Abstract
Staphylococcus aureus is a significant infectious threat to global public health. Acquisition or synthesis of heme is required for S. aureus to capture energy through respiration, but an excess of this critical cofactor is toxic to bacteria. S. aureus employs the heme sensor system (HssRS) to overcome heme toxicity; however, the mechanism of heme sensing is not defined. Here, we describe the identification of a small molecule activator of HssRS that induces endogenous heme biosynthesis by perturbing central metabolism. This molecule is toxic to fermenting S. aureus, including clinically relevant small colony variants. The utility of targeting fermenting bacteria is exemplified by the fact that this compound prevents the emergence of antibiotic resistance, enhances phagocyte killing, and reduces S. aureus pathogenesis. Not only is this small molecule a powerful tool for studying bacterial heme biosynthesis and central metabolism; it also establishes targeting of fermentation as a viable antibacterial strategy.
Keywords: glycolysis, high-throughput screen (HTS), two-component system (TCS), heme oxygenase
The high incidence of hospital- and community-acquired infections caused by methicillin-resistant Staphylococcus aureus (MRSA) underscores the importance of identifying novel targets for the treatment of this microbial threat (1). Upon breaching the epithelium of its host, S. aureus has the potential to infect virtually any tissue. This adaptability reflects the ability of S. aureus to sense a variety of environmental signals and integrate them into its metabolic program, enabling growth in diverse host niches.
To cause disease, S. aureus requires nutrient iron as a cofactor for proteins involved in replication, metabolism, and protection against reactive oxygen species (2). Within vertebrates, the majority of iron is sequestered from invading pathogens as heme bound to hemoglobin. S. aureus captures hemoglobin and transports heme into the cell (3). S. aureus also synthesizes heme endogenously through the coordinated effort of enzymes encoded by the hemAXCDBL, hemEHY, and hemN operons (4). The ability to exogenously acquire and endogenously synthesize heme allows S. aureus to satisfy cellular iron and heme requirements in diverse environments. This strategy is typical for most bacterial pathogens and reflects the integral role of heme in metabolism and physiology; however, the distinct contributions of endogenous and exogenous heme to the cellular physiology of bacteria are unknown.
Heme is a cofactor required for respiration. Furthermore, S. aureus respiration requires that the bacteria either synthesize or assimilate the electron carrier menaquinone (MK) and that a terminal electron acceptor be available. During respiration, reducing equivalents derived from the oxidation of carbon sources are donated to MK. The shuttling of electrons by MK through the electron transport chain generates a proton motive force (pmf) across the cytoplasmic membrane. The energy stored in the pmf powers ATP synthesis and nutrient import. When heme, MK, or terminal electron acceptors are absent, S. aureus generates energy through fermentation. Fermentation employs substrate-level phosphorylation, which produces acid end products, to generate ATP and maintain the redox balance of the cell.
Small colony variants (SCVs) of S. aureus are spontaneous, slow-growing mutants associated with persistent and recurrent S. aureus infections that are recalcitrant to antibiotic therapy (5). Their slow growth is often due to lesions that eliminate respiration, such as inactivation of MK or heme biosynthesis. SCVs are not exclusive to S. aureus as they have been isolated in other pathogens, including Escherichia coli, Neisseria gonorrhoeae, Pseudomonas aeruginosa, and Salmonella enterica serovar Typhimurium (5, 6).
Without heme, many central metabolic pathways and enzymes cannot function; however, excess heme is toxic due to its reactive nature. Prior exposure of S. aureus to subinhibitory concentrations of heme increases heme tolerance (7). This adaptation is due to the increased expression of the heme regulated transporter HrtAB, an efflux pump that protects the bacteria from heme toxicity. The increased expression of hrtAB is mediated by the heme sensor system (Hss) two-component system (TCS). Comprised of the HssS-sensor kinase and HssR-response regulator, HssRS is activated when the bacteria are exposed to heme through an as-yet-unidentified mechanism (7). Functional hss/hrt systems have been described in members of the genera Staphylococcus, Bacillus, Listeria, Enterococcus, and Streptococcus, establishing HssRS as a conserved heme responsive system in Gram-positive bacteria (7, 8).
In this study, we probed the mechanism of HssS stimulation by identifying small molecule activators of HssS. The most potent compound, VU0038882 (‘882), activates HssRS by inducing endogenous heme biosynthesis in S. aureus, leading to increased intracellular heme levels. The metabolic alterations induced by ‘882 are toxic to fermenting S. aureus. Therapeutic development of ‘882 has revealed that this compound is synergistic with known respiration inhibitors, is highly antimicrobial against fermenting bacteria including SCVs, and virtually eliminates the development of resistance to aminoglycoside antibiotics. Notably, a derivative of ‘882 reduces bacterial burdens in a systemic model of staphylococcal infection, underscoring the therapeutic value of targeting bacterial fermentation.
Results
High-Throughput Screen Identifies Activators of HssRS.
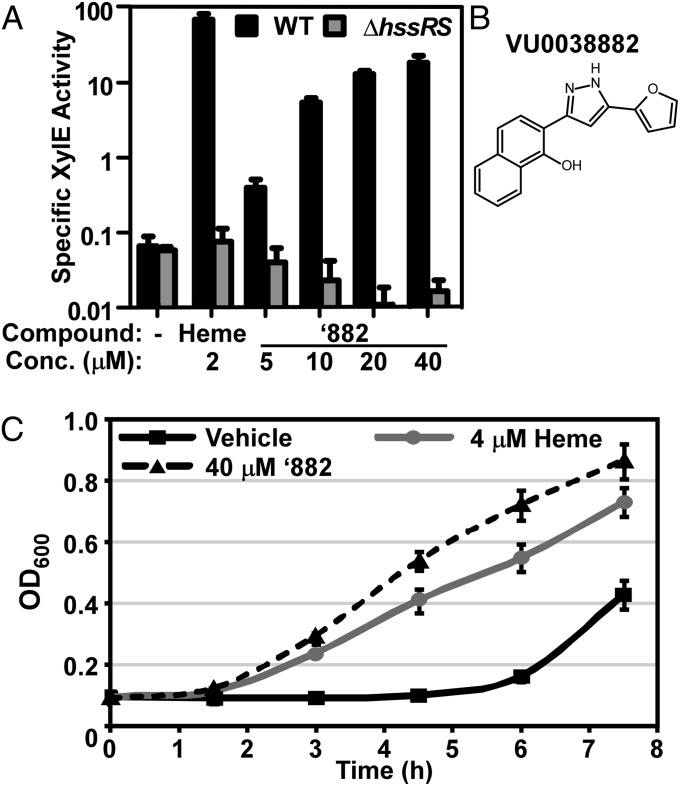
To perform a high-throughput screen (HTS) for small molecule activators of S. aureus HssRS, we created an hrtAB-driven expression system in the pXen-1 vector, which contains the Photorhabdus luminescens luciferase operon (luxABCDE) that produces blue-green light when expressed (Fig. S1A). This construct was used to screen a library of ∼160,000 small molecules and resulted in the identification of 250 positive hits. Based on luminescence values, the top 110 hits were subjected to a secondary screen using an xylE reporter assay to eliminate compounds that generated nonspecific luminescence (7). Hits that passed this secondary screen were further tested in a tertiary screen for their ability to adapt S. aureus to heme toxicity by growth curve analyses (7). Of all of the compounds screened, ‘882 was the most potent activator of hrtAB expression. ‘882 activates the hrtAB promoter in a dose-responsive manner requiring HssRS and preadapts S. aureus for heme toxicity (Fig. 1). These properties were observed for both commercially purchased and independently synthesized preparations of ‘882 (Fig. S2). These results establish ‘882 as a small molecule activator of the HssRS-dependent heme stress response.
Fig. 1.
A high-throughput screen identifies small molecule activators of HssRS. (A) A secondary screen consisting of an XylE reporter assay verified the activity of the top 110 hits from the primary screen, including ‘882. Triplicate cultures of S. aureus WT and ∆hssR transformed with the hrtAB promoter-xylE fusion-containing plasmid (phrt.xylE) were grown in the presence of the indicated additive, and XylE activity was measured. (B) The structure of lead compound VU0038882 (‘882). (C) ‘882 was confirmed as the top hit in a tertiary screen that measured the ability of the compound to preadapt S. aureus for growth in 20 μM heme. Triplicate cultures of WT S. aureus were grown overnight in medium containing the indicated additive and subcultured into medium containing 20 μM heme. Growth was monitored by measuring the optical density at 600 nm (OD600) over time. (A and C) Error bars represent one SD from the mean.
‘882 Stimulates Heme Biosynthesis to Activate HssRS.
To determine the mechanism by which ‘882 activates HssRS, the residues required for HssS heme sensing were identified. An alignment of the HssS extracytoplasmic domain from members of the Firmicutes encoding putative hss/hrt systems was used to predict residues required for heme sensing in S. aureus. Alanine substitution mutants were generated at 10 highly conserved residues and 1 nonconserved residue (Fig. S3A). These HssS variants were expressed in S. aureus ∆hssS, and their expression levels were compared with wild type by immunoblot (Fig. S3B). Next, their responsiveness to heme was quantified by XylE activity and growth curve analyses (Fig. S3 C–E). Analogous to Bacillus anthracis HssS, residues T125, F128, and R163 are required for heme sensing and HssS function in S. aureus (9). To gain insight into the mechanism by which ‘882 activates HssS, the ability of ‘882 to activate HssS substitution mutants with heme sensing defects was probed. R94A, T125A, and F165A were chosen for this analysis as they represent three mutations that reduce HssS heme sensing to different degrees. The impact of these mutations on HssS activation by ‘882 mirrored that observed upon heme exposure (Fig. S3F). This result indicates that heme and ‘882 trigger HssS signaling through similar residues despite being structurally distinct.
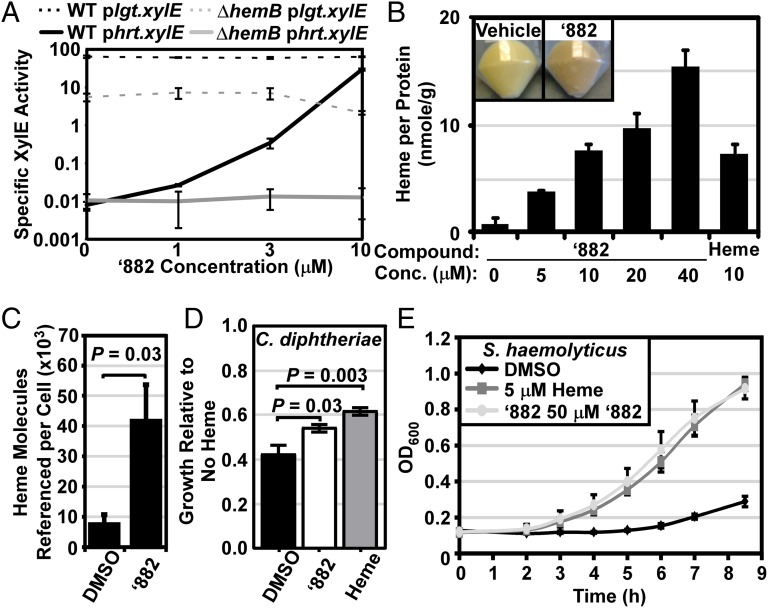
This observation suggests that ‘882 triggers HssRS through a mechanism similar to that of heme. A potential explanation is that ‘882 is a small molecule activator of endogenous heme synthesis. To test this model, the hrtAB promoter-xylE fusion reporter plasmid (phrt.xylE) was transformed into the heme auxotroph hemB::ermC (∆hemB), and HssRS activation was evaluated upon ‘882 exposure (10). In contrast to wild-type S. aureus, ‘882 does not activate HssRS in ∆hemB (Fig. 2A). The loss of ‘882-mediated activation of HssRS in ∆hemB is not due to the slower growth rate of this strain as XylE activity from a constitutively expressed xylE (plgt.xylE) is only modestly decreased (Fig. 2A). Furthermore, exogenous heme activates HssRS in ∆hemB, indicating that ∆hemB HssRS is still able to sense heme (Fig. S1B). These observations are consistent with a model whereby ‘882 exposure leads to an increase in intracellular heme and subsequent HssS activation. In support of this model, intracellular heme levels increase in a dose-responsive manner in bacteria treated with ‘882 (Fig. 2 B and C). Moreover, ‘882 exposure leads to a darkening of S. aureus pellets indicative of massive heme accumulation in these cells (Fig. 2B, Inset). The activation of HssS by ‘882 is not due to enzymatic degradation of heme as ‘882 still activates HssS in a strain of S. aureus lacking all heme oxygenases (Fig. S1C) (11).
Fig. 2.
‘882 acts through endogenous heme biosynthesis to activate HssRS and stimulate heme production. (A) S. aureus wild type (WT, black lines) and the heme auxotroph hemB::ermC (∆hemB, gray lines) were transformed with plasmids constitutively expressing XylE (plgt.xylE, dashed lines) or with xylE under the control of the hrtAB promoter (phrt.xylE, solid lines). Triplicate cultures of these strains were grown in the presence of ‘882, and XylE activity was measured. (B) Heme levels in triplicate cultures of S. aureus treated with the indicated additive were quantified using the pyridine hemochromogen assay and normalized to the concentration of protein in the whole cell lysates. (Inset) Pellets from a culture grown with 40 µM ‘882 are darker compared with vehicle-treated S. aureus. (A and B) Error bars represent 1 SD from the mean. (C) Exact-mass mass spectrometric analysis was used in conjunction with ultra-high performance liquid chromatography (UPLC) to detect and quantify heme in protoplasts from cells treated with 40 µM ‘882 or vehicle grown in triplicate. Measured heme molecules were referenced to estimated cfus per pellet, factoring in dilutions. Dead or lysed cells would also contribute to the measurement. Therefore the measured numbers are considered upper estimates. Samples measured in duplicate or triplicate injections had typical errors of <5% between analytical replicates. Error bars represent the SD, and significance was calculated using a two-tailed Student’s t test. (D) Adaptation by heme and ‘882 in C. diphtheriae was tested by growth analyses. Triplicate cultures were grown overnight in medium containing vehicle, 5 µM heme or 50 µM ‘882 and subcultured into medium containing 15 μM heme. The cfus were enumerated 2.5 h after inoculation and normalized to cultures unexposed to heme. Shown is the average of five replicates; error bars represent SEM and significance was determined by a two-tailed Student’s t test. (E) Adaptation by heme and ‘882 in S. haemolyticus was tested by growth analyses. Triplicate cultures were grown overnight in medium containing the indicated additive and subcultured into medium containing 30 μM heme. Growth was monitored by measuring the optical density at 600 nm (OD600) over time. Error bars represent 1 SD from the mean.
To determine whether the endogenous heme produced as a result of ‘882 exposure is available for use in cellular processes, cytoplasmic heme availability was measured by quantifying intracellular levels of the cytoplasmic iron-regulated surface determinate system heme oxygenase, IsdG. In the absence of exogenous heme, IsdG is rapidly degraded; however, heme binding stabilizes IsdG and reduces its proteolytic degradation (11). Therefore, the abundance of IsdG reflects the cytoplasmic levels of heme. Following ‘882 treatment, the intracellular abundance of IsdG increased in a dose-dependent manner (Fig. S1 D and E). IsdG is not stabilized when S. aureus ∆hemB is grown in the presence of ‘882, demonstrating that stabilization of IsdG requires endogenous heme (Fig. S1 D and E). Moreover, IsdG abundance increases in ∆hemB exposed to exogenous heme, indicating that heme-dependent stabilization of IsdG is not generally disrupted in this strain (Fig. S1 D and E). Taken together, these experiments reveal that ‘882 exposure increases cytoplasmic heme availability.
To test whether other bacterial heme sensing proteins monitor endogenous heme, the ability of the Corynebacterium diphtheriae ChrAS and Staphylococcus haemolyticus HssRS TCSs to sense ‘882 by adaptation growth curve was examined (7, 12, 13). Due to the slow growth and low optical density achieved by C. diphtheriae, enumerating colony forming units (cfus), a more sensitive measure of growth, was used to assess the adaptation of this pathogen. Pretreatment of both species with either heme or ‘882 improved survival in heme compared with nonadapted cultures (Fig. 2 D and E). These data suggest that ‘882 may stimulate heme biosynthesis in multiple Gram-positive bacteria and support the hypothesis that the ability to respond to both endogenous and exogenous heme is a conserved function of bacterial heme sensor systems.
‘882 Diminishes Fermentative Activity of S. aureus.
To define the mechanism by which ‘882 manipulates heme biosynthesis, an S. aureus transposon library was screened for mutants unable to sense ‘882 or heme by growth curve adaptation (7, 14). Of the ∼7,000 mutants screened, only one strain was completely unable to be preadapted for heme toxicity by ‘882; this strain contained a transposon in the glutamate-1-semialdehyde aminotransferase (hemL) gene involved in heme biosynthesis. Forty-four additional mutants were found to have a defect in ‘882 sensing, and, of those, 17 were also deficient in heme sensing (Table S1). A number of genes required for sensing heme and/or ‘882 are involved in central metabolic pathways including a predicted α-d-1,4-glucosidase (malA), the catabolite control protein (ccpA), respiratory response two-component system (srrAB), branched-chain amino acid amino transferase (ilvE), bifunctional pyrimidine regulator/uracil phosphoribosyltransferase (pryR), and pyridoxal 5′-phosphate synthase glutamine amidotransferase subunit (pdxT) (2, 15, 16). Consistent with these observations, bacteria treated with ‘882 had reduced glycolytic activity as indicated by slower acidification of the medium that correlated with delayed consumption of d-glucose and reduced production of l- and d-lactate (Fig. 3). These observations suggest that, as ‘882 induces endogenous heme biosynthesis, it also reduces the glycolytic or fermentative capacity of S. aureus. These results support a model whereby the metabolic state of S. aureus and heme homeostasis are functionally interconnected.
Fig. 3.
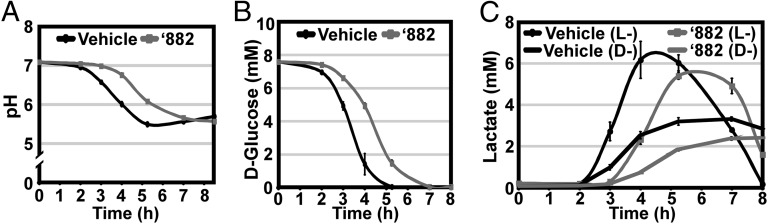
‘882 diminishes fermentative activity. S. aureus was grown in triplicate under aerobic conditions in the presence of vehicle (black lines) or 40 µM ‘882 (gray lines). At the indicated time intervals, culture supernatants were sampled and the (A) pH, (B) d-glucose, and (C) d- and l-lactate were quantified. Error bars represent 1 SD from the mean.
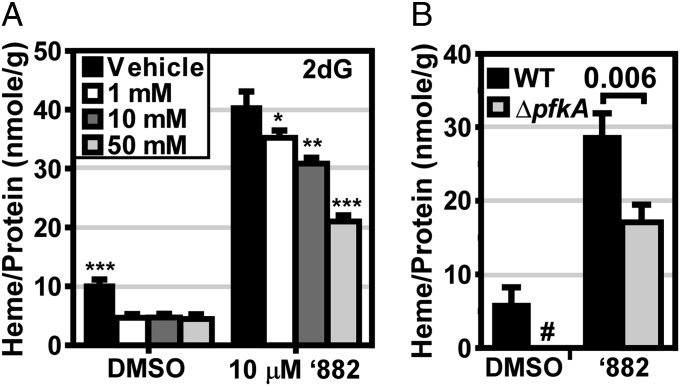
To test whether heme biosynthesis interfaces with central metabolism, we first assessed the effect of 2-deoxyglucose (2dG) on heme biosynthesis. The glucose analog 2dG primarily inhibits the phosphoglucoisomerase reaction, the second step in glycolysis (17). Treatment of S. aureus with 2dG reduces endogenous heme levels and antagonizes ‘882 activity (Fig. 4A). Deletion of 6-phosphofructokinase (pfkA), the third enzyme in glycolysis, results in a loss of glucose uptake and decreased acid end product secretion in S. aureus (Fig. S4 A and B). In agreement with the effect of 2dG on heme biosynthesis, both basal and ‘882-induced heme biosynthesis are suppressed in ΔpfkA (Fig. 4B). These data implicate a product of glycolysis in stimulating heme biosynthesis, strengthening the observed link between the regulation of heme biosynthesis and central metabolism.
Fig. 4.
Glycolytic activity regulates heme biosynthesis. S. aureus was grown in triplicate in the presence of vehicle or 10 µM ‘882. Heme levels were quantified using the pyridine hemochromogen assay and normalized to the concentration of protein in the whole cell lysates. (A) Cultures were treated with the indicated dose of 2-deoxyglucose (2dG). *P ≤ 0.05, **P ≤ 0.001, ***P ≤ 0.0001. (B) Wild-type (WT) and ΔpfkA Newman were cultured in TSB + 1% pyruvate. #, the signal was below the limit of detection. (A and B) Error bars represent the SEM from three independent experiments. Statistical significance was determined using an unpaired Student’s t test.
‘882 Is Bacteriostatic to Fermenting S. aureus.
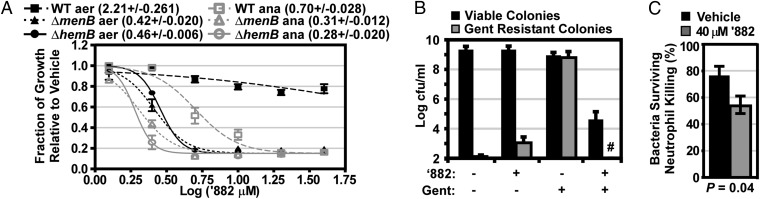
The ‘882-dependent suppression of fermentative activity in aerobic cultures suggests that the compound may inhibit fermentative growth. Indeed, when S. aureus was grown anaerobically in the presence of ‘882, growth was dramatically reduced (Fig. 5A). In addition, a strain bearing a lesion in the MK biosynthesis gene menB (∆menB), which cannot respire and solely ferments to produce energy, is more susceptible to ‘882 toxicity than wild type (Fig. 5A). ‘882 is bacteriostatic to ∆menB both aerobically and anaerobically (Fig. S4C). Moreover, when ‘882 is combined with the respiratory poison potassium cyanide (KCN), S. aureus growth is inhibited, even under aerobic conditions (Fig. S4D). Taken together, these data indicate that ‘882 inhibits S. aureus fermentative growth and that respiration is protective against ‘882 toxicity. Notably, ‘882 inhibits the growth of C. diphtheriae, supporting the conserved activity of ‘882 in heme sensing organisms (Fig. S4E). Furthermore, ∆hemB, an SCV deficient in heme biosynthesis, is also sensitive to ‘882, indicating that heme accumulation is not the source of toxicity, but rather a result of ‘882 perturbing S. aureus metabolism (Fig. 5A).
Fig. 5.
‘882 inhibits fermenting S. aureus. (A) S. aureus wild type (WT, dashed lines), the menaquinone auxotroph (∆menB, dotted lines), and the heme auxotroph hemB::ermC (∆hemB, solid lines) were grown in triplicate under aerobic (aer, black lines) and anaerobic (ana, gray lines) conditions in the presence of the indicated log of the concentration of ‘882 (µM). After 18 h, the absorbance at 600 nm (OD600) was measured and normalized to vehicle (DMSO)-treated bacteria. Curves were fit by nonlinear regression analysis and absolute logIC50 values were calculated in Prism with the top set at 1.0 and the bottom set at 0.15. LogIC50 values are indicated in parentheses in the figure key ±SEM. All logIC50 values were statistically different from WT aerobic logIC50 when analyzed by one-way ANOVA with a Dunnett post test (P < 0.001). Error bars represent the SEM. (B) Triplicate cultures of S. aureus were grown in the presence of the indicated additive. After 24 h, cfus were enumerated on tryptic soy agar (TSA) containing 5 μg/mL gentamicin (Gent) and plain TSA with a limit of detection of 100 cfus/mL (minimum y value); #, colonies were not identified above the limit of detection. Shown is the average of three independent experiments. Error bars represent 1 SD from the mean. (C) S. aureus was grown in the presence of vehicle (DMSO) or 40 µM ‘882 and coated in serum. Murine polymorphonuclear leukocytes (PMNs) were elicited with casein and harvested from the peritoneum. The ability of neutrophils to kill S. aureus in the presence of ‘882 was assessed by comparing cfus recovered from neutrophil-exposed S. aureus with those recovered from identical conditions lacking neutrophils. The mean of at least six independent experiments performed in triplicate are represented by the data; error bars represent SEM and significance was determined by a two-tailed Student’s t test.
‘882 Prevents the Evolution of Antibiotic Resistance.
S. aureus SCVs are obligate fermenters that emerge in response to aminoglycoside treatment (5). Because ‘882 is toxic to fermenting staphylococci, we hypothesized that ‘882 would prevent the outgrowth of antibiotic resistant SCVs in the presence of aminoglycosides. To test this hypothesis, S. aureus was grown in the presence of vehicle, gentamicin, ‘882, or a combination of gentamicin and ‘882, and the evolution of gentamicin resistance was monitored. Bacteria treated with gentamicin alone became resistant to gentamicin whereas ‘882 treatment alone did not affect the number of viable or gentamicin-resistant bacteria (Fig. 5B). Combining ‘882 with gentamicin resulted in a 5-log reduction in bacterial viability and eliminated all detectable gentamicin-resistant colonies (Fig. 5B). This improvement in gentamicin activity suggests that targeting fermentation can be used as an adjunctive therapy with antibiotics that are active against respiring S. aureus to improve antibacterial action and reduce the emergence of antibiotic resistance.
‘882 Enhances Innate Immune Function.
Neutrophils are the first immune cells to respond to the site of a S. aureus infection (18). As part of the innate defense program, they secrete noxious chemicals to kill invading pathogens (18). One component of this toxic milieu is nitric oxide (NO·), which inactivates iron-containing proteins critical for respiration (19). In agreement with the observation that ‘882 is particularly toxic to fermenting S. aureus, simultaneous exposure of S. aureus to ‘882 and NO· is more antibacterial than treatment with either molecule alone (Fig. S4F). Additionally, ‘882 improves neutrophil-dependent killing of S. aureus but does not overtly affect neutrophil viability (Fig. 5C). These results support the notion that ‘882 may have therapeutic efficacy against bacterial invaders by augmenting the killing activity of neutrophils.
Derivative of ‘882 Reduces S. aureus Pathogenesis in Vivo.
Abscesses, which are purulent lesions containing bacteria and neutrophils surrounded by a pseudocapsule, are a hallmark of S. aureus infections (20). It is likely that fermentation is critical to staphylococcal pathogenesis because abscesses are thought to be anaerobic environments (21). Testing this hypothesis is challenging as S. aureus fermentation is branched, so genetic inactivation of fermentation is difficult. The toxic effect of ‘882 toward fermenting S. aureus provides an opportunity to gain initial insights into the role of fermentation in pathogenesis. However, ‘882 has a metabolically labile furan group that may form protein adducts and deplete glutathione in vivo (22). Thus, the furan was replaced with a 2-fluorophenyl group to create a more biologically stable derivative, VU0420373 (‘373) (Figs. S5 and S6A). ‘373 activates HssRS as evidenced by its ability to increase the expression of the xylE reporter gene and preadapt S. aureus for heme toxicity (Fig. S6 B and C). In addition, ‘373 inhibits the growth of S. aureus ∆menB, although to a lesser extent than ‘882 (Fig. S6D).
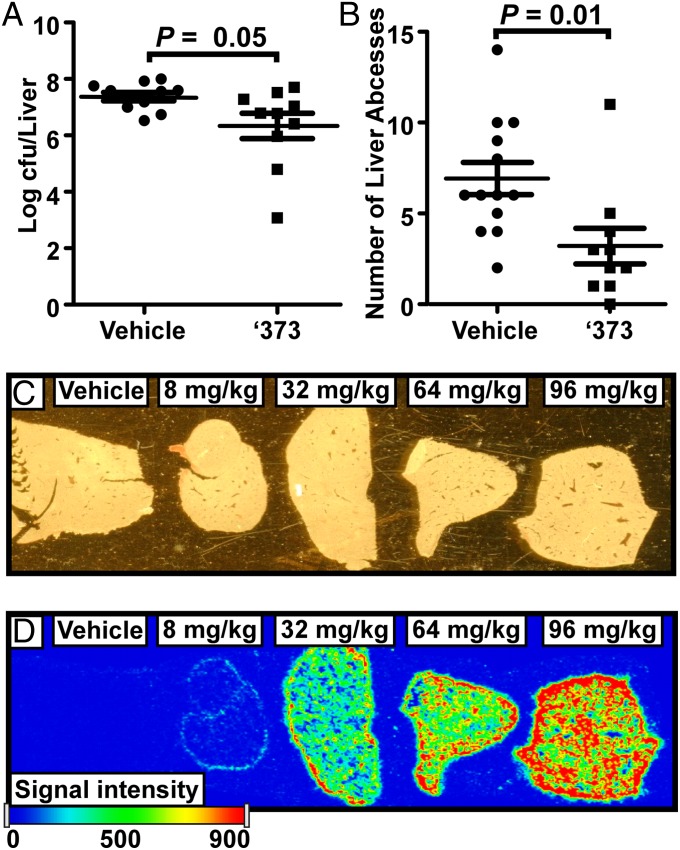
Intraperitoneal administration of ‘373 to mice i.v. infected with S. aureus resulted in a significant 1-log decrease in cfus recovered from the livers of ‘373-treated animals compared with vehicle (Fig. 6A). Notably, ‘373 treatment reduced tissue pathology associated with infection as demonstrated by a significant reduction in the number of liver abscesses (Fig. 6B). This liver-specific reduction in bacterial burden and inflammation correlates with an accumulation of ‘373 in the livers of treated animals as shown by Imaging Mass Spectrometry (IMS) (Fig. 6C, D and Fig. S7 A–C) (23). To quantify the tissue level of ‘373, portions of the livers were excised, and '373 concentrations were determined by HPLC-MS (Fig. S7D). A linear relationship between tissue level and dose of ‘373 administered was observed. Average spectral intensity extrapolated from IMS data of '373 reflected a dose–response relationship similar to that observed by HPLC-MS, confirming the suitability of IMS for monitoring tissue levels of '373 (Fig. S7E). Both the HPLC-MS and IMS data support the notion that the therapeutic effect of ‘373 is due to the interaction of the compound with S. aureus as it is found at the site where bacterial growth is restricted. Although the therapeutic benefits of ‘373 could be due to alternative activities of this molecule, these results provide evidence that targeting fermentation in a facultative anaerobe may be a viable therapeutic strategy. The therapeutic value of this strategy is supported by the observation that S. aureus generates resistance to ‘882 in anaerobic conditions with a frequency of ∼1 in 10−7 cfus.
Fig. 6.
‘373 reduces S. aureus pathogenesis in vivo. Mice infected retroorbitally with S. aureus were treated intraperitoneally with vehicle [10% (vol/vol) Tween 80] or ‘373. After 96 h, mice were euthanized and (A) cfus and (B) surface abscesses were enumerated from the livers. Each marker represents an individual mouse. Data were collected from three independent experiments resulting in n = 13 for vehicle and n = 12 for ‘373-treated mice once the highest and lowest values were removed from each group. The horizontal line indicates the mean and the error bars represent the SEM. Statistical significance was determined by a two-tailed Student’s t test. Livers from mice treated with the indicated dose of ‘373 were harvested 24 h postinfection (1 h after the second treatment), sectioned, and mounted on MALDI target plates (C). (D) Tissue sections were imaged by MALDI-MS/MS for accumulation of a fragment of ‘373 by MALDI-MS/MS (m/z 255.2→134). Spectra were acquired at 10 microscans per step. Five laser shots were acquired per pixel, and pixels were obtained every 100 µm.
Discussion
Here, we describe an HTS that identified small molecule activators of the S. aureus heme sensor system HssRS. The most potent hit, ‘882, increases endogenous heme levels and activates HssRS through the heme biosynthesis pathway. This effect appears to be due to a perturbation of the metabolic state of S. aureus as transposon insertions targeting genes involved in metabolism contribute to the ability of the bacteria to sense ‘882. This hypothesis is supported by the observations that ‘882 reduces fermentative processes and is bacteriostatic to fermenting staphylococci. Targeting staphylococcal fermentation has therapeutic potential as it augments innate immune function and prevents outgrowth of antibiotic resistant colonies in vitro, and reduces S. aureus liver colonization and associated inflammation in vivo.
Using ‘882 as a probe has revealed that HssRS responds to both exogenous and endogenous heme accumulation. The discovery that S. aureus monitors intracellular heme status through HssRS suggests that other bacterial heme sensing systems may also sense intracellular heme. This hypothesis is supported by the observation that C. diphtheriae and S. haemolyticus are also adapted for heme toxicity by ‘882 (12). The fact that ‘882 is sensed by C. diphtheriae and S. haemolyticus indicates that the target of ‘882 is present in multiple pathogens, establishing this molecule as a powerful probe for studying intracellular heme metabolism. The utility of ‘882 as a probe is further supported by the observation that IsdG binds endogenously synthesized heme following stimulation with ‘882. This result implicates bacterial heme oxygenases in intracellular heme turnover, a result that is likely generalizable to all bacterial heme oxygenases as P. aeruginosa HemO has also been shown to act on endogenous heme (24). Expanding the function of bacterial heme degrading enzymes beyond nutrient iron acquisition establishes a mechanism by which bacteria can adjust iron and heme levels to satisfy cellular needs (3, 11).
Heme biosynthesis is typically regulated by cellular iron or heme levels although there is some evidence that it is tied to central metabolism (25, 26). The perturbation of heme homeostasis and central metabolism by ‘882 suggests that the two cellular processes are coordinated in S. aureus. Strengthening this hypothesis, both chemical and genetic inhibition of the upper steps in glycolysis result in suppressed basal and ‘882-induced heme levels. These data suggest that an intact glycolytic pathway is required for ‘882-dependent activation of heme biosynthesis, further supporting a functional interconnection between central metabolism and heme biosynthesis in S. aureus. An integration of heme homeostasis with central metabolism is consistent with the fact that heme and iron are critical cofactors for many enzymes involved in energy conversion. Further studies using ‘882 could dissect the interplay between heme and central metabolism in both S. aureus and other pathogens.
The activity of ‘882 against fermenting bacteria has broad therapeutic potential. ‘882 is bacteriostatic to SCVs, which often emerge during recurrent and persistent S. aureus infections (5). This therapeutic strategy may be generalizable across other infectious diseases because N. gonorrhoeae, E. coli, P. aeruginosa, and S. Typhimurium can also spawn SCVs (5, 6). In addition to being used as a treatment for persistent infections, derivatives of ‘882 could have utility in combinatorial therapy with antibiotics that target respiring bacteria. As a proof of principle, cotreating S. aureus with gentamicin and ‘882 in vitro reduces bacterial viability and the outgrowth of antibiotic resistant populations. Although gentamicin is typically used in combination with other primary antibiotic agents, it is often conjugated to beads and used as the primary antibiotic agent at the site of osteomyelitis infections. SCVs often arise during the prolonged antibiotic regimen required for treatment of osteomyelitis. Therefore, it is possible that an ‘882–gentamicin dual therapy could be used to improve therapy for osteomyelitis by preventing the generation of SCVs during infection. If ‘882 proves synergistic with other primary antistaphylococcal agents such as β-lactams and vancomycin, it is possible that ‘882 could be used more broadly as an adjuvant to expand the antibiotic armamentarium that is effective against S. aureus infections.
The majority of bacterial pathogens use some combination of fermentation and respiration to produce ATP, suggesting that both are important for colonization and persistence (27). The importance of S. aureus fermentative pathways during infection is demonstrated by the fact that ‘373 reduces abscess formation and S. aureus growth in the livers of systemically infected mice. The observed effects of ‘373 on S. aureus pathogenesis support the notion that the abscess is an anaerobic environment and that bacterial fermentation is required for efficient colonization (21). Furthermore, the modest effect ‘373 has on S. aureus pathogenesis in vivo, despite its diminished bacteriostatic activity in vitro, highlights the promise of more potent, biologically compatible derivatives of ‘882 as novel therapeutics.
In summary, we have identified a small molecule that diminishes fermentative processes in S. aureus, stimulates endogenous heme biosynthesis, and activates the HssRS heme sensor. This molecule has twofold utility as both a probe of bacterial physiology and an unconventional therapeutic. Tools for stimulating endogenous heme biosynthesis are limited, and these probes, which stimulate heme biosynthesis across genera, will provide insight into conserved mechanisms of heme regulation. As a therapeutic, further studies confirming the mechanisms by which ‘882-derived compounds reduce S. aureus pathogenesis and associated inflammation in vivo will lay the groundwork for improved strategies for treating recalcitrant infections.
Materials and Methods
Descriptions of the bacterial strains and growth conditions, high-throughput screen, XylE reporter assay, growth curve analyses, metabolite detection, heme quantification, transposon site identification, gentamicin resistance assay, neutrophil killing assay, murine infection, IMS and HPLC-MS analysis of ‘373, small molecule synthesis, and statistical analyses are in the SI Materials and Methods. Primers used for generating ΔpfkA and identifying transposon integration sites are listed in Table S2. All animal experiments were reviewed and approved by Vanderbilt University's Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We thank members of the E.P.S. laboratory for critical reading of the manuscript, Daniel Dorset and the Vanderbilt High-Throughput Screening Facility for assistance in the HTS, Dr. Friedrich Götz for S. aureus 8325-4 hemB::ermC, Xenogen for the pXen-1 plasmid, Dr. Carrie Jones for guidance in formulating the compounds used in animal studies, and Kathryn Haley for the purified IsdG used as a protein standard. We thank Amanda McCoy for her work generating the transposon library. We thank Brian Hachey and M. Lisa Manier of the Mass Spectrometry Research Center for assistance with HPLC-MS quantitation. This work was supported by the Searle Scholars Program, National Institutes of Health (NIH) Grant U54 AI057157-06 from the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense, and NIH Grants AI069233 and AI073843. L.A.M. was supported by NIH Grant T32 HL069765. B.F.D. was supported by NIH Grant T32 GM065086. J.L.M. acknowledges the support of Vanderbilt Chemical Biology Interface Training Program Grant T32 GM008320). T.E.K.-F. recognizes support from NIH Grant F32 AI100480 and an American Heart Association Postdoctoral Fellowship. J.L.D. acknowledges support from NIH Grant R01GM090260. R.M.C. acknowledges the support of NIH/ National Institute of General Medical Sciences Grant 8p41GM103391-3 (formerly NIH/National Center for Research Resources Grant 1P41RR031461). E.P.S. is a Burroughs Wellcome Investigator in the Pathogenesis of Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303674110/-/DCSupplemental.
References
- 1.Pantosti A, Venditti M. What is MRSA? Eur Respir J. 2009;34(5):1190–1196. doi: 10.1183/09031936.00007709. [DOI] [PubMed] [Google Scholar]
- 2.Somerville GA, Proctor RA. At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiol Mol Biol Rev. 2009;73(2):233–248. doi: 10.1128/MMBR.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazmanian SK, et al. Passage of heme-iron across the envelope of Staphylococcus aureus. Science. 2003;299(5608):906–909. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 4.Johansson P, Hederstedt L. Organization of genes for tetrapyrrole biosynthesis in gram-positive bacteria. Microbiology. 1999;145(Pt 3):529–538. doi: 10.1099/13500872-145-3-529. [DOI] [PubMed] [Google Scholar]
- 5.Proctor RA, et al. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. 2006;4(4):295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 6.Bryan LE, Kwan S. Aminoglycoside-resistant mutants of Pseudomonas aeruginosa deficient in cytochrome d, nitrite reductase, and aerobic transport. Antimicrob Agents Chemother. 1981;19(6):958–964. doi: 10.1128/aac.19.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres VJ, et al. A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe. 2007;1(2):109–119. doi: 10.1016/j.chom.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez A, et al. Two coregulated efflux transporters modulate intracellular heme and protoporphyrin IX availability in Streptococcus agalactiae. PLoS Pathog. 2010;6(4):e1000860. doi: 10.1371/journal.ppat.1000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stauff DL, Skaar EP. Bacillus anthracis HssRS signalling to HrtAB regulates haem resistance during infection. Mol Microbiol. 2009;72(3):763–778. doi: 10.1111/j.1365-2958.2009.06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Eiff C, et al. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J Bacteriol. 1997;179(15):4706–4712. doi: 10.1128/jb.179.15.4706-4712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reniere ML, Skaar EP. Staphylococcus aureus haem oxygenases are differentially regulated by iron and haem. Mol Microbiol. 2008;69(5):1304–1315. doi: 10.1111/j.1365-2958.2008.06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt MP. Identification of a two-component signal transduction system from Corynebacterium diphtheriae that activates gene expression in response to the presence of heme and hemoglobin. J Bacteriol. 1999;181(17):5330–5340. doi: 10.1128/jb.181.17.5330-5340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stauff DL, Torres VJ, Skaar EP. Signaling and DNA-binding activities of the Staphylococcus aureus HssR-HssS two-component system required for heme sensing. J Biol Chem. 2007;282(36):26111–26121. doi: 10.1074/jbc.M703797200. [DOI] [PubMed] [Google Scholar]
- 14.Youngman PJ, Perkins JB, Losick R. Genetic transposition and insertional mutagenesis in Bacillus subtilis with Streptococcus faecalis transposon Tn917. Proc Natl Acad Sci USA. 1983;80(8):2305–2309. doi: 10.1073/pnas.80.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIllmurray MB, Lascelles J. Anaerobiosis and the activity of enzymes of pyrimidine biosynthesis in Staphylococcus aureus. J Gen Microbiol. 1970;64(3):269–277. doi: 10.1099/00221287-64-3-269. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick TB, et al. Two independent routes of de novo vitamin B6 biosynthesis: Not that different after all. Biochem J. 2007;407(1):1–13. doi: 10.1042/BJ20070765. [DOI] [PubMed] [Google Scholar]
- 17.Wick AN, Drury DR, Nakada HI, Wolfe JB. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957;224(2):963–969. [PubMed] [Google Scholar]
- 18.DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am. 2009;23(1):17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson AR, Libby SJ, Fang FC. A nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunity. Science. 2008;319(5870):1672–1676. doi: 10.1126/science.1155207. [DOI] [PubMed] [Google Scholar]
- 20.Cheng AG, DeDent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011;19(5):225–232. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park MK, Myers RAM, Marzella L. Oxygen tensions and infections: Modulation of microbial growth, activity of antimicrobial agents, and immunologic responses. Clin Infect Dis. 1992;14(3):720–740. doi: 10.1093/clinids/14.3.720. [DOI] [PubMed] [Google Scholar]
- 22.Burka LT, Washburn KD, Irwin RD. Disposition of [14C]furan in the male F344 rat. J Toxicol Environ Health. 1991;34(2):245–257. doi: 10.1080/15287399109531564. [DOI] [PubMed] [Google Scholar]
- 23.Manier ML, et al. Reagent precoated targets for rapid in-tissue derivatization of the anti-tuberculosis drug isoniazid followed by MALDI imaging mass spectrometry. J Am Soc Mass Spectrom. 2011;22(8):1409–1419. doi: 10.1007/s13361-011-0150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker KD, Barkovits K, Wilks A. Metabolic flux of extracellular heme uptake in Pseudomonas aeruginosa is driven by the iron-regulated heme oxygenase (HemO) J Biol Chem. 2012;287(22):18342–18350. doi: 10.1074/jbc.M112.359265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doss M, Philipp-Dormston WK. Regulatory link between lactate dehydrogenase and biosynthesis of porphyrin and heme in microorganisms. Enzyme. 1973;16(1):28–41. doi: 10.1159/000459359. [DOI] [PubMed] [Google Scholar]
- 26.Frunzke J, Gätgens C, Brocker M, Bott M. Control of heme homeostasis in Corynebacterium glutamicum by the two-component system HrrSA. J Bacteriol. 2011;193(5):1212–1221. doi: 10.1128/JB.01130-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurtshuk PJ. Bacterial metabolism. In: Baron S, editor. Medical Microbiology. 4th Ed. Galveston, TX: Univ of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.