Abstract
In addition to their devastating effects on global biodiversity, mass extinctions have had a long-term influence on the history of life by eliminating dominant lineages that suppressed ecological change. Here, we test whether the end-Permian mass extinction (252.3 Ma) affected the distribution of tetrapod faunas within the southern hemisphere and apply quantitative methods to analyze four components of biogeographic structure: connectedness, clustering, range size, and endemism. For all four components, we detected increased provincialism between our Permian and Triassic datasets. In southern Pangea, a more homogeneous and broadly distributed fauna in the Late Permian (Wuchiapingian, ∼257 Ma) was replaced by a provincial and biogeographically fragmented fauna by Middle Triassic times (Anisian, ∼242 Ma). Importantly in the Triassic, lower latitude basins in Tanzania and Zambia included dinosaur predecessors and other archosaurs unknown elsewhere. The recognition of heterogeneous tetrapod communities in the Triassic implies that the end-Permian mass extinction afforded ecologically marginalized lineages the ecospace to diversify, and that biotic controls (i.e., evolutionary incumbency) were fundamentally reset. Archosaurs, which began diversifying in the Early Triassic, were likely beneficiaries of this ecological release and remained dominant for much of the later Mesozoic.
Keywords: biogeography, complex networks, macroevolution, biotic recovery, paleoecology
Mass extinctions are thought to reshape the composition and ecological structure of communities on a scale unparalleled by background extinction (1, 2). Within the terrestrial realm, the replacement of dinosaur-dominated communities by those of mammals after the end-Cretaceous extinction is perhaps the best-known example of such wholesale faunal reshuffling. By contrast, understanding the effects of the more massive end-Permian extinction on terrestrial community structure has been hampered by the paucity of high-quality geographic data, with nearly all studies restricted to sequences from single basins in Russia (3, 4) or South Africa (5–8). Although some broad similarities have emerged, such as the progressive aridification of the two basins (3, 8) and a heightened diversity of temnospondyl amphibians in the recovery interval (9), limited geographic sampling in each hemisphere constrains the ability of paleontologists to distinguish regional patterns from those characteristic of the individual basins. Moreover, these broadly separated areas are uninformative regarding large-scale (i.e., continent-level) patterns of faunal evolution.
Here we examine tetrapod faunal composition in five fossiliferous areas in southern Pangea approximately 5 million years before and 10 million years after the end-Permian mass extinction. We analyze changes in biogeographic structure with four metrics of taxon occurrence data. First, biogeographic connectedness (BC) quantifies the proportion of taxon-locality occurrences relative to the maximum number of such occurrences possible. Thus, at its extremes, faunas or floras where species are distributed across all localities show high connectedness, whereas when each species occurs at only a single locality, BC has a minimum value. A second, related factor of biogeographic structure is the propensity for species to have correlated geographic ranges. To measure this factor, we used a network clustering algorithm that measures the potential to compress a taxon-locality bipartite network into subunits. These measures have several advantages over most current biogeographic methods (10, 11) because the summary measures follow directly from the occurrence data of the taxa included, rather than being filtered through a similarity measure. We also analyzed the average number of basins in which species occur as well as the proportion of endemic taxa per locality. These four measures quantify complementary aspects of large-scale biogeographic structure across the Permo-Triassic boundary.
Data Collection
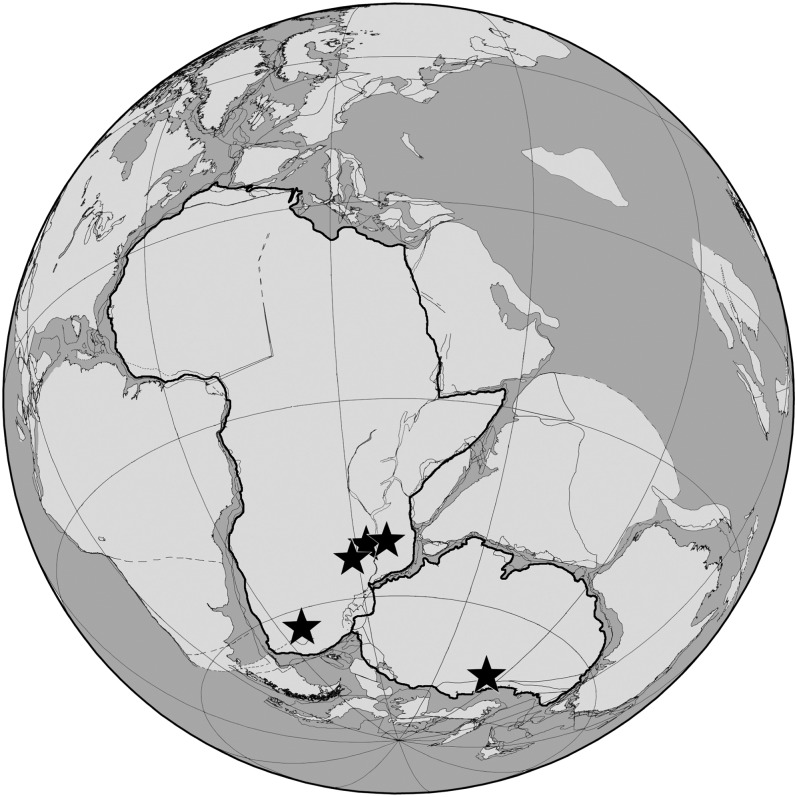
Tetrapod occurrence data were collected from the Upper Permian Cistecephalus Assemblage Zone (AZ) and the primarily Middle Triassic Cynognathus AZ of South Africa and their temporal equivalents (SI Text). These two zones are sufficiently separated from the end-Permian mass extinction to represent reasonable glimpses of pre- and postextinction assemblages. Indeed, the Cynognathus AZ is considered the first postextinction biozone to feature a stable global carbon cycle (12, 13). In addition to the Karoo, we collected data on the composition of tetrapod faunas in four other areas preserving fossiliferous Upper Permian and Middle Triassic beds, namely the (i) Luangwa Basin of Zambia, (ii) Ruhuhu Basin of Tanzania, (iii) Chiweta beds of Malawi, and (iv) Beacon Basin of Antarctica (see Fig. 1 for relative positions in southern Pangea).
Fig. 1.
Paleogeographic map of southern Pangea with stars indicating the positions of the five Permian and Triassic fossiliferous areas analyzed (from left to right: Karoo Basin of South Africa; Luangwa Basin of Zambia; Chiweta beds of Malawi; Ruhuhu Basin of Tanzania; Beacon Basin of Antarctica). Corresponding faunal data from India and Namibia are discussed in the SI Text. Modern outlines of Africa (excluding Madagascar) and Antarctica (excluding East Antarctica) are highlighted. Early Triassic paleogeography (∼250 Ma) is based on data originally published by Lawver et al. (40).
Data from the literature were extensively updated by over a decade of fieldwork and firsthand research of historical collections, both of which have yielded extensively revised occurrence lists as well as a variety of new taxa (14–18) (SI Text). Importantly, fieldwork has been able to precisely document the stratigraphic position of fossil assemblages such that the fossiliferous Permian beds of Malawi, Tanzania, and Zambia are now considered equivalent to the Cistecephalus AZ in South Africa (15, 17). In addition, our occurrence data benefit from the personal study of all of the relevant fossils and thus do not rely on the literature being up-to-date. We restricted our analysis to species-level taxa, as this is the most appropriate level for biogeographic analysis. However, the current state of gorgonopsid therapsid taxonomy hampered our ability to generate a species-level taxonomic list for each region for this group, and therefore we excluded this clade from our analysis. Based on our fieldwork, however, we suspect that at least two medium-sized gorgonopsids are shared between the four Permian areas used here. In total, we gathered occurrence data for 62 Permian and 68 Triassic species (Tables S1 and S2).
Network Methods for Assessing Biogeographic Structure
A number of measures have been proposed to summarize real-world networks. For example, in food webs species connectance is the number of observed relationships divided by the number that could conceivably occur (19), a property that is more generally referred to as the density of a network. To study biogeographic structure with bipartite (i.e., taxon-locality) occurrence networks, two key network statistics are required. First, to summarize the overlap of the geographic ranges of taxa, we rescale the density measure for species-locality bipartite networks. Second, to summarize network clustering (20), or the extent to which species are grouped biogeographically, we use the higher-order relationships (i.e., taxon-taxon cooccurrences) in the occurrence network.
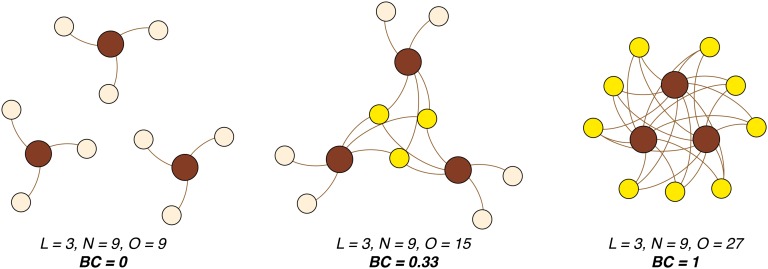
Biogeographic data are inherently bipartite (i.e., taxon-locality occurrences). The density of such a bipartite network can be visualized with the simple case given in Fig. 2. More formally, we rescale the bipartite density so that it is undefined when only a single locality is included (connectedness cannot be assessed for a single locality), and require all taxa to be present in at least one locality. When rescaled, the bipartite density measures the connectedness of the localities by the occurrence relationships of taxa. The rescaled bipartite density, which we introduce here as BC, is written
 |
where O is the number of links in the occurrence network (number of occurrences), N is the number of taxa, and L is the number of localities. The numerator is the number of occurrences of taxa beyond a single locality (hence why N is subtracted from O), and the denominator is the number of occurrences that could conceivably occur (LN), minus one occurrence for each species because each species must occur at least once. This measure is bounded between 0 (when O = N) and 1 (when O = LN), which correspond to extreme occurrence network topologies of minimum and maximum homogeneity, respectively (Fig. 2). Between these extremes, networks with a high proportion of endemic taxa will have values closer to zero, whereas more cosmopolitan faunas will have higher values (Fig. 2). The measure does not assume that L be a specific spatial scale, or that the taxonomic rank of N be constrained to the species level.
Fig. 2.
Bipartite networks exemplifying minimum to maximum scores of BC. Brown circles denote localities (geographic areas), tan circles denote taxa that occur at a single locality, and yellow circles denote taxa present at two or more localities. A taxon is connected to a locality if it occurs there.
BC is not a sufficient measure on its own to summarize biogeographic structure because a fauna or flora with different biogeographic structure but equivalent geographic range distributions can produce the same value. To more completely assess biogeographic structure, we used the map equation, an information-theoretic approach for clustering networks (20–22). When a network can be compressed into subunits with minimal information loss, it has rich cluster structure. For our purpose, a bipartite occurrence network with a low code length (measured in bits) can be compressed into more distinct biogeographic subunits than a bipartite occurrence network that clusters with a high coding length. Thus, changes in map equation scores serve as a useful proxy for differences in biogeographic clustering.
Results
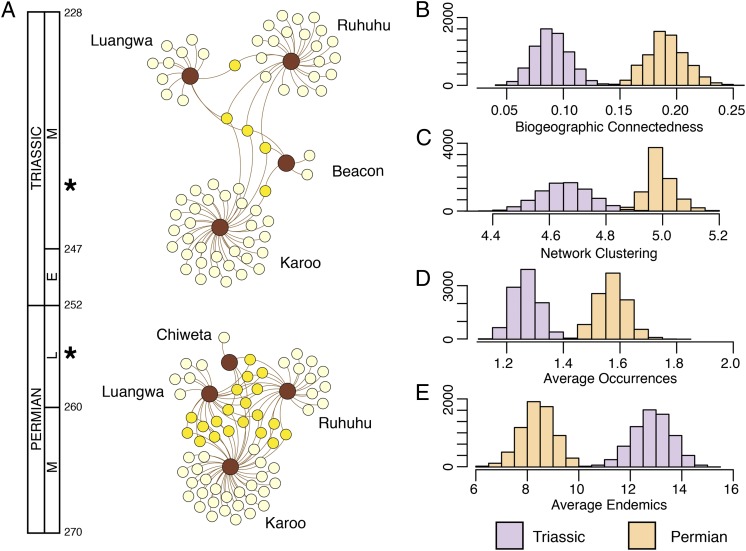
Fig. 3A shows the bipartite networks generated from our faunal occurrence data. The Late Permian network includes a substantial percentage of species (21 of 62) that are shared by at least two basins. It is worth noting that the South African Karoo has a very long history of paleontological work (23–25), which helps to explain its large number of endemic taxa (n = 26) compared with the comparatively under-sampled Malawian, Tanzanian, and Zambian basins. In contrast, the Middle Triassic network shows clear geographic clustering and very few species shared between regions (5 of 68), with only the cynodont therapsid Diademodon shared by all four. Equally interesting is the remarkably divergent nature of the Tanzanian and, to a lesser degree Zambian, fossil record. Crown group archosaurs are diverse and abundant in the Manda beds of Tanzania (16), which is in stark contrast to their absence from contemporaneous South African strata.
Fig. 3.
Biogeographic structure of southern Pangean tetrapod assemblages before and after the Permian-Triassic mass extinction. (A) Late Permian terrestrial vertebrate assemblages show high connectedness and are similar across regions. Early Middle Triassic faunas show increased biogeographic separation that corresponds to faunal differentiation among fossiliferous basins. Asterisks denote approximate faunal horizon on the geological timescale (geochronology from ref. 41). Color codes correspond to those in Fig. 2. E, Early; L, Late; M, Middle. (B–E) Histograms depicting the distribution of bootstrap analyses of four measures of biogeographic structure. Values for network clustering are given in bits (22), with higher values indicating less distinct subunits in the bipartite network. Recovery from the end-Permian crisis resulted in significant differences in biogeographic connectedness, network clustering, range size, and proportion of endemics (Welch two sample t test, P << 0.01 for each; bootstrap data were normally distributed), indicating increased provincialism in the Triassic.
Our analysis revealed a decrease in BC from 0.16 in the Late Permian to 0.04 in the Middle Triassic. Correspondingly, our analysis showed increased network clustering across the mass-extinction boundary, with code length decreasing from 4.99 to 4.66. To test for significance, we resampled the occurrence data, such that for each presence of a species in a locality, we removed that presence with 5% probability. If a species was reduced to zero occurrences, it did not count toward the sample size. For each absence of a species in a locality, we included the species with 5% probability. Ten-thousand bootstrap replicates were reanalyzed and showed statistically significant differences between the Permian and Triassic in both the BC and code-length values (P < 0.01) (Fig. 3 B and C). Our data show that on average, Middle Triassic tetrapod species had smaller geographic ranges, measured as the number of locality (namely, geologic basin) occurrences, compared with their Late Permian forebears (Fig. 3D). Permian tetrapods of southern Pangea occurred in an average of 1.49 basins, whereas Triassic species occurred in an average of 1.13 basins. Bootstrapping the underlying occurrence datasets suggests that these differences are significantly different (P < 0.01). We also examined the proportion of species endemic to a single geographic locality. In the Permian, just over one-third (0.37) of the species studied occurred in a single locality, whereas in the Triassic the same value rose to almost three-quarters (0.73). Again, bootstrapping indicates that this change is significant (P < 0.01) (Fig. 3E). All four measures of biogeographic structure support the proposal that Triassic tetrapod assemblages of southern Pangea were significantly more heterogeneous and provincial than their Permian antecedents.
Discussion
Analyzing Biogeographic Structure.
Quantitative studies of community similarity and biogeographic structure frequently make use of a combination of similarity or distance metrics and cluster analyses (10, 11). A wide range of similarity and distance metrics have been proposed, which use differing definitions of similarity and make various assumptions about issues such as the weighting of joint occurrences or mismatches. However, it is often unclear which metric or metrics might be most appropriate in a given case, and when different metrics give differing results it is often difficult to determine which result is more strongly supported. These metrics also tend to obscure the actual geographic ranges of the constituent species and can be susceptible to differences in the number of species per basin (a problem for paleontological analyses that consistently underestimate richness). When combined with the variety of methods available for clustering, a researcher may be faced with an impractically large number of conflicting dendrograms, each based on different assumptions and emphasizing various aspects of faunal similarity (10).
The network-based method we adopted here has several advantages over more traditional approaches. For example, it uses the occurrence distribution data directly, instead of filtering it through a distance calculation, and can also take into account indirect connections between communities (i.e., species that do not cooccur in a given location but are likely part of a larger regional assemblage on the basis of cooccurrences with more widely distributed species). The use of descriptive network statistics also provides more detailed insight into the underlying structure of biogeographic assemblages, and network diagrams provide an easy-to-interpret means of representing similarities and differences between faunas at various localities. In the current case, our network results show that the higher dissimilarity of Triassic communities relative to Permian ones was driven by a significant decrease in the geographic ranges of taxa and a fundamental change in the nature of the faunal connections between the basins we investigated. Interestingly, these results predict that the biostratigraphic correlation of mid-Triassic rocks should be more difficult than those of the Permian, as widespread tetrapod species became less common.
Permo-Triassic Crisis.
The end-Permian event had a devastating effect on global biodiversity, with estimates of over 90% species extinction among marine invertebrates and over 70% in terrestrial animals (but significantly less among plants) (3–7, 26–28). Recent work has suggested that recovery was delayed because of prolonged environmental disturbance, manifested by wide fluctuations in the global carbon cycle that persisted for most of the Early Triassic (13, 29), and that substantial ecosystem diversity was not regained until about 8-million-years later in the Middle Triassic (12, 13, 30, 31). On land, the recovery of terrestrial vertebrates has been studied extensively in the Karoo Basin of South Africa. In that basin, only four therapsid genera are known to have survived the end-Permian extinction (5–7, 32), which implies that immigration substantially contributed to postextinction faunas. Indeed, coeval rocks in Antarctica have produced fossils suggestive of a high-latitude refugium (14, 33).
The lack of well-sampled, coeval basins has hamstrung the attempts of vertebrate paleontologists to understand how the end-Permian mass extinction affected the geographic distribution of tetrapod faunas. As a result, patterns documented in the Karoo Basin have necessarily been considered representative of the southern hemisphere as a whole (5, 12, 24). Our work has shown that the four basins preserving Upper Permian fossils (Malawi, South Africa, Tanzania, Zambia) were, despite markedly different basin architecture and fill, characterized by a single, highly interconnected community during the Permian. Furthermore, the tetrapod fauna was broadly distributed (e.g., ∼2,600 km between the Tanzanian and South African basins) and dominated by dicynodont herbivores, with taxa such as Pristerodon, Oudenodon, Dicynodontoides, and Endothiodon being especially wide-ranging (17).
Postextinction terrestrial vertebrate community structure changed in southern Pangea in two important ways. First, as previously shown for tetrapods (3, 4, 12, 17) the taxonomic composition of assemblages preserved in each basin underwent wholesale revision; there are no species (or genera) found in both Upper Permian and Middle Triassic rocks. Among basins, a second, larger-scale transition also occurred. Our results demonstrate the balkanization of Triassic terrestrial vertebrate faunas, with different communities present in each basin (although the depauperate Antarctic fauna is the least differentiated from that of the Karoo). Compared with the Permian data from the same basins, the number of shared species is dramatically reduced and proportion of endemics increased. Even the Middle Triassic of Antarctica, which is by far the least well understood (14, 34), has two unique species of five total. Data for the Lower Triassic “disaster interval” are more limited, but suggest a relatively cosmopolitan tetrapod fauna with Lystrosaurus as its hallmark. Thus, the transition from widespread to endemic tetrapod assemblages did not directly stem from the extinction event, but instead coincided with the restoration of ecological stability during the recovery process (30, 35) (SI Text).
Evolutionary Incumbency and the Rise of Archosaurs.
The timing of the archosaur radiation has received renewed attention with the recent discovery of Middle Triassic dinosauriforms (16, 18, 36) as well as Early Triassic footprints ascribed to dinosauromorphs (37) and body fossils of poposauroid pseudosuchians (38). All of these findings suggest that crown-group archosaur diversification was more intimately related to recovery from the end-Permian mass extinction than previously suspected. Importantly, this early diversification included not only phylogenetic diversification, with at least five archosaur ghost lineages drawn back to the Early Triassic, but ecological expansion into a variety of carnivorous, omnivorous, and herbivorous niches (16, 39).
Decimation of therapsid-dominated Late Permian ecosystems left vacant ecospace that was only partially filled in the earliest Triassic by Lystrosaurus and its associated fauna (23–25, 35). Lystrosaurus is broadly distributed (e.g., China, India, Russia, South Africa) and considered a disaster taxon adapted to the perturbed environments typifying most of the Early Triassic (4, 7, 33). Proterosuchus, an early archosauriform, cooccurs with Lystrosaurus and is found in the first 10 m of the Triassic in South Africa (5, 6), despite having no Permian predecessors there. Middle Triassic archosauriforms, such as Erythrosuchus and Euparkeria, are similarly found in the Karoo, but despite intensive collecting for over a century, unambiguous crown-group archosaurs have yet to be recovered from that basin. Our research in the Middle Triassic of Tanzania and Zambia has uncovered an unsuspected taxonomic and ecological diversity of archosaurs (16, 18, 36), indicating that the Karoo may not serve as a useful model system for understanding postextinction diversification. Although data rich, patterns of vertebrate recovery established within the Karoo Basin of South Africa and the south Urals of Russia fail to capture the geographic complexity of the recovery process. Data from undersampled basins show that the composition of tetrapod faunas varied to a greater degree in the Triassic than in the Permian, and that the intervening mass extinction likely dislodged therapsid incumbents and set the stage for a spatially heterogeneous recovery.
Supplementary Material
Acknowledgments
We thank L. Nampunju, C. Sanaane, and A. Tibaijuka for facilitating fieldwork in Tanzania; A. Goulding, J. Museba, K. Mwamulowe, and S. Tolan, for support in Zambia; and the governments of Tanzania and Zambia for permission to conduct fieldwork. This research would not have been possible without access to fossils housed at a variety of museums and institutions. For access to collections, we thank the following museum personnel: C. Mehling and M. Norell of the American Museum of Natural History; B. Rubidge and B. Zipfel of the Bernard Price Institute for Palaeontological Research; M. Maisch at the Geologisch-Paläontologisches Institut Tübingen; P. Barrett, S. Chapman, and A. Milner of the Natural History Museum of the United Kingdom; T. Kemp of the Oxford University Museum of Natural History; S. Kaal of the Iziko South African Museum; and R. Asher and M. Lowe of the University of Cambridge Museum of Zoology. This research was funded by National Geographic Society Grants CRE 8571-088 (to J.S.S.) and CRE 7787-05 and 8962-11 (to C.A.S.); The Grainger Foundation (K.D.A.); the Field Museum/IDP, Inc. African Partner’s Program (K.D.A.); the Evolving Earth Foundation (S.J.N.); National Science Foundation Grants EAR 1024036, ANT 0838762, and 1146399 (to C.A.S.); and the National Research Council (R.M.H.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302323110/-/DCSupplemental.
References
- 1.Jablonski D. Background and mass extinctions: The alternation of macroevolutionary regimes. Science. 1986;231(4734):129–133. doi: 10.1126/science.231.4734.129. [DOI] [PubMed] [Google Scholar]
- 2.Jablonski D. Mass extinctions and macroevolution. Paleobiology. 2005;31(Suppl 2):192–210. [Google Scholar]
- 3.Benton MJ, Tverdokhlebov VP, Surkov MV. Ecosystem remodelling among vertebrates at the Permian-Triassic boundary in Russia. Nature. 2004;432(7013):97–100. doi: 10.1038/nature02950. [DOI] [PubMed] [Google Scholar]
- 4.Sahney S, Benton MJ. Recovery from the most profound mass extinction of all time. Proc Biol Sci. 2008;275(1636):759–765. doi: 10.1098/rspb.2007.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward PD, et al. Abrupt and gradual extinction among Late Permian land vertebrates in the Karoo basin, South Africa. Science. 2005;307(5710):709–714. doi: 10.1126/science.1107068. [DOI] [PubMed] [Google Scholar]
- 6.Smith RMH, Botha J. The recovery of terrestrial vertebrate diversity in the South African Karoo Basin after the end-Permian extinction. C R Palevol. 2005;4(6-7):623–636. [Google Scholar]
- 7.Botha J, Smith RMH. Rapid vertebrate recuperation in the Karoo Basin of South Africa following the end-Permian extinction. J Afr Earth Sci. 2006;45(4-5):502–514. [Google Scholar]
- 8.Smith RMH. Changing fluvial environments across the Permian-Triassic boundary in the Karoo Basin, South Africa and possible causes of tetrapod extinctions. Palaeogeogr Palaeoclimatol Palaeoecol. 1995;117(1-2):81–104. [Google Scholar]
- 9.Ruta M, Benton MJ. Calibrated diversity, tree topology and the mother of mass extinctions: The lesson of temnospondyls. Palaeontology. 2008;51(6):1261–1288. [Google Scholar]
- 10.Shi GR. Multivariate data analysis in palaeoecology and palaeobiogeography—A review. Palaeogeogr Palaeoclimatol Palaeoecol. 1993;105(3-4):199–234. [Google Scholar]
- 11.Kreft H, Jetz W. A framework for delineating biogeographical regions based on species distributions. J Biogeogr. 2010;37(11):2029–2053. [Google Scholar]
- 12.Irmis RB, Whiteside JH. Delayed recovery of non-marine tetrapods after the end-Permian mass extinction tracks global carbon cycle. Proc Biol Sci. 2012;279(1732):1310–1318. doi: 10.1098/rspb.2011.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Payne JL, et al. Large perturbations of the carbon cycle during recovery from the end-permian extinction. Science. 2004;305(5683):506–509. doi: 10.1126/science.1097023. [DOI] [PubMed] [Google Scholar]
- 14.Sidor CA, Damiani R, Hammer WR. A new Triassic temnospondyl from Antarctica and a review of Fremouw Formation biostratigraphy. J Vertebr Paleontol. 2008;28(3):656–663. [Google Scholar]
- 15.Sidor CA, et al. Tetrapod fauna of the lowermost Usili Formation (Songea Group, Ruhuhu Basin) of southern Tanzania, with a new burnetiid record. J Vertebr Paleontol. 2010;30(3):696–703. [Google Scholar]
- 16.Nesbitt SJ, et al. Ecologically distinct dinosaurian sister group shows early diversification of Ornithodira. Nature. 2010;464(7285):95–98. doi: 10.1038/nature08718. [DOI] [PubMed] [Google Scholar]
- 17.Angielczyk KD, et al. Permian and Triassic dicynodont (Therapsida: Anomodontia) faunas of the Luangwa Basin, Zambia: Taxonomic update and implications for dicynodont biogeography and biostratigraphy. In: Kammerer CF, Angielczyk KD, Fröbisch J, editors. The Early Evolutionary History of Synapsida. Dordrecht: Springer; in press. [Google Scholar]
- 18.Peecook BR, et al. A new silesaurid from the upper Ntawere Formation of Zambia (Middle Triassic) demonstrates the rapid diversification of Silesauridae (Avemetarsalia: Dinosauriformes) J Vertebr Paleontol. 2013;33 in press. [Google Scholar]
- 19.Dunne JA, Williams RJ, Martinez ND. Food-web structure and network theory: The role of connectance and size. Proc Natl Acad Sci USA. 2002;99(20):12917–12922. doi: 10.1073/pnas.192407699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortunato S. Community detection in graphs. Phys Rep. 2010;486(3-5):75–174. [Google Scholar]
- 21.Lancichinetti A, Fortunato S. Community detection algorithms: A comparative analysis. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;80(5 Pt 2):056117. doi: 10.1103/PhysRevE.80.056117. [DOI] [PubMed] [Google Scholar]
- 22.Rosvall M, Bergstrom CT. Maps of random walks on complex networks reveal community structure. Proc Natl Acad Sci USA. 2008;105(4):1118–1123. doi: 10.1073/pnas.0706851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubidge BS. Re-uniting lost continents—Fossil reptiles from the ancient Karoo and their wanderlust. S Afr J Geol. 2005;108(1):135–172. [Google Scholar]
- 24.Smith R, Rubidge B, van der Walt M. Therapsid biodiversity patterns and paleoenvironments of the Karoo Basin, South Africa. In: Chinsamy-Turan A, editor. Forerunners of Mammals: Radiation, Histology, Biology. Indianapolis: Indiana Univ Press; 2011. pp. 30–62. [Google Scholar]
- 25.Hancox PJ, Rubidge BS. Breakthroughs in the biodiversity, biogeography, biostratigraphy, and basin analysis of the Beaufort Group. J Afr Earth Sci. 2001;33(3-4):563–577. [Google Scholar]
- 26.Jin YG, et al. Pattern of marine mass extinction near the Permian-Triassic boundary in South China. Science. 2000;289(5478):432–436. doi: 10.1126/science.289.5478.432. [DOI] [PubMed] [Google Scholar]
- 27.King GM. Terrestrial tetrapods and the end Permian event: A comparison of analyses. Hist Biol. 1991;5(2-4):239–255. [Google Scholar]
- 28.McElwain JC, Punyasena SW. Mass extinction events and the plant fossil record. Trends Ecol Evol. 2007;22(10):548–557. doi: 10.1016/j.tree.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Shen S-Z, et al. Calibrating the end-Permian mass extinction. Science. 2011;334(6061):1367–1372. doi: 10.1126/science.1213454. [DOI] [PubMed] [Google Scholar]
- 30.Roopnarine PD, Angielczyk KD, Wang SC, Hertog R. Trophic network models explain instability of Early Triassic terrestrial communities. Proc Biol Sci. 2007;274(1622):2077–2086. doi: 10.1098/rspb.2007.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z-Q, Benton MJ. The timing and pattern of biotic recovery following the end-Permian mass extinction. Nat Geosci. 2012;5:375–383. [Google Scholar]
- 32.Huttenlocker AK, Sidor CA, Smith RMH. A new specimen of Promoschorhynchus (Therapsida: Therocephalia: Akidnognathidae) from the Early Triassic of South Africa and its implications for theriodont survivorship across the Permian-Triassic boundary. J Vertebr Paleontol. 2011;31(2):405–421. [Google Scholar]
- 33.Fröbisch J, Angielczyk KD, Sidor CA. The Triassic dicynodont Kombuisia (Synapsida, Anomodontia) from Antarctica, a refuge from the terrestrial Permian-Triassic mass extinction. Naturwissenschaften. 2010;97(2):187–196. doi: 10.1007/s00114-009-0626-6. [DOI] [PubMed] [Google Scholar]
- 34.Hammer WR. Triassic terrestrial vertebrate faunas of Antarctica. In: Taylor TN, Taylor EL, editors. Antarctic Paleobiology: Its role in the Reconstruction of Gondwana. New York: Springer; 1990. pp. 42–50. [Google Scholar]
- 35.Roopnarine PD, Angielczyk KD. The evolutionary palaeoecology of species and the tragedy of the commons. Biol Lett. 2012;8(1):147–150. doi: 10.1098/rsbl.2011.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nesbitt SJ, Barrett PM, Werning S, Sidor CA, Charig AJ. The oldest dinosaur? A Middle Triassic dinosauriform from Tanzania. Biol Lett. 2013;9(1):20120949. doi: 10.1098/rsbl.2012.0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brusatte SL, Niedźwiedzki G, Butler RJ. Footprints pull origin and diversification of dinosaur stem lineage deep into Early Triassic. Proc Biol Sci. 2011;278(1708):1107–1113. doi: 10.1098/rspb.2010.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler RJ, et al. The sail-backed reptile Ctenosauriscus from the latest Early Triassic of Germany and the timing and biogeography of the early archosaur radiation. PLoS ONE. 2011;6(10):e25693. doi: 10.1371/journal.pone.0025693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nesbitt SJ. The early evolution of archosaurs: Relationships and the origin of major clades. Bull Am Mus Nat Hist. 2011;352:1–292. [Google Scholar]
- 40.Lawver LA, Dalziel IWD, Norton IO, Ganagan LM. The PLATES 2009 atlas of plate reconstructions (750 Ma to present day), PLATES Progress Report No. 325-0509. Univ of Texas Technical Report. 2009;196:1–32. [Google Scholar]
- 41.Rubidge BS, Erwin DH, Ramezani J, Bowring SA, de Klerk WJ. High-precision temporal calibration of Late Permian vertebrate biostratigraphy: U-Pb zircon constraints from the Karoo Supergroup, South Africa. Geology. 2013 doi: 10.1130/G33622.1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.