Abstract
We report here the generation of antibody agonists from intracellular combinatorial libraries that transdifferentiate human stem cells. Antibodies that are agonists for the granulocyte colony stimulating factor receptor were selected from intracellular libraries on the basis of their ability to activate signaling pathways in reporter cells. We used a specialized “near neighbor” approach in which the entire antibody library and its target receptor are cointegrated into the plasma membranes of a population of reporter cells. This format favors unusual interactions between receptors and their protein ligands and ensures that the antibody acts in an autocrine manner on the cells that produce it. Unlike the natural granulocyte-colony stimulating factor that activates cells to differentiate along a predetermined pathway, the isolated agonist antibodies transdifferentiated human myeloid lineage CD34+ bone marrow cells into neural progenitors. This transdifferentiation by agonist antibodies is different from more commonly used methods because initiation is agenetic. Antibodies that act at the plasma membrane may have therapeutic potential as agents that transdifferentiate autologous cells.
Keywords: neuralgenisis, phenotypic selections, growth factors
Recently we developed a method in which combinatorial antibody libraries are rendered infectious for eukaryotic cells by incorporating them into lentiviruses. This method enables direct selection of agonists that regulate cellular phenotypes from the very cells expressing the agonist antibodies: i.e., in an autocrine manner (1). The power of these infectious libraries stems from several unique features. First, as for other antibody libraries, one is able to select from vast numbers of candidates (2–7). Secondly, the infected eukaryotic cell, itself, becomes the reporter system. This direct linkage of genotype and phenotype greatly facilitates the discovery of functional antibodies such as agonists. Finally, there is the possibility of adding protein signals or anchoring sequences to the expressed antibodies, such that they can be secreted, directed to subcellular compartments, or anchored in the plasma membrane, respectively. Antibodies anchored in the plasma membrane are designed particularly to activate colocalized neighboring receptors (“near neighbor” libraries).
To demonstrate the power of this methodology, we used combinatorial antibody libraries in their near neighbor format to coexpress antibodies and the granulocyte colony-stimulating factor receptor (G-CSFR). Remarkably, using this method, we isolated an agonist antibody to the G-CSFR that can induce human CD34+ stem cells to form neural progenitor cells. Because CD34+ stem cells are of the myeloid lineage, this antibody appears to induce a transdifferentiation process (8, 9).
Results
Construction of Near Neighbor Antibody Libraries.
Many different antibody formats that address diverse cellular compartments can be used for intracellular combinatorial libraries (10). In our previous studies using intracellular libraries coupled to single cell selection systems, we generated many antibodies that were phenocopies of the natural agonists. Because antibody agonists have the potential to bind to receptors in a way that is different from the natural agonists, they are potentially capable of pleiotropic effects. To favor the selection of antibodies that bind to receptors in unusual ways, we generated a format in which members of the combinatorial antibody libraries are integrated into the plasma membranes of target cells (Fig. 1). Selections using anchored antibodies are based on an autocrine mechanism in which one ensures that the antibody acts on the cell that produced it.
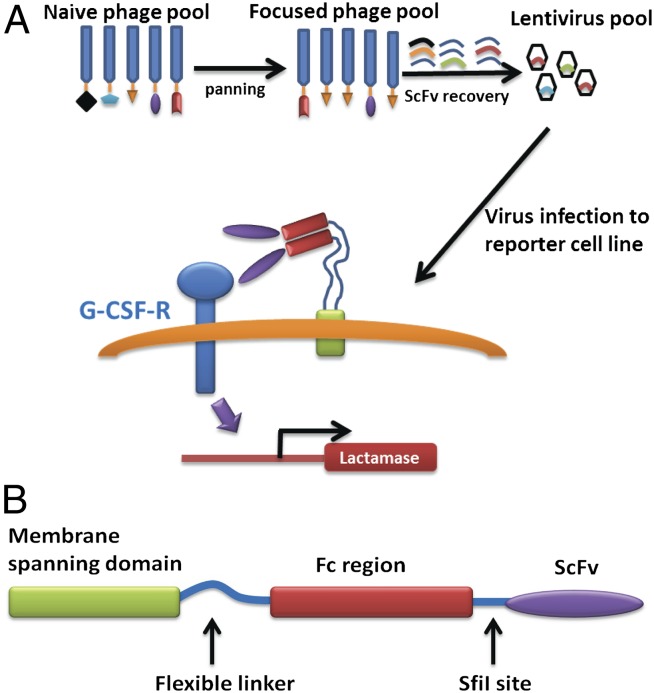
Fig. 1.
(A) Schematic illustration of the strategy for selecting the G-CSF receptor agonist antibody. A naïve phage library was used to pan against the purified receptor ectodomain. This pool that was enriched for antibodies that bound to the G-CSFR was converted to a membrane bound version in lentivirus. A reporter cell line was infected with these lentiviruses and individual cells were selected for β-lactamase gene expression. (B) Topology of the membrane-tethered antibody (MTA) fusion protein.
A single chain ScFv that is dimerized by the appended Fc domains is linked via a flexible linker to a platelet-derived growth factor receptor (PDGFR) membrane-spanning domain such that the antibody molecules are integrated as dimers into the plasma membrane with their binding sites facing the solvent. We termed these libraries near neighbor libraries because the binding of the antibody is likely constrained to available regions of neighboring molecules. The central concept was that by using near neighbor libraries, unusual antibodies that are not seen frequently when selections are carried out in solution might be favored because of the coupling of constrained reaction geometries to a very high effective molarity for the interacting pairs. The method also has the important advantage that the target receptor is present in its natural milieu, thus, ensuring the presence of physiologically relevant conformations.
As a proof of principle, we tested the potential for an antibody that is a known thrombopoietin (TPO) phenocopy in its soluble form to function when it is coexpressed and anchored in the plasma membrane along with its thrombopoietin receptor (TPOR) target. The antibody still functioned as an agonist when it was integrated into the plasma membrane suggesting that it could activate a neighboring TPOR (Fig. S1 A and B). Two separate assays were used. In one, a FRET fluorescence reporter assay that measured activation of the signal transduction pathway was studied (Fig. S1A). The second assay measured stimulation of cell growth (Fig. S1B). To confirm that the antibody activated the same cell that expressed it, and not an adjacent one by cell–cell interaction, cells were plated at a low density so they could be studied individually. When the culture was exposed to the FRET substrate, cells in isolation were found to be activated, strongly suggesting that the membrane bound antibody activated the cell expressing it by binding to a neighboring receptor (Fig. 2 A and B). No activation was observed in cells infected with a virus expressing red fluorescent protein alone (Fig. 2 C and D).
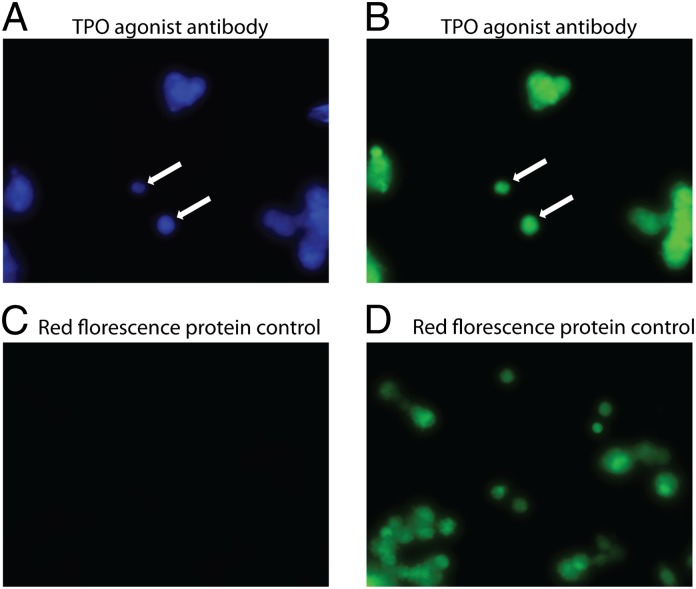
Fig. 2.
Activation does not require contact with neighboring cells. (A) TPOR reporter cells were activated after infection with lentivirus expressing the TPOR agonist antibody (3D9). All of the reporter cells constitutively express the EGFP. The cells were incubated with the CCF4-AM FRET substrate and visualized by fluorescent emission. (B) Same field visualized after excitation of EGFP at 488 nm. (C) As a control the TPOR reporter cells were infected by lentivirus expressing the tomato fluorescent protein on the membrane. After infection, the cells were incubated with the CCF4-AM substrate and visualized after UV excitation. (D) Same view of cells infected with virus encoding the membrane tethered Tomato protein alone after excitation at 488 nm.
Isolation of G-CSF Antibody Phenocopies.
To increase the potential for isolation of unusual antibodies, a dual selection strategy was used. In the first step, antibodies that bound to the G-CSFR ectodomain in solution were selected from a combinatorial library expressed in phage that contained about 1.0 × 109 members. The purpose of this step was to select binding antibodies from a large diversity system to enter the highest number of candidates into the more stringent secondary screen. We expect this enriched library to have large numbers of antibodies to easily available epitopes and fewer to other regions. The secondary near neighbor screen that is based on function rather than simple binding, was designed to both isolate directly those members of the preselected library that are agonists and, uncover those, perhaps rare, antibodies with unusual functions. Thus, the antibodies that were preselected in phage after two rounds of panning were converted into a plasma membrane binding format and transferred to lentivirus for infection of reporter cells. The entire pool of these selected antibodies that had about 5.0 × 105 members was used to infect reporter cell lines where an anchored G-CSFR was colocalized in the plasma membrane. In these reporter cell lines either the growth of BaF3 cells or induction of a FRET signal in SIE/BLA/SIG cells was strictly dependent on the presence of a G-CSFR agonist (Fig. S2 A and B). Both infection with the antibody library or exposure to authentic G-CSF activated the G-CSFR in these cells (Fig. S3 A–D). Again, infection with a virus encoding the red fluorescent protein alone did not activate the cells.
Although the selected antibodies were agonists when integrated into the plasma membrane, it was important to demonstrate that they were also functional as soluble proteins in a fashion that is similar to the endogenous GCSF agonist that is a secreted growth factor. To study this, the selected antibody genes were converted to a secretory format in an expression vector that was used to transfect HEK-293 cells in 96-well plates. The secreted antibodies were harvested and tested for their ability to bind to the G-CSFR and activate reporter cells. Sequencing of the antibody genes from the positive clones revealed that they all derived from a single clone, which indicates strongly that a selectable event occurred. This clone was present in the original pool at a frequency of less than 10% as determined by sequence of the antibody genes from 50 randomly picked bacterial colonies, and could easily have been lost if the selection was confined to more rounds of panning in phage. An analysis of the affinity purified antibody by Western blotting and FACS showed that it bound strongly to the G-CSFR (Fig. 3A) and was an agonist (Fig. 3 B–D). Additional studies showed that the purified antibody induced both G-CSFR downstream signaling and growth in the reporter cell lines (Fig. S4 A and B).
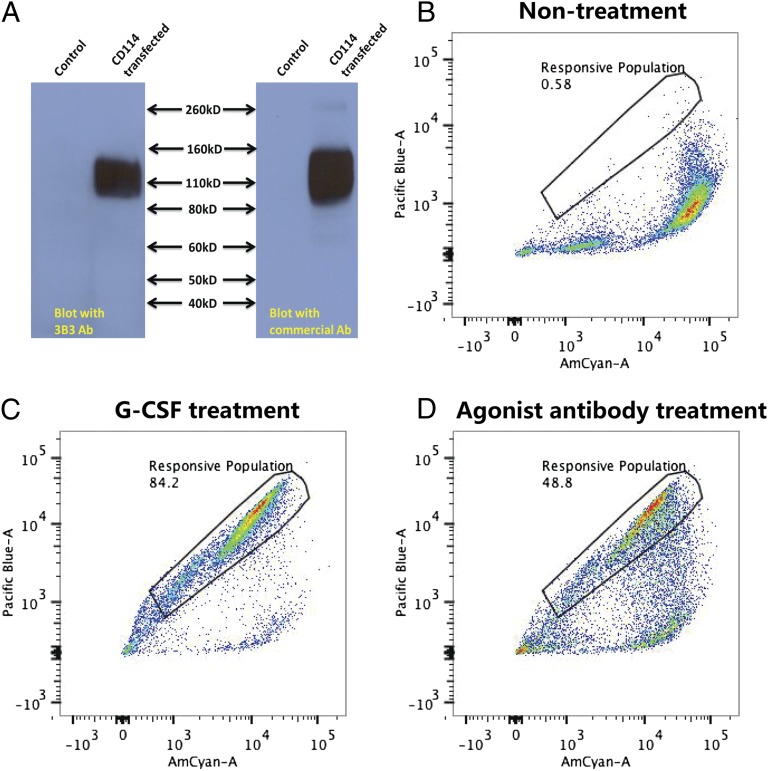
Fig. 3.
The G-CSFR is recognized and activated by the selected antibody. (A) Lysates of control cells and cells transfected with the G-CSFR (CD114) were analyzed by Western blotting against either the agonist antibody (3B3) or a commercial antibody to the G- CSFR. (B–D). Cells treated differently were incubated with the CCF4-AM substrate and subjected to FACS based on the FRET signal. (B) Cells without antibody treatment. (C) Cells treated with G-CSF. (D) Cells treated with 10 μg/mL 3B3 agonist antibody.
To complete the cycle, the antibody was reconverted to its membrane-tethered format (MTA) in lentivirus and after infection was again capable of activating selectively the reporter cells (Fig. S5 A–C).
To determine whether antibody and its receptor target actually colocalize in the plasma membrane, fluorescence microscopy study of cells expressing both the G-CSFR and the selected antibody clone were carried out. These studies showed that both the antibody molecules and the G-CSFR are simultaneously expressed strongly on the plasma membrane (Fig. 4 A–D) and colocalize in the classical patches induced by cross linking (Fig. 4 E–H) (10). Receptor activation by either G-CSF or the agonist antibody was again strictly dependent on the presence of the G-CSFR. There was no activation of mock-transfected cells by either G-CSF or the agonist antibodies (Fig. S6 A and B).
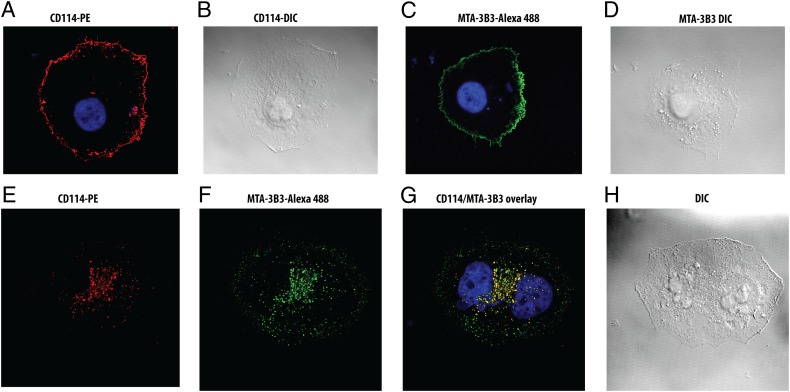
Fig. 4.
Distribution and colocalization of the receptor and plasma membrane tethered antibody. (A and B). The G-CSFR (CD114) is labeled by a commercial G-CSFR binding antibody conjugated to PE (red). Differential interference contrast (DIC) image shows the morphology of the cells. (C and D). The membrane tethered agonist antibody (MTA-3B3) was stained with a Alexa Fluor-488 conjugated commercial antibody recognizing its Fc domain. (E–H) The receptor and antibody when cross-linked respectively by their fluorophore conjugated antibodies show a “patching” pattern. (G) The overlaid image shows colocalization of the antibody and its G-CSFR target.
Transdifferentiation of Human Stem Cells.
Because the main purpose of developing near neighbor combinatorial antibody libraries was to select agonists that might act in unusual ways, we tested the ability of these G-CSFR binding antibodies in their soluble format to activate human CD34+ stem cells. We thought that they could possibly be more potent and/or drive differentiation further along pathways of the myeloid lineage. However, what actually happened was that, unlike the natural ligand, the antibodies initiated neurogenesis in these stem cells.
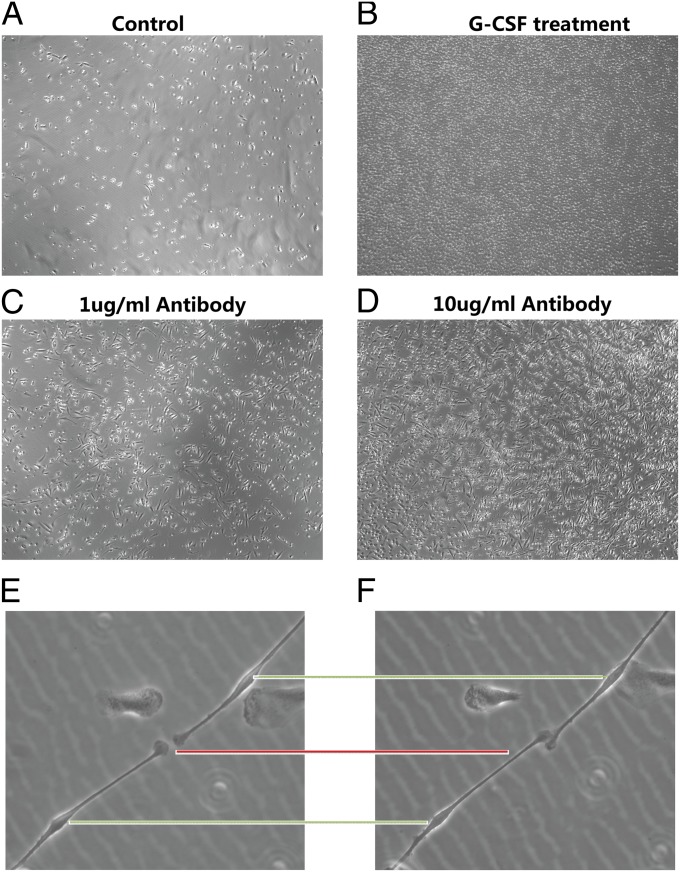
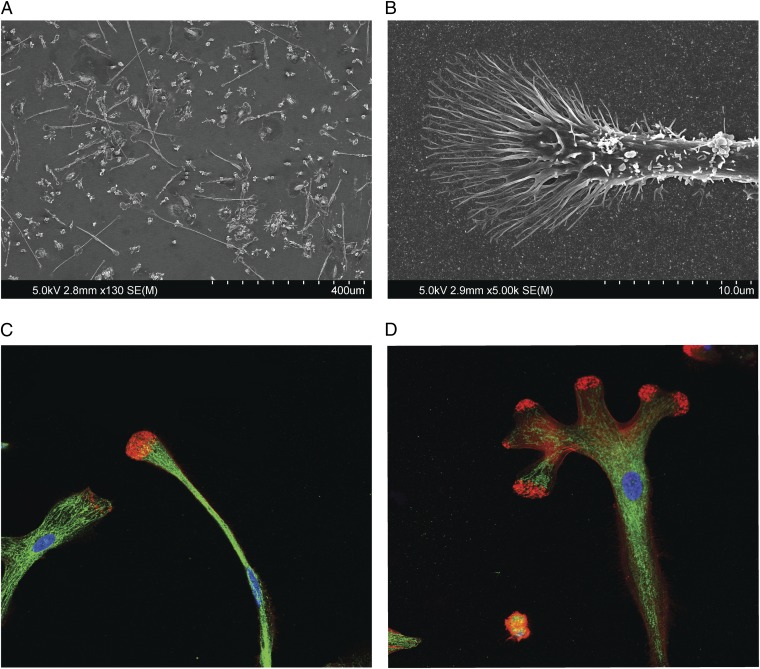
CD34+ stem cells were isolated on separate occasions by cell sorting from five individuals. The sorted cells had a purity of 96–99%. In the first experiment, one half of the cells were treated with G-CSF and the other half was treated with the selected soluble antibody. As, expected, G-CSF caused proliferation of cells that did not attach to the dish and had morphologies characteristic of the myeloid lineage. By contrast, the antibodies caused widespread formation of cells that attached to the dish. Attachment started at 8 d, and by 14 d, about 40% of the cells attached and appeared to have characteristics most consistent with those of cells undergoing neural development (Figs. 5 A–F and 6A and Fig. S7 A–C). These cells were mobile, had long neuritis, and exhibited multiple morphologies including bipolar configurations and extensive development of pyramidal shaped growth cones (Fig. 5 E and F). Scanning electron microscopy was carried out on these cells to obtain further morphological details. These studies showed that the growth cones on the neurite tips had a classical morphology with extensive formation of filopodia and lamellipodia (Fig. 6 A and B).
Fig. 5.
(A–D) Representative views of the CD34+ cells that attached to uncoated glass coverslips after 2 wk of treatment. (E and F) Cells led by neurite extension are mobile. The image in F was taken 1 h after the image in E. Green lines indicate the aligned cell body and the red line indicates the leading edge of the growth cone.
Fig. 6.
(A) SEM study of the cells attached after 2 wk to the uncoated coverslips showing extensive neurite formation. (B) Representative SEM image of the tip of a neurite showing classical growth cone morphology. (C and D) Representative growth cone and neurite staining for the localization of actin and neurospecific tubulin (Tuj-1). Actin is labeled by Phalloidin-eFluor 570 (red) and Tuj-1 is labeled with Alexa Fluor-488 (green).
Induction of neural cells was observed in repeated experiments from different donors of CD34+ cells. Similar morphological features were not observed when the same cell populations were treated with G-CSF, irrelevant antibodies, or with agonist antibodies to other receptors on CD34+ cells such as integrins. To confirm that these cells were of neural lineage, they were studied by fluorescent microscopy for the presence of neuronal tubulin (Tuj1) as a highly specific neuronal marker, nestin as a transient marker of neural progenitor cells, F-actin as a component of growth cones, and CD29 as a marker of cells of other lineages, including fibroblasts. Intense staining of the cells was observed with antibodies to F-actin, Tuj1 tubulin and nestin (Fig. 6 C and D and Fig. S8 A–C). By contrast, the cells did not express the fibroblast marker, CD29. The F-actin, and the neurospecific Tuj1 protein were organized into the canonical patterns observed in the growth cones of neural progenitor cells (11, 12). Tuj1 tubulin extended into the growth cone margins and terminated at the actin bundles. The actin filaments at the tips of the growth cones ranged in morphology from spike-like to a more blunted arrangement.
The intensity of the tubulin stain allowed one to observe clearly the remarkable length of the developing neuritis, which is a distinguishing feature of cells of the neuronal lineage (Fig. S8 D and E). Another feature of neuroblasts in culture is their growth cone motility. To study this feature, cells were observed at 1-h intervals. Even in this short interval, extensive motility of the growth cones accompanied by neurite extension could be observed easily (Fig. 5 E and F).
Differential Activation of Signal Transduction Pathways and Gene Expression.
Because the antibodies apparently transdifferentiated stem cells, one might expect that the activation of signal transduction pathways would be different from those used generally by cytokines such as G-CSF. To test this possibility, freshly isolated human CD34+ bone marrow stem cells were treated with either G-CSF or the agonist antibody, and the activation of the major G-CSF receptor signaling pathways such as STAT5, Protein Kinase B (AKT), and ERK were analyzed using selective anti-phospho-antibodies. G-CSF induced phosphorylation of STAT5, AKT, and ERK, whereas the antibody induced stronger phosphorylation of AKT but did not affect STAT5 or ERK (Fig. S9A). Thus, the cellular fate we observe may be the result of a concerted enhancement of one signal transduction pathway coupled with the loss others. Interestingly, the PI3 kinase/AKT pathway is known to be important in neurogenesis (13–15).
A more comprehensive analysis of the induction of gene expression was carried out using a RT-PCR array based analysis of neurogenesis in which the expression of 84 genes known to be important for neurogenesis were studied. The results are expressed as the ratio of induction by the antibody or G-CSF to that of untreated cells (Fig. S9B). The expression of many genes important to neurogenesis are up-regulated by the antibody relative to those of uninduced cells or cells induced by G-CSF. This up-regulated expression includes genes encoding proteins that promote neurite outgrowth and adhesion (NRP1, NRP2, and NRCAM) and notch signaling (NRP2, HEY1, DLL1, and ADORA1), among others. Although some induction of gene expression is common to G-CSF and the antibody, many are unique and highly activated by the antibody alone (Fig. S9B). An interesting gene whose expression is up-regulated far more than any of the others except ADORA1 (229-fold) is the gene involved in Norrie disease (NDP). This gene encodes a secreted protein that is a ligand in the Wnt/beta-catenin pathway and may play a role in the early development of the neuroectoderm (16–18).
Discussion
The most important consequence of these studies is that they suggest that antibody agonists and the natural ligand that bind to the same receptor can induce different cell fates from an identical starting cell population. In the case of GPCRs and cytokine receptors, pluripotency of signaling is a growing area of pharmacology where one aims at finding agonists that bias signaling via a receptor to a particular down streaming pathway (19–23). To accomplish this, one needs to test a large number of agonists. Although this is relatively easy for small molecule ligands, the generation and study of a large and diverse library of protein agonists is more problematic. Our method may provide a solution to this problem in that it facilitates study of a large number of potential protein agonists that bind to different regions of the receptor and favor alternative down stream signaling pathways. This effect is dramatically illustrated here because both the antibody and G-CSF bind to the G-CSFR and induce cell proliferation, but only the antibody initiates neurogenesis.
The special way that these agonists were generated may be relevant to their unusual function. Because the antibodies are integrated into the plasma membrane during the selection process, their binding is limited to near neighbors that occur because of stochastic proximity in a fluid membrane, or are the result of heretofore-unrecognized specialized membrane ensembles. Also, the local concentration, and thus the effective molarity, of the interacting partners may be much higher than that attainable in solution. When such enforcement of the configuration and proximity of the interacting partners is coupled with the power of single cell phenotypic selections, antibodies that have new and, possibility, rare potentials may be selected. In terms of phenotypic selections of rare events, it should be noted that screening autocrine systems by FACS allows one to assay two million events an hour. Furthermore, because such interactions occur in the natural milieu of the receptor, they have a higher potential for physiological relevance.
The reasons that unusual antibodies are generated not withstanding, an understanding of their mechanism is of considerable interest. There are several possibilities. The most obvious is that the CD34+ population of cells is heterogeneous and consists of cells with various potentials for differentiation. However, if this were simply the case, one would expect that the natural ligand would also induce neurogenesis and activate the same genes. Thus, although both agonists bind to the same receptor in CD34+ stem cells, only one, the antibody, induces formation of neural progenitors. Also, the antibody induces expression of a different set of genes.
A likely explanation for the transdifferentiation is that we are observing a kinetic effect based the intensity and duration of the signal. This effect, in turn, is related to the stability and/or configuration of the “agonist occupied” receptor-antibody complex relative to G-CSF. The general concept is that the read out of signaling from the same receptor can differ because it depends on the integration of multiple factors including the strength and duration of the signal. We know that, during development and differentiation, the interpretation of signal strength is especially important in the response to morphogenic gradients (24–29). It is of interest that some of the genes, such as NDP, whose expression is the most up-regulated by the antibody, belong to pathways such as the Wnt/beta-catenin cascade, whose induction is sensitive to the duration and concentration of the signal (26). Although we are not dealing with physiological generation of signaling gradients, differences in the molecules themselves, in terms of their half-lives or how they interact with cellular pathways may, nevertheless, regulate their signaling potential. For instance, it has been suggested that receptor-mediated endocytosis may play a role in the regulation of cell signaling by controlling the persistence or availability of a ligand (30). In this sense, the antibody signal may be more sustainable than that of G-CSF because its half-life in the presence of receptor baring cells is days to weeks compared with only hours for G-CSF.
Another possibility is that binding to diverse regions of the receptor ectodomain can cause unique conformational changes or cross-linking that activates its signal transduction domain in different ways. These could be cis-effects where the receptors operate on their own, albeit with different consequences. There is the interesting possibility that we are observing a trans-effect in that a conformation change in the G-CSFR allows it to bind to and selectively activate a neighboring receptor such that a receptor-receptor interaction leads to engagement of a different pathway. In this case, the role of the antibody is to initiate this cascade. Alternatively, the G-CSFR and its neighbor could both be activated such that their synergy is responsible for the different cell fates in much the same way that induction of multiple transcription factors leads to cellular pluripotentiallity (31).
The work of others both lends support to our studies and indicates future directions, especially in terms of the therapeutic potential of antibodies that transdifferentiate autologous cells. Several authors have shown that the G-CSFR is present on a variety of neurons and this receptor and its G-CSF ligand colocalize in adult neurons, especially in the cortex, hippocampus, cerebellum, subventricular zone, and olfactory bulb. It has been suggested that this receptor-ligand system constitutes an autocrine system that, when activated, promotes neurogenesis (32, 33). Interestingly, the G-CSFR is also strongly expressed in radial glia cells (34).
Regardless of the role of G-CSF in the complicated milieu of the brain, the presence of the G-CSFR in adult neurons is consistent with our finding that it may play a role in their ontogeny. In terms of biased signal transduction, Tian et al. (35) demonstrated that a series of G-CSFR deletion mutants can activate different JAK–STAT pathways, leading to activation of distinct genetic programs. Thus, antibody agonists that are markedly different from the natural ligand could be expected to induce even more drastic changes in the properties of the signal-transducing domain of the receptor than that observed for simple deletion mutants. In the cytokine area, Kondo et al. (36) showed that lymphoid progenitors transfected with the Il-2 and the GM-CSF receptors were converted into cells of the myeloid lineage with a concomitant loss of their lymphoid potential when they were exposed to GM-CSF. The findings of Buzanska et al. (37) and Gage and colleagues (38) are, perhaps, more relevant to our report. Buzanska et al. reported that umbilical cord blood-derived cells of mesenchymal lineage can be reprogramed to neuronal fates when grown in neuronal media plus EGF, and Gage and colleagues showed that the ectopic expression of Sox2 and c-Myc in CD133+ cord-blood stem cells is capable of inducing neuronal-like cells.
Finally, as therapeutics, antibodies have the advantage that they are long lived and do not need to enter cells to function. Thus, antibodies could transdifferentiate autologous stem cells in vivo or in vitro to generate differentiated cells that are self. Such cells might be useful in a variety of ways, including the repair of an injured region of the brain or spinal cord.
Materials and Methods
Establishment of Reporter Cell Lines.
CellSensor SIE-bla HEK293T Cells (Life Technology K1649) were infected by lentivirus expressing wild-type G-CSF receptor at multiplicity of infection (MOI) = 5 to generate the stable cell line, SIE/BLA/SIG. The response to G-CSF was determined by detection of the FRET signal of CCF4-AM in accordance with the manufacturer’s instructions. To construct the growth-based reporter cell line, Ba/F3 cells were infected by lentivirus expressing the wild-type G-CSF receptor at a MOI = 5 to generate the BaF3-114 cells. The growth response of the cell line was determined 2 d after treatments by a MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay.
Analysis of Activation of the SIE-bla Reporter Cell Line.
After treatments, cells were incubated with the CCF4-AM substrate (Life Technology K1089) for 2 h. The FRET signal was excited at 409 nm, and the emissions were analyzed at 460 nm (Am-Cyan) and 530 nm (Pacific Blue). The activated cells are blue.
Patching.
Cells expressing antigen on the surface were incubated with fluorophore conjugated specific antibodies for 1 h in culture medium followed by fixation without permeabilization. The samples were analyzed by confocal microscopy.
Differentiation of CD34+ Cells in Culture.
Fresh bone marrow CD34+ cells were purchased from Allcells (catalog no. ABM010). The purity is greater than 96% as determined by FACS. The cells were washed with SFEM (Stem Cell 09600) and kept in SFEM with 50 ng/mL SCF (c-kit), 20 ng/mL IL-3, and 20 ng/mL IL-6 for 2 wk without a change of media. In total, 15,000 cells in a volume of 2 mL were studied in triplicate.
Immunoflorescence Analysis of Neural Markers.
The cells were fixed by 4% (vol/vol) PFA for 20 min and incubated with specific antibodies, followed by fluorophore conjugated secondary antibody staining. Tuj-1 antibody is from Covance (catalog no. MRB-435P), Nestin antibody is from BD Bioscience (catalog no. 611658), Phalloidin eFluor 570 is from eBioscience (catalog no. 41–6559). Alexa Fluor-488–conjugated secondary antibodies are from Life Technology.
Supplementary Material
Acknowledgments
We thank Fred Gage, Irv Weissman, Paul Greengard, Ronald Lindsay, Jim Rothman, and Tamas Bartfai for many helpful discussions and suggestions. Kristin Baldwin, Fred Gage, and Paul Greengard kindly provided vetted antibodies to neurogenesis markers. We thank Malcolm Wood for all the help with the SEM studies.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306263110/-/DCSupplemental.
References
- 1.Zhang H, Wilson IA, Lerner RA. Selectifon of antibodies that regulate phenotype from intracellular combinatorial antibody libraries. Proc Natl Acad Sci USA. 2012;109(39):15728–15733. doi: 10.1073/pnas.1214275109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huse WD, et al. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989;246(4935):1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- 3.Orlandi R, Güssow DH, Jones PT, Winter G. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86(10):3833–3837. doi: 10.1073/pnas.86.10.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sastry L, et al. Cloning of the immunological repertoire in Escherichia coli for generation of monoclonal catalytic antibodies: Construction of a heavy chain variable region-specific cDNA library. Proc Natl Acad Sci USA. 1989;86(15):5728–5732. doi: 10.1073/pnas.86.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature. 1990;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 6.Barbas CF, 3rd, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: The gene III site. Proc Natl Acad Sci USA. 1991;88(18):7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerner RA. Manufacturing immunity to disease in a test tube: The magic bullet realized. Angew Chem Int Ed Engl. 2006;45(48):8106–8125. doi: 10.1002/anie.200603381. [DOI] [PubMed] [Google Scholar]
- 8.Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: Origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 9.Graf T. Historical origins of transdifferentiation and reprogramming. Cell Stem Cell. 2011;9(6):504–516. doi: 10.1016/j.stem.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 10.de Petris S, Raff MC. Normal distribution, patching and capping of lymphocyte surface immunoglobulin studied by electron microscopy. Nat New Biol. 1973;241(113):257–259. doi: 10.1038/newbio241257a0. [DOI] [PubMed] [Google Scholar]
- 11.Okabe S, Hirokawa N. Actin dynamics in growth cones. J Neurosci. 1991;11(7):1918–1929. doi: 10.1523/JNEUROSCI.11-07-01918.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Letourneau PC. Differences in the organization of actin in the growth cones compared with the neurites of cultured neurons from chick embryos. J Cell Biol. 1983;97(4):963–973. doi: 10.1083/jcb.97.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Gang Zhang Z, Lan Zhang R, Chopp M. Activation of the PI3-K/Akt pathway mediates cGMP enhanced-neurogenesis in the adult progenitor cells derived from the subventricular zone. J Cereb Blood Flow Metab. 2005;25(9):1150–1158. doi: 10.1038/sj.jcbfm.9600112. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, et al. Dehydroepiandrosterone (DHEA) and its sulfated derivative (DHEAS) regulate apoptosis during neurogenesis by triggering the Akt signaling pathway in opposing ways. Brain Res Mol Brain Res. 2002;98(1-2):58–66. doi: 10.1016/s0169-328x(01)00315-1. [DOI] [PubMed] [Google Scholar]
- 15.Torroglosa A, et al. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells. 2007;25(1):88–97. doi: 10.1634/stemcells.2006-0131. [DOI] [PubMed] [Google Scholar]
- 16.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 17.McNeill B, et al. Hedgehog regulates Norrie disease protein to drive neural progenitor self-renewal. Hum Mol Genet. 2013;22(5):1005–1016. doi: 10.1093/hmg/dds505. [DOI] [PubMed] [Google Scholar]
- 18.Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: A major site of neurogenesis. Proc Natl Acad Sci USA. 2004;101(9):3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stallaert W, Dorn JF, van der Westhuizen E, Audet M, Bouvier M. Impedance responses reveal β2-adrenergic receptor signaling pluridimensionality and allow classification of ligands with distinct signaling profiles. PLoS ONE. 2012;7(1):e29420. doi: 10.1371/journal.pone.0029420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther. 2011;336(2):296–302. doi: 10.1124/jpet.110.173948. [DOI] [PubMed] [Google Scholar]
- 21.Stallaert W, Christopoulos A, Bouvier M. Ligand functional selectivity and quantitative pharmacology at G protein-coupled receptors. Expert Opin Drug Discov. 2011;6(8):811–825. doi: 10.1517/17460441.2011.586691. [DOI] [PubMed] [Google Scholar]
- 22.Galandrin S, Oligny-Longpré G, Bouvier M. The evasive nature of drug efficacy: Implications for drug discovery. Trends Pharmacol Sci. 2007;28(8):423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: The impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev. 2010;62(2):265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagès F, Kerridge S. Morphogen gradients. A question of time or concentration? Trends Genet. 2000;16(1):40–44. doi: 10.1016/s0168-9525(99)01880-6. [DOI] [PubMed] [Google Scholar]
- 25.Dessaud E, et al. Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen sonic hedgehog. PLoS Biol. 2010;8(6):e1000382. doi: 10.1371/journal.pbio.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dessaud E, et al. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450(7170):717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- 27.Balaskas N, et al. Gene regulatory logic for reading the Sonic Hedgehog signaling gradient in the vertebrate neural tube. Cell. 2012;148(1-2):273–284. doi: 10.1016/j.cell.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harfe BD, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118(4):517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118(4):505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 30.Sigismund S, et al. Endocytosis and signaling: Cell logistics shape the eukaryotic cell plan. Physiol Rev. 2012;92(1):273–366. doi: 10.1152/physrev.00005.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Diederich K, Schäbitz W-R, Minnerup J. Seeing old friends from a different angle: Novel properties of hematopoietic growth factors in the healthy and diseased brain. Hippocampus. 2012;22(5):1051–1057. doi: 10.1002/hipo.20904. [DOI] [PubMed] [Google Scholar]
- 33.Schneider A, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115(8):2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirsch F, Krüger C, Schneider A. The receptor for granulocyte-colony stimulating factor (G-CSF) is expressed in radial glia during development of the nervous system. BMC Dev Biol. 2008;8:32–39. doi: 10.1186/1471-213X-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian S-S, et al. Multiple signaling pathways induced by granulocyte colony-stimulating factor involving activation of JAKs, STAT5, and/or STAT3 are required for regulation of three distinct classes of immediate early genes. Blood. 1996;88(12):4435–4444. [PubMed] [Google Scholar]
- 36.Kondo M, et al. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407(6802):383–386. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 37.Buzańska L, Machaj EK, Zabłocka B, Pojda Z, Domańska-Janik K. Human cord blood-derived cells attain neuronal and glial features in vitro. J Cell Sci. 2002;115(Pt 10):2131–2138. doi: 10.1242/jcs.115.10.2131. [DOI] [PubMed] [Google Scholar]
- 38.Giorgetti A, et al. Cord blood-derived neuronal cells by ectopic expression of Sox2 and c-Myc. Proc Natl Acad Sci USA. 2012;109(31):12556–12561. doi: 10.1073/pnas.1209523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.