs4 in PFC reveals that Rgs4 actions in this brain region contribute to this phenotype (n = 5–7 per group; *P < 0.05 between treatments **P < 0.05 for treatment versus genotype, dose as in B). (D) Rgs4KO mice in the spared nerve injury neuropathic pain model show reduced allodynia in response to a low ketamine dose (3 mg/kg i.p., 3 h before test), which has no effect in Rgs4WT mice (n = 7 per group, *P < 0.01 between genotypes). Data are expressed as average ± SEM and analyzed by using two-way ANOVA followed by Bonferroni post hoc test. (E and F) Rgs4 levels are reduced in PFC, but not NAc, 1 h after SNC80 (5 mg/kg) or 3 h after ketamine (5 mg/kg) administration (n = 6–7 per group, P < 0.05) of C57BL/6 mice. Data are expressed as average ± SEM and analyzed by using t test. OD, optical density; SAL, saline.
Discussion
Our work provides insight into the cellular mechanisms of antidepressant drug actions and identifies Rgs4 as a unique key modulator of treatment responsiveness. We show that Rgs4 is a positive modulator of both antidepressant and antiallodynic actions of several classes of monoamine-targeting antidepressants, including a TCA, SSRI, and NRI. In striking contrast, Rgs4 exerts an opposite influence on the ability of two nonmonoamine-based drugs—ketamine and a delta opioid receptor agonist—to induce similar behavioral responses. Importantly, our studies of mouse brain demonstrate that these monoamine versus nonmonoamine-targeting antidepressants likewise have opposite effects on Rgs4 expression levels in distinct brain regions, with the actions of the monoamine-based drugs validated in human postmortem brain tissue.
We previously developed tools to examine the brain region-specific actions of Rgs4 and demonstrated that, at the level of the NAc, Rgs4 is a negative modulator of opiate reward but promotes the analgesic actions of certain opiate agonists (18). Given the wide distribution of Rgs4, the lack of pharmacological agents that target the protein, and the potential developmental deficits associated with global deletion of Rgs4, conditional deletion or overexpression approaches represent crucial tools for these studies, because they permit investigation of Rgs4 function within specific brain regions of adult animals. We show that constitutive genetic ablation of Rgs4 attenuates acute responses to low doses of TCA, SSRI, and NRI antidepressants in the forced swim assay as well as chronic responses to a TCAs in the hyponeophagia assay. Using conditional interventions, we show that the NAc is one critical region that mediates these actions in adult animals without developmental influences: Selective knockdown of Rgs4 from NAc of adult mice similarly attenuates antidepressant-like effects of a TCA, whereas overexpression of Rgs4 in adult NAc potentiates these actions. Although these findings do not rule out effects of Rgs4 in other brain structures, our data demonstrate that Rgs4 acting in NAc plays a positive modulatory role in antidepressant responses. Thus, agents favoring Rgs4 stability or increasing the protein’s activity may constitute unique targets for antidepressant drug development. Future studies should examine the function of Rgs4 in other brain regions involved in stress and depression and evaluate the influence of Rgs4 on specific neurotransmitter systems in mediating these effects.
TCA and SNRI antidepressants are also widely used in the treatment of chronic neuropathic pain, where they effectively treat both pain and the oft-associated depressive symptoms (21). It is thus very interesting that the ability of the TCA, DMI, to exert antiallodynic effects in a mouse model of neuropathic pain was also decreased upon constitutive knockout of Rgs4. Loss of Rgs4, however, had no effect on baseline pain responses in the absence of the antidepressant, as we have observed (18). Our biochemical and behavioral findings support the notion that induction of Rgs4 is a critical step in the mechanisms by which monoamine targeting antidepressants treat symptoms of depression and neuropathic pain. In this study, we demonstrate that chronic administration of monoamine-based antidepressants up-regulates Rgs4 in NAc. Notably, dopamine D1 or D2 receptor antagonists induce Rgs4 expression in caudate-putamen, whereas opiates reduce Rgs4 levels in NAc (2, 18, 29, 30). Interestingly, the psychostimulant amphetamine down-regulates Rgs4 in caudate-putamen but not NAc, whereas NAc Rgs4 levels are only reduced following an amphetamine challenge in chronically treated animals (30). Moreover, although amphetamine and monoamine-based antidepressants both increase monoamine levels in NAc, these drugs regulate Rgs4 levels in opposite directions. Thus, Rgs4 shows complex brain region-, context-, and treatment-specific regulation, consistent with its modulation of numerous GPCRs, including several adrenergic, dopaminergic, serotonergic, opiate, muscarinic, and metabotropic glutamate receptors (mGluRs), which are either Gαi or Gαq coupled (2–9, 31, 32). Further work is needed to understand the complex interactions between Rgs4 and several other signaling proteins that control receptor function (2, 8, 9, 18, 30, 31) and how these mechanisms influence therapeutic-like responses to monoamine-based antidepressant medications.
Given the fact that more than one-half of all depressed patients do not respond fully to available monoamine-based antidepressants, there has been great interest in developing drugs with novel mechanisms of action. One of the most promising is ketamine, which has been shown to exert rapid and robust antidepressant and antisuicidal effects in treatment-resistant patients in several clinical trials (23–27). Ketamine is also used for the treatment of certain chronic pain conditions (28). It was therefore of interest to study whether Rgs4 exerts a similar action on the behavioral effects of ketamine compared with traditional monoamine-based drugs. Surprisingly, in striking contrast to the latter, ketamine had no effect on Rgs4 levels in NAc, but reduced Rgs4 expression in PFC. Moreover, although knockout of Rgs4 attenuated the antidepressant- and antiallodynic-like effects of standard antidepressants, it potentiated both actions of ketamine. This effect was mediated at least in part via the PFC, because local knockdown of Rgs4 from this brain region replicated the potentiated behavioral responses to ketamine observed in constitutive knockouts. Several studies have demonstrated the ability of GPCRs to inhibit NMDA glutamate receptor currents in PFC (7, 8). Specifically, Rgs4 in PFC negatively modulates α1- and α2-adrenergic receptor subtypes, but only Rgs4 modulation of α1 receptors involves complexes with the scaffold protein, spinophilin (8). Similar receptor specificity has been reported for Rgs4 regulation of the 5-hydroxytryptamine 1A (5-HT1A) serotonin receptor (but not the D4 dopamine receptor) in NMDA receptor function in PFC (7). We speculate that increased GPCR activity in PFC as a result of Rgs4 knockout leads to suppression of NMDA receptors, which are also the targets for ketamine. Based on this hypothesis, ketamine potentiates its own action by decreasing Rgs4 levels in PFC and, thereby, enhancing GPCR-mediated inhibition of NMDA currents. Rgs4 regulation of group I mGluRs (6, 31, 32) might further influence ketamine’s behavioral effects. Future studies should investigate the mechanism by which Rgs4 modulates Gαi or Gαq signal transduction in PFC and its role in NMDA receptor function and other synaptic events.
Our study reports similar effects with another nonmonoamine-based putative antidepressant, SNC80, which also reduced Rgs4 levels in PFC and whose antidepressant-like effects were potentiated upon global Rgs4 knockout. It would be interesting to see a much broader screen of drugs, of diverse monoamine- and nonmonoamine-based actions, with established or putative antidepressant or antiallodynic properties, for their effects on Rgs4 expression in brain as well as the influence of Rgs4 in modulating their behavioral effects in a range of animal depression and pain models.
Results from this study have direct implications for pharmaceutical development. The ability of Rgs4 to oppositely influence both the antidepressant- and antiallodynic-like actions of DMI and of ketamine coincide with the drugs’ opposite effects on Rgs4 expression levels in brain. Our data suggest that drugs that stabilize or otherwise potentiate the actions of RGS4 would be useful as an adjunct to monoamine-based antidepressants to promote their treatment of depression and neuropathic pain, whereas drugs that antagonize RGS4 would be useful as an adjunct to ketamine and perhaps other nonmonoamine-based medications. Importantly, these inferences are based not only on local actions of Rgs4 within a particular brain region, but also on global deletions of Rgs4, consistent with the potential utility of systemically administered Rgs4-directed agents. There has been considerable interest in generating small molecules that regulate Rgs4 activity (e.g., ref. 33). It would be interesting to study the effect of such molecules in depression and pain assays, as well as to study the influence of known genetic variations in the RGS4 gene, which have been shown to cause differences in RGS4 activity, in regulating an individual’s responsiveness to monoamine- and nonmonoamine-based antidepressant treatments.
In summary, this work provides evidence on the cellular mechanisms governing the behavioral actions of a wide range of antidepressant medications. We show that monoamine-targeting antidepressants, as well as drugs targeting the delta opioid or NMDA glutamate receptor, exert their cellular actions through Rgs4-modulated pathways, but via very different mechanisms. These data support earlier findings on the diverse tissue-, cell type-, and receptor-selective actions of Rgs proteins (10, 11) and point to Rgs4 complexes in the brain as important targets for the treatment of severe chronic affective and pain disorders.
Methods
Animal Studies and Drug Treatments.
For all behavioral assays, we used 2-mo-old male Rgs4KO mice and their Rgs4WT littermates (18), except for studies on neuropathic pain that were performed in female mice. For viral infections, we used 2- to 3-mo-old male C57BL/6 or floxed Rgs4 male mice. Conditional deletion of Rgs4 was achieved via application of viruses expressing Cre into particular brain regions of floxed Rgs4 mice, as described (18). The mutant mice used were on DBA background, which explains differences in some of the baseline responses. Mice were housed in a 12-h dark/light cycle according to the Animal Care and Use Committees of Mount Sinai and University of Crete. DMI (Sigma Aldrich) stock solutions were prepared in water and final dilutions were made in saline. FLX, RBX, and ketamine (Sigma Aldrich) were diluted in saline. SNC80 (Sigma Aldrich) was diluted in 1 M HCl to a concentration of 40 mg/mL and then to saline for the final concentration.
SNI Model of Neuropathic Pain.
Mice were anesthetized with avertine (250 mg/kg), and the left limb was placed in a lateral position and immobilized. Using the knee as a landmark, an ∼1-cm incision was made in the longitudinal direction (34). The skin was cut, muscles were bluntly dissected, and the sciatic nerve was exposed distal to its trifurcation. Muscles and connective tissue were cleaned and the sural, common peroneal, and tibial nerves were exposed. Sural and common peroneal nerves were ligated (6.0 silk, Ethicon) and transected; the tibial nerve was left intact. Muscle and skin were closed by using a 4.0 silk suture (Ethicon).
Behavioral Paradigms.
Forced swim testing was conducted by placing mice in 4-L beakers containing ∼3 L of tap water at a temperature of 24 ± 1 °C. An independent experienced observer recorded immobility times for 5.5 min, starting 30 s after the beginning of the assay (35). For hyponeophagia experiments, animals were food deprived for 48 h and then placed into an open field, containing food in the center. The latency to eat the food was monitored for 5-min periods (36).
Stereotaxic Surgery and Viral-Mediated Gene Transfer.
AAV-Rgs4, AAV-GFP, and AAV-Cre were produced by using a triple transfection helper-free method in HEK cells and purified, as described (18, 37). Stereotaxic coordinates for vector injections into NAc were as follows: anteriorposterior +1.6 mm, lateral ± 1.5 mm, and dorsoventral −4.4 mm at an angle of 10° from the midline (relative to Bregma); for PFC, coordinates were as follows: +1.8 mm, lateral ± 0.2 mm, and dorsoventral −2.8 (prelimbic), and −3 mm (infralimbic) relative to Bregma. For all stereotaxic surgeries, mice were anesthetized with avertine and experiments were performed 2 wk later.
Western Blot Analysis.
Tissue from NAc and PFC was dissected, frozen on dry ice, and subsequently sonicated in buffer containing 1% SDS and 0.1% protease and phosphatase inhibitors and proteasome inhibitor (MG132; Sigma Aldrich) as described (18). Membranes were incubated in solutions of primary antibody: rabbit anti-RGS4 (1:1,000; S. Mumby, University of Texas Southwestern Medical Center; see Fig. S3 for antibody specificity), rabbit anti-RGS6/7 (Wyeth-Ayerst Pharmaceuticals), mouse anti–phospho-CREB (Ser-133) or total CREB (1:1,000; Cell Signaling), rabbit anti-phospho-Akt (Ser473) or total Akt (1:1,000; Cell Signaling), or mouse anti-β-tubulin antibody (1:20.000, Sigma-Aldrich). After further washes in tris-buffered saline-tween, membranes were incubated with peroxidase labeled goat anti-rabbit IgG or horse anti-mouse IgG (1:20,000; Invitrogen). Bands were visualized with SuperSignal West Dura substrate (Pierce).
Studies on Human Postmortem NAc.
Human brain specimens were obtained from the Dallas Brain Collection as described (38).
Information About qPCR Studies.
RNA isolation, RNA integrity number determination, and PCR, can be found on SI Methods and Table S1.
Statistical Analysis.
Two-way ANOVA was used to examine significant effects of treatment over genotype for all studies by using the forced swim, hyponeophagia, and Von Frey assays. Significant post hoc effects were revealed by the Bonferroni post hoc test. Two-way ANOVA or unpaired two-tailed Student t tests were used for comparisons between groups in Western blot analyses. One-way ANOVA was used for group comparisons in the qPCR experiments.
Supplementary Material
Acknowledgments
M.S. and A.V. are students of the Cellular and Genetic Etiology, Diagnosis and Treatment of Human Diseases Graduate Program at the University of Crete, Greece. V.M. is a student of the Molecular Biology and Biomedicine Graduate Program, University of Crete, Greece.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.T.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214696110/-/DCSupplemental.
References
- 1.Argoff CE. The coexistence of neuropathic pain, sleep, and psychiatric disorders: A novel treatment approach. Clin J Pain. 2007;23(1):15–22. doi: 10.1097/01.ajp.0000210945.27052.b3. [DOI] [PubMed] [Google Scholar]
- 2.Taymans JM, et al. Dopamine receptor-mediated regulation of RGS2 and RGS4 mRNA differentially depends on ascending dopamine projections and time. Eur J Neurosci. 2004;19(8):2249–2260. doi: 10.1111/j.0953-816X.2004.03336.x. [DOI] [PubMed] [Google Scholar]
- 3.Ding J, et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;9(6):832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 4.Gold SJ, et al. Regulation of RGS proteins by chronic morphine in rat locus coeruleus. Eur J Neurosci. 2003;17(5):971–980. doi: 10.1046/j.1460-9568.2003.02529.x. [DOI] [PubMed] [Google Scholar]
- 5.Georgoussi Z, et al. Selective interactions between G protein subunits and RGS4 with the C-terminal domains of the mu- and delta-opioid receptors regulate opioid receptor signaling. Cell Signal. 2006;18(6):771–782. doi: 10.1016/j.cellsig.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Volk DW, Eggan SM, Lewis DA. Alterations in metabotropic glutamate receptor 1α and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. Am J Psychiatry. 2010;167(12):1489–1498. doi: 10.1176/appi.ajp.2010.10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu Z, Jiang Q, Yan Z. RGS4 modulates serotonin signaling in prefrontal cortex and links to serotonin dysfunction in a rat model of schizophrenia. Mol Pharmacol. 2007;71(4):1030–1039. doi: 10.1124/mol.106.032490. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, et al. Adrenergic modulation of NMDA receptors in prefrontal cortex is differentially regulated by RGS proteins and spinophilin. Proc Natl Acad Sci USA. 2006;103(48):18338–18343. doi: 10.1073/pnas.0604560103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalli A, Druey KM, Milligan G. The regulator of G protein signaling RGS4 selectively enhances alpha 2A-adreoreceptor stimulation of the GTPase activity of Go1alpha and Gi2alpha. J Biol Chem. 2000;275(31):23693–23699. doi: 10.1074/jbc.M910395199. [DOI] [PubMed] [Google Scholar]
- 10.Hollinger S, Hepler JR. Cellular regulation of RGS proteins: Modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54(3):527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- 11.Terzi D, Stergiou E, King SL, Zachariou V. Regulators of G protein signaling in neuropsychiatric disorders. Prog Mol Biol Transl Sci. 2009;86:299–333. doi: 10.1016/S1877-1173(09)86010-9. [DOI] [PubMed] [Google Scholar]
- 12.Levitt P, Ebert P, Mirnics K, Nimgaonkar VL, Lewis DA. Making the case for a candidate vulnerability gene in schizophrenia: Convergent evidence for regulator of G-protein signaling 4 (RGS4) Biol Psychiatry. 2006;60(6):534–537. doi: 10.1016/j.biopsych.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Buckholtz JW, et al. Allelic variation in RGS4 impacts functional and structural connectivity in the human brain. J Neurosci. 2007;27(7):1584–1593. doi: 10.1523/JNEUROSCI.5112-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding L, Hegde AN. Expression of RGS4 splice variants in dorsolateral prefrontal cortex of schizophrenic and bipolar disorder patients. Biol Psychiatry. 2009;65(6):541–545. doi: 10.1016/j.biopsych.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Lerner TN, Kreitzer AC. RGS4 is required for dopaminergic control of striatal LTD and susceptibility to parkinsonian motor deficits. Neuron. 2012;73(2):347–359. doi: 10.1016/j.neuron.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: Region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17(20):8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni YG, et al. Region-specific regulation of RGS4 (Regulator of G-protein-signaling protein type 4) in brain by stress and glucocorticoids: in Vivo and in vitro studies. J Neurosci. 1999;19(10):3674–3680. doi: 10.1523/JNEUROSCI.19-10-03674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han MH, et al. Brain region specific actions of regulator of G protein signaling 4 oppose morphine reward and dependence but promote analgesia. Biol Psychiatry. 2010;67(8):761–769. doi: 10.1016/j.biopsych.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Covington HE, 3rd, et al. A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron. 2011;71(4):656–670. doi: 10.1016/j.neuron.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuzawa-Yanagida K, et al. Usefulness of antidepressants for improving the neuropathic pain-like state and pain-induced anxiety through actions at different brain sites. Neuropsychopharmacology. 2008;33(8):1952–1965. doi: 10.1038/sj.npp.1301590. [DOI] [PubMed] [Google Scholar]
- 22.Broom DC, et al. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002;26(6):744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- 23.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 24.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. aan het Rot M, et al. (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psych 67(2):139–145. [DOI] [PubMed]
- 26.DiazGranados N, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71(12):1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murrough JW, Perez AM, Mathew SJ, Charney DS. A case of sustained remission following an acute course of ketamine in treatment-resistant depression. J Clin Psychiatry. 2011;72(3):414–415. doi: 10.4088/JCP.10l06447blu. [DOI] [PubMed] [Google Scholar]
- 28.Blonk MI, Koder BG, van den Bemt PM, Huygen FJ. Use of oral ketamine in chronic pain management: A review. Eur J Pain. 2010;14(5):466–472. doi: 10.1016/j.ejpain.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Taymans JM, Leysen JE, Langlois X. Striatal gene expression of RGS2 and RGS4 is specifically mediated by dopamine D1 and D2 receptors: Clues for RGS2 and RGS4 functions. J Neurochem. 2003;84(5):1118–1127. doi: 10.1046/j.1471-4159.2003.01610.x. [DOI] [PubMed] [Google Scholar]
- 30.Schwendt M, McGinty JF. Regulator of G-protein signaling 4 interacts with metabotropic glutamate receptor subtype 5 in rat striatum: Relevance to amphetamine behavioral sensitization. J Pharmacol Exp Ther. 2007;323(2):650–657. doi: 10.1124/jpet.107.128561. [DOI] [PubMed] [Google Scholar]
- 31.Saugstad JA, Marino MJ, Folk JA, Hepler JR, Conn PJ. RGS4 inhibits signaling by group I metabotropic glutamate receptors. J Neurosci. 1998;18(3):905–913. doi: 10.1523/JNEUROSCI.18-03-00905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwendt M, Sigmon SA, McGinty JF. RGS4 overexpression in the rat dorsal striatum modulates mGluR5- and amphetamine-mediated behavior and signaling. Psychopharmacology (Berl) 2012;221(4):621–635. doi: 10.1007/s00213-011-2606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner EM, Blazer LL, Neubig RR, Husbands SM. Small molecule inhibitors of regulator of G protein signalling (RGS) proteins. ACS Med Chem Lett. 2012;3(2):146–150. doi: 10.1021/ml200263y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terzi D, Cao Y, Agrimaki I, Martemyanov KA, Zachariou V. R7BP modulates opiate analgesia and tolerance but not withdrawal. Neuropsychopharmacology. 2012;37(4):1005–1012. doi: 10.1038/npp.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnan V, et al. Calcium-sensitive adenylyl cyclases in depression and anxiety: Behavioral and biochemical consequences of isoform targeting. Biol Psychiatry. 2008;64(4):336–343. doi: 10.1016/j.biopsych.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onksen JL, Brown EJ, Blendy JA. Selective deletion of a cell cycle checkpoint kinase (ATR) reduces neurogenesis and alters responses in rodent models of behavioral affect. Neuropsychopharmacology. 2011;36(5):960–969. doi: 10.1038/npp.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9(12):1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 38.Stan AD, et al. Human postmortem tissue: What quality markers matter? Brain Res. 2006;1123(1):1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.