Abstract
Many of the axons that carry messages to the thalamus for relay to the cerebral cortex are branched in a pattern long known from Golgi preparations. They send one branch to the thalamus and the other to motor centers of the brainstem or spinal cord. Because the thalamic branches necessarily carry copies of the motor instructions their messages have the properties of efference copies. That is, they can be regarded as providing reliable information about impending instructions contributing to movements that will produce changes in inputs to receptors, thus allowing neural centers to compensate for these changes of input. We consider how a sensory pathway like the medial lemniscus, the spinothalamic tract or the optic tract can also be seen to act as a pathway for an efference copy. The direct connections that ascending and cortical inputs to the thalamus also establish to motor outputs create sensorimotor relationships that provide cortex with a model of activity in lower circuits and link the sensory and the motor sides of behavior more tightly than can be expected from motor outputs with a single, central origin. These transthalamic connectional patterns differ from classical models of separate neural pathways for carrying efference copies of actions generated at higher levels, and introduce some different functional possibilities.
Keywords: Efference copy, Corollary discharge, Sensorimotor link, Embodied perception
1. Introduction: early evidence from Golgi preparations
The patterns of axonal branching revealed by the Golgi method and described in significant detail by Cajal form the subject of this essay. They were early recognized as important. The Golgi method allowed investigators to trace axons for long distances, find branching points and often trace individual branches to several distinct end-stations, demonstrating functionally significant links. However, the full functional implications of these branching patterns could not be understood until the nature of axonal conduction was known. Cajal recognized that axonal conduction depended on neural impulses but his view of the conduction of impulses along the branches of an axon was based on a mistaken hydraulic model of the axon. He considered that, “At any point along an axon and its collaterals, the amount of energy associated with a particular input is proportional to the diameter of the process in question.” (Ramon y Cajal, 1995, translation of Ramon y Cajal, 1911, p. 428). He argued that “the sum of the diameters of collaterals supplied by a dorsal root fiber (see Fig. 1) is far greater than the diameter of the terminal arborization in the posterior column nuclei arising from the same root fiber.” (italics added). This led him to conclude that most of the information went to the collaterals, which mediated the fastest spinal reflexes. He wrote that “This is how the longest sensory fibers, which end in the dorsal column nuclei, relay that part of the activity giving rise to conscious sensations.” (p 429). Although we can see that on the basis of contemporary knowledge the hydraulic model does not provide an accurate view of how the “conscious sensations” transmitted by the posterior column fibers relate to the spinal reflexes, we should not ignore Cajal's insight: that the branching pattern must represent some close relationship between the messages that are sent through the posterior column nuclei to the thalamus and cortex on the one hand and those that play a role in spinal reflexes, on the other. The role of branched axons in defining what exactly is happening in the brain during a sensory experience forms the subject of this essay. It is an important problem because essentially all of the axons that carry messages to the thalamus for relay to the cortex come from branched axons comparable to those illustrated by Cajal, with one branch supplying the thalamus or a relay to the thalamus and other branches supplying brainstem or spinal centers with connections to motor outputs (Guillery and Sherman, 2002; Guillery, 2003). The argument we present now is a tentative interpretation, based on the anatomy of the pathways and information about impulse conduction along branched axons. It raises a number of problems that currently have no answers in terms of clear experimental evidence about specific pathways and their demonstrable functions. Our aim is to point to many areas where more knowledge about the distribution and the functional organization of the motor branches is needed, and where the specific actions of thalamocortical axons on cortical circuits need to be defined.
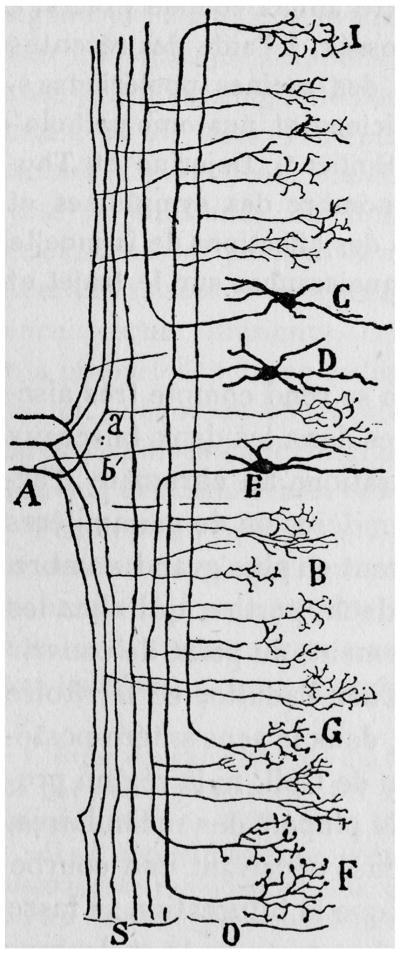
Fig. 1.
Drawing from Ramon y Cajal (1952, French translation) to show some of the branching patterns of incoming dorsal root axons that are entering the spinal cord at A and sending their ascending branches toward the posterior column nuclei at the top of the figure. They give off branches (e.g. a′, b′) that form dense terminal arbors in the region of the cord that contains cells (C,D,E) with further intraspinal connections.
2. Relating the ascending “sensory” axon to the innervation of spinal motor centers
Fig. 1 shows one of Cajal's drawings of the intraspinal branching patterns of the dorsal root inputs and Fig. 2 shows another of his drawings for the trigeminal nerve. In Fig. 1 the axons ascending towards the top of the figure on their way to the posterior column nuclei are shown with many spinal branches within the cord. Fig. 2 shows that nerve cells of the spinal nucleus of the trigeminal nerve (F) send axons towards the top of the figure on their way to the thalamus and also innervate two motor nuclei, the facial (D) and the hypoglossal (E) nuclei in the lower part of the figure, as well as the motor nucleus of the trigeminal nerve (C) in the upper part of the figure.
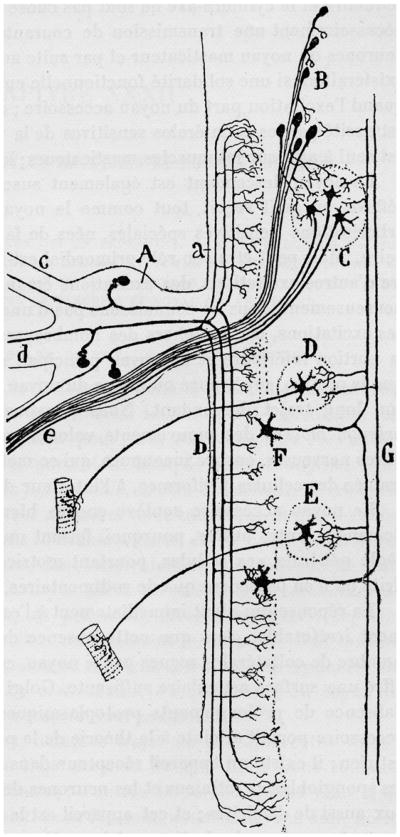
Fig. 2.
Drawing from Ramon y Cajal (1952, French translation) to show some of the branching patterns of incoming axons from the trigeminal nerve which enter the figure on the left. C, motor nucleus of the trigeminal nerve; D, facial nucleus; E, hypoglossal nucleus; F, spinal nucleus of the trigeminal nerve; G, spinothalamic axons.
Fig. 3 shows Sherrington's (1906) representation of the spinal connections of the scratch reflex. This figure illustrates a complex reflex reaction that survives a low cervical transection, and perhaps for this reason the figure does not show the ascending branches of the dorsal root axons whose peripheral branches innervate the two hairs shown in the lower left part of the figure and whose ascending axons were cut in Sherrington's experiment. Cajal's evidence indicates that such an ascending branch must have been present as does the more recent and detailed review of Lu and Willis (1999).
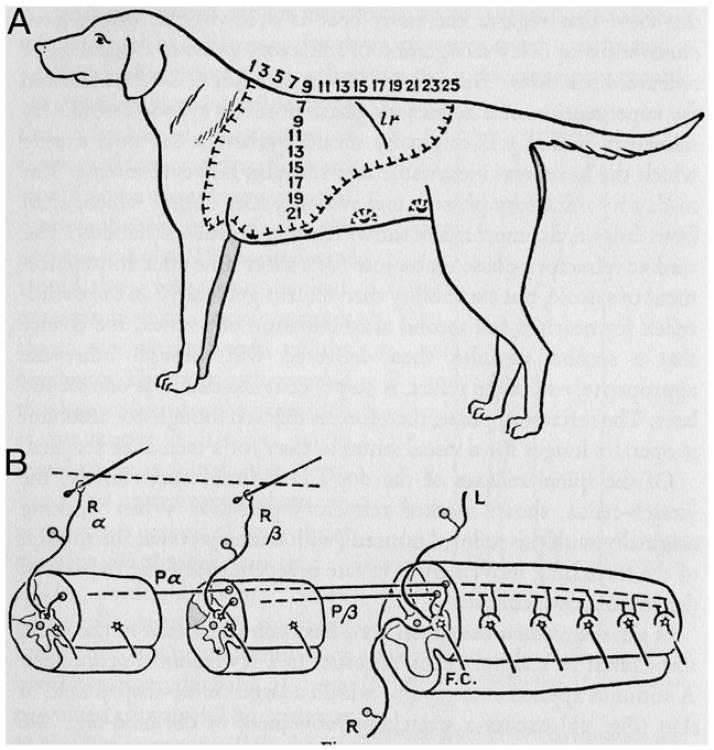
Fig. 3.
Diagram from Sherrington (1906) to show the major spinal connections of the scratch reflex. The two dorsal root ganglion cells that innervate the two hairs at the bottom left of the figure send branches to the spinal cord neurons and also have an ascending axon that was cut in this experiment and is not shown. Further details in the text.
These three figures illustrate anatomical relationships that were already well known in the 19th century but whose functional relationships are still puzzling.
The functional significance of these branched axons depends on more recent knowledge about the nature of the action potential (see Adrian, 1928; Hodgkin, 1964) and on experimental evidence about the way in which action potentials are distributed to the branches of anaxon. Impulses that pass along a branching axon enter each of the daughter branches and essentially the same pattern of impulses is transmitted along each branch (Cox et al. 2000; Raastad and Shepherd 2003). Some branch point failures may occur, particularly at high frequencies, but at a constant temperature, the message in one branch bears a consistent relationship to the message passed into the other. The relevance of the constant temperature suggests that the following discussion, of how activity in one branch relates to that in other branches, may apply to homeotherms but not to poikilotherms. That is an open question but since we are dealing with afferents to the thalamus, which is strongly developed in homeotherms, this point may be important. It is possible that branched axons in mammals can perform in ways that those of our cold-blooded ancestors cannot, and that this difference has been exploited in mammals in the pathways that carry messages to the thalamus for relay to the cortex; this perhaps presents a useful area for comparisons between the pathways of homeo- and poikilotherms.
Figs. 1 and 2 and the information in the previous paragraph indicate that the pattern of impulses sent along the ascending branches to the posterior column nuclei and through them to the thalamus and cortex must bear a constant relationship to the patterns of impulses sent to the spinal motor centers for walking, running, swimming, simple postural or defensive reflexes such as the scratch reflex and also for movements of the jaws. That is, the ascending fibers carry messages that represent not only the sensory inputs but they also represent the concurrent contributions to spinal or brainstem motor centers.
It is unreasonable to assume that the nature of the message passed to the sensory cortex, which is a faithful copy of the message(s) being passed to the spinal or brainstem circuitry, is read by the cortex as merely representing the sensory events and that the information already on its way to the spinal circuitry is ignored by the cortical circuits. The problem is to understand the significance of a single message that can be read to represent two different events, one occurring first in the sensory receptor and the other due to contribute to an event that will occur shortly in the motor apparatus.
One way of considering the dual significance of the single transthalamic message which represents the afferent message and the copy of the motor instruction, is to think of an army general who receives a message: “The enemy is advancing towards the river. A squadron has been sent to destroy the bridge.” The first part is a sensory message, the second part is motor. The general cannot afford to ignore either part of the message. He should immediately send one copy of the message to the officer in charge of observing the progress of the enemy and another to the officer concerned with the control of river crossings. Each of the subordinates will read the whole message and react in accordance with their particular responsibilities.
3. Efference copy or corollary discharge
Copies of motor instructions have a long history in neuroscience (see Grüsser, 1995). They have played an important role in accounting for an organism's ability to distinguish whether changes in sensory inputs are produced by changes in the environment or by movements produced by the organism that affect how a receptor responds to stimuli: a good example is in the effects that eye movements have on retinal receptors. In 1950 two important articles (Sperry, 1950; von Holst and Mittelstaedt, 1950) discussed the importance of such copies in relation to the circling behavior that resulted when the eyes of a fish (Sperry)or the head of an insect (von Holst and Mittelstaedt) were rotated through 180°. The proposed neural circuit included a copy of the motor instruction (named “corollary discharge” or “efference copy”) that was forwarded to a center where this instruction could be compared with the new afferent signal that was produced by the movement; this signal was called an “adjustment of the sensorium” by Sperry and “reafference” by von Holst and Mittelstaedt (see Fig. 4). The important point is that the efference copy and the reafference can be compared, as indicated by the plus and minus signs in Fig. 4, and the result sent to the centers concerned with initiating the movement. If the efference copy and the reafference match, no further action is needed, but if they don't match, then a further adjustment in the motor instruction may be required. The afference and the reafference signal are both coming from the same receptor and travel along the same axons, one after the other. The time interval between the signals will be critical for each system, and it is necessary to assume that the system is making a comparison between two time points separated by a precisely defined interval. If the sum of the efference copy and the reafference is not zero there is an uncertainty that the brain has to resolve: is the second signal about a change in the external stimulus or does it represent an error of the compensatory movement? If the sum is zero then there is still a degree of uncertainty but the probability of getting a zero is likely to be very low if there is any movement of the receptors or in the environment. The important point is that anything other than a zero calls for action,probably based on further information from other centers.
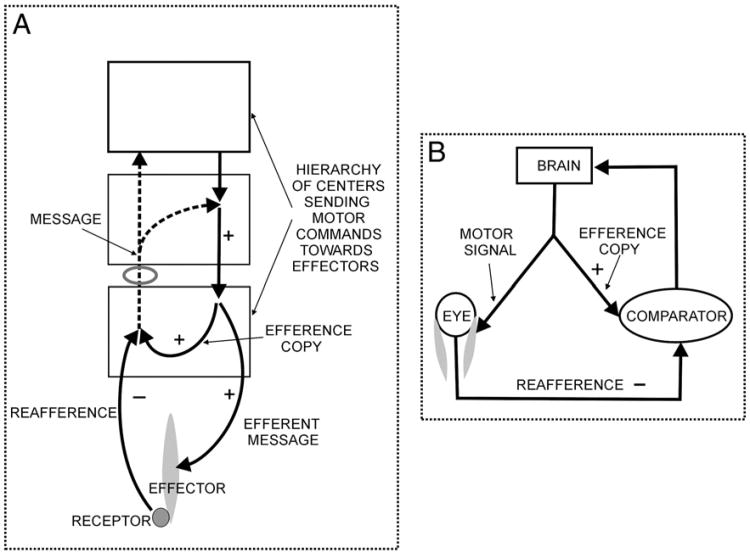
Fig. 4.
Schema based with some modifications on A: that of von Holst and Mittelstaedt (1950) and B: that of Perrone and Krauzlis (2008), who present it as a diagram to represent the corollary discharge/efference copy theory. Both figures have been modified to make the comparison easier. Note that whereas von Holst and Mittelstaedt appear to have the message after the comparison going back to a motor center, Perrone and Krauzlis in the original figure send it back as a sensory input to “perception of movement” to the right of the brain and outside the brain. This has been modified to go back to the brain in this figure.
Although Sperry and von Holst and Mittelstaedt used the visual pathways and the control of ocular movements in their accounts, the concepts of efference copy and reafference have been applied to these and to other motor control systems, with a variety of different precise meanings attached to the terms. There have been many studies of efference copies1 in a number of different systems, often with a particular focus on visual or limb movements or cerebellar pathways (e.g. Kandel et al., 1991; Bridgeman, 2007; Miall and Wolpert, 1996; Perrone and Krauzlis (2008); Sommer and Wurtz, 2008, Klier and Angelaki, 2008). The proposed pathways are often theoretical, with the actual anatomy of the pathways generally not clearly defined or completely undefined, and a number of different diagrams have been published (see Fig. 4 for two examples).
The implied value of an efference copy is that it provides information of body movements, information needed to distinguish actual changes in the environment from those that are self-induced. One advantage of efference copy over proprioceptive feedback of bodily movements is that its signal is earlier and actually anticipates movements. Efference copy messages and those from propioceptors must be integrated to provide the complete analysis required to sort environmental changes from self-induced ones. Typical of more conventional ideas of efference copy is the recent evidence presented by Sommer and Wurtz (2004a,b, 2008). They describe a pathway in the monkey from the superior colliculus to the medial dorsal nucleus of the thalamus and thence to the frontal eye field of cortex that they argue provides an efference copy (although their term is “corollary discharge”) for impending saccades. If this input from midbrain to thalamus is, indeed, relayed to the cortex, a plausible but as yet unproven point, then we predict that the collicular axons involved are branches of axons that also innervate oculomotor centers. In this regard, this view of efference copy (or corollary discharge) fits neatly with the views outlined in this essay, although we distinguish the brainstem circuits, which are responsible for the actual performance of the compensatory motor actions from the transthalamic copies of the brainstem actions, which serve to keep cortex informed about what the lower centers are doing. An important additional piece of evidence concerning these transthalamic pathways is the occurrence in several visual cortical areas of nerve cells whose receptive fields shift in position in accurate anticipation of a saccade. (Duhamel et al., 1992; Colby et al., 1996; Sommer and Wurtz, 2004a, b, 2008). This can be seen as evidence that cortical cells are kept informed about instructions for movements that are currently being generated at lower levels.
An efference copy is an instruction for a movement and must be clearly distinguished from the movement itself. It is not a movement. Even if the axon that represents the efference copy innervates a ventral horn cell whose axon goes directly to a muscle, this ventral horn cell is likely to have several different inputs and the movement it produces will almost certainly depend on more than one muscle. We are not looking at efference copies in individual axons as providing information about movements. They provide information about a contribution to the production of a movement, which will almost certainly depend on multiple factors, factors that can vary from one moment to the next.
Much of the circuitry that allows for a distinction between the movement of the receptor and the movement of the stimulus is likely to be taken care of at levels that do not involve thalamus and cortex. The proposals from Sperry and von Holst and Mittelstaedt are based on organisms, flies and fish, that must have solved the problem with non-cortical mechanisms. The fish will probably have relied on tectal and spinal circuits or, more likely, on the circuits that link the tectum to the oculomotor and spinal outputs. It is important to realize that mammals have these same circuits, largely preserved through evolution. The inputs to thalamocortical circuits considered in what follows should not be thought of as producing the messages that need to be passed to the muscles. They represent a record of what the lower levels are doing, providing the cortex with continuous information about sensorimotor events.
Usually the discussion of efference copies refers to motor instructions that are generated centrally; Klier and Angelaki (2008) define them as “copies of voluntary, outgoing motor commands that are generated whenever we make a movement”. This is a far narrower view than that implied by the original proposals of Sperry and von Holst and Mittelstaedt, which were based on reflex movements in fish and insects. We have learnt that the term “efference copy” means something rather special to many people who are puzzled by the idea of an efference copy being carried in a sensory axon. We are using the term to describe a situation where an axon that acts on motor centers also sends a branch to some other, generally higher center.Weargue that the rules that apply to conduction of impulses at branch points, and summarized above, lead to the conclusion that a copy of the motor instruction must also be sent along the non-motor branch. The possibility of copies of motor instructions reaching the thalamus from the dorsal root ganglia or the optic nerve as efference copies introduces a novel view and deserves careful consideration.
When a new sensory receptor is acquired in the course of evolution it will have no survival value if it lacks a motor output. Probably this relates to the fact that many receptors appear on cilia. The early reflex motor connections of receptor inputs can be seen as an evolutionary necessity. These pathways can only later sprout ascending, “sensory” connections. It appears from the accounts of branching sensory inputs described above that many (probably all) sensory pathways maintain their early motor connections. The view that sensory pathways cannot be, themselves, directly involved in reporting motor functions, often expressed by colleagues in discussions, is not tenable. This is a crucial point in the following presentation. Pathways that carry messages from peripheral receptors to the brain at their early entry to the brain serve motor functions, with “early” here referring to the evolutionary history and also to the topographical relationships of the entering axons. The ascending branches that develop at later stages of evolution must then carry a copy of the early motor connections, and these copies have to be seen as efference copies in the literal meaning of the words. They serve to keep higher levels informed of sensorimotor events at lower levels.
We realize that the term “efference copy” is often used to describe a message originating from a motor center, and so seems inappropriate for a message sent along a sensory pathway such as the ascending spinal or trigeminal pathways or the optic nerve. However, a major point that needs to be recognized is that this view needs reconsideration, because these sensory pathways also have the phylogenetically old motor connections and thus do, indeed, carry copies of motor instructions.
A crucial point about any efference copy is that it must be a faithful representation of the original motor instruction. It need not be an exact copy. If it is a reliable representation that can be adjusted by a multiplier somewhere in the circuit, then it can function in the way intended. It looks as though, for a homeotherm, the best way of producing a reliable representation is by a branching axon. This is also the best way for the investigator to identify a pathway that carries a reliable representation. Any other type of connection has a disadvantage. It is not easy to see how a reliable representation not involving branched axons can be produced consistently by the organism and for the investigator it becomes important to demonstrate that there really is a reliable representation. If an efference copy does not arise from a single cell through a branched axon, then it must arise from two separate nerve cells in the relevant center and their activity patterns over a long term must demonstrably have the required relationship. It may be difficult to establish that long term, accurately matching activity patterns exist when different cells or populations must separately encode them, whereas branching patterns, which have been known for more than a century from Golgi preparations and more recently from other experimental approaches, provide the most reliable means of transmitting exact copies of any message, and this is a requirement for an effective efference copy.
If the ascending axon that goes to the posterior column nuclei is now looked at from the point of view of its relationship to the branches that are innervating spinal reflex mechanisms, it has to be regarded as an efference copy. It carries a reliable representation of the instructions that produce the reflex movements of the limbs (or jaws for the trigeminal nucleus). This is information that higher levels can use to generate new motor instructions. Previous studies have generally (e.g. Sommer and Wurtz, 2008; Bridgeman, 2007; Perrone and Krauzlis, 2008, and see Fig. 4) treated efference copies or corollary discharges as messages that are carried by a distinct pathway to a separate center, where the efference copy and the reafference can be compared. The issue that needs to be explored is how a single axon can be carrying the afferent signal, and the copy of the motor instruction at the same time.
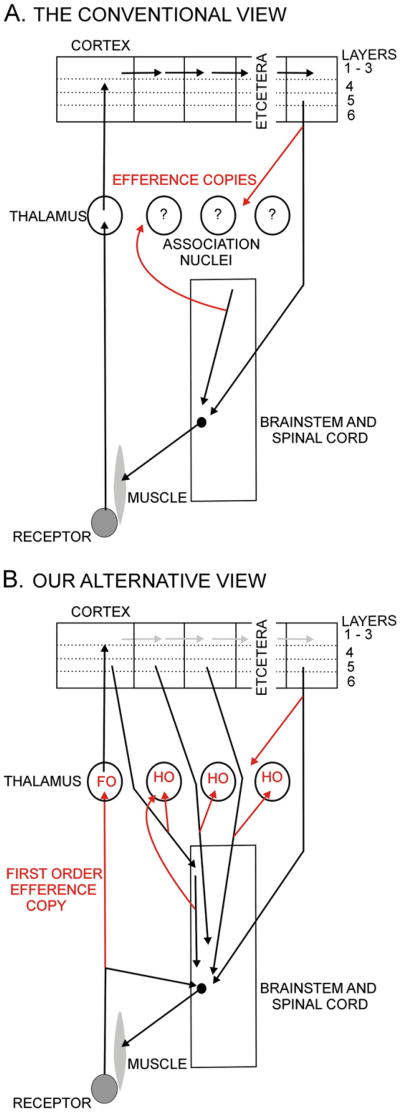
The medial lemniscus and the optic tract are sensory pathways bringing messages to the brain about the outside world. That is their sole function for any textbook that deals with neuroscience. However, the evidence summarized in the previous sections argues strongly that these pathways are also carrying a different type of information. This is a strange and surprising conclusion that deserves some detailed discussion. In Fig. 5 we compare the dominant contemporary view of thalamocortical pathways with the view we are proposing. While both include an efference copy in a corticofugal axon that sends a branch to the thalamus, it is the ascending branch to the thalamus, labeled first order (FO) efference copy, that, in the words of one colleague, “may raise a few questioning eyebrows and perhaps a snicker or two”. We call this a first order efference copy, and the others higher order (HO) efference copies for reasons explained in the next section.
Fig. 5.
Two views of thalamocortical pathways. The upper figure illustrates a motor instruction to the lower motor center, coming either from the cortex or from the upper parts of the brainstem. Each can send an efference copy shown in red to the thalamus. The lower figure shows the afferents to the thalamus, also in red, all serving essentially the same function as copies of motor instructions. Abbreviations: FO first order, HO higher order.
4. The common occurrence of branched driver inputs to the thalamus and their possible functional significance
For the ascending spinal pathways considered so far there is no question that the message they carry is relayed to the cortex. Although the terminals of thalamic afferents that carry messages for relay to the cortex, the “drivers” (Sherman and Guillery, 1998), represent only a minority of the afferents in any one thalamic nucleus (generally less than 10%), they have a characteristic light and electron microscopical appearance in all major thalamic nuclei and are characterized by comparable patterns of multiple synaptic junctions, with well defined transmitter and receptor characteristics.(Sherman and Guillery, 1998).
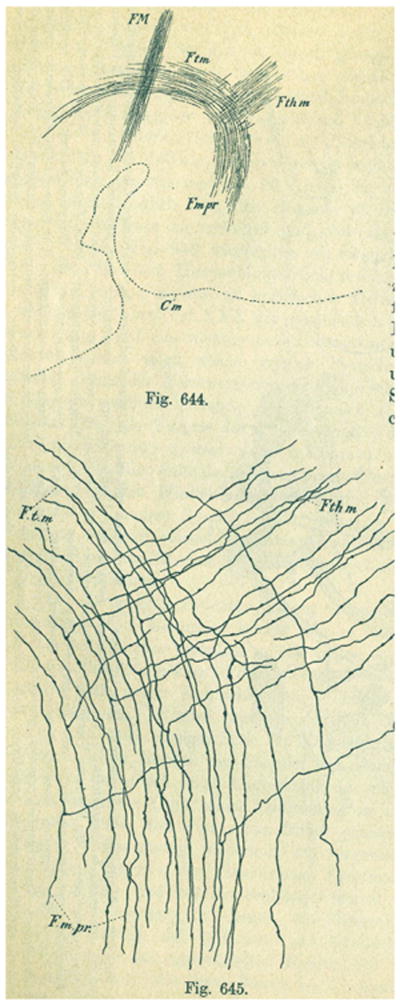
Figs. 6 and 7 show two more of Cajal's drawings of Golgi preparations that demonstrate branching patterns in some other driver afferents to the thalamus. Specifically they show axons going to the thalamus from the mamillary bodies (Fig. 6) and the cerebellum (Fig. 7). These are both pathways that have the characteristic driver2 terminals in the thalamus (Harding, 1973; Rinvik and Grofova, 1974; Kultas-Ilinsky and Ilinsky, 1991; Somogyi et al, 1978, Petrof and Sherman, 2009). They show a branching pattern comparable to that of the ascending somatosensory inputs.
Fig. 6.
Ramon y Cajal's (1911) illustration of the thalamic branches given off by the mamillotegmental tract. The upper figure (644) is a sagittal section (anterior to right, dorsal up) that shows the principal mamillary tract (Fmpr) giving off the mamillothalamic tract (Fthm) anteriorly and continuing posteriorly as the mamillotegmental tract (Ftm). FM, habenulo-peduncular tract. The lower figure (645) shows the detail of the branching.
Fig. 7.
Ramon y Cajal's (1911) illustrations of the thalamic branches given off by the superior cerebellar peduncle. The upper figure shows the overall branching pattern. O. Deep cerebellar nuclei, A, superior cerebellar peduncle, a, b, c branches given off on the way to the thalamus. In the lower figure, A shows the axons from the dentate nucleus; B shows the axons that go to the brainstem, which are labeled “a” in the left of figure and C shows the axons that go to the red nucleus and thalamus.
The visual pathways, which provided much of the original stimulus for discussions of efference copies and corollary discharges, albeit in fish and flies in which thalamocortical circuits play no role, provide another example of branching inputs to the thalamus. The axons of retinal ganglion cells in all mammalian non-primate species that have been studied are all branched, sending one branch to the midbrain and the other to the lateral geniculate nucleus (summarized in Guillery, 2003). In the midbrain most of the axons go to the superior colliculus, a phylogenetically old structure concerned with the control of head and eye position, that is, with the control of gaze. Other axons go to the pretectal areas which are primarily concerned with pupillary control and accommodation, although they also relate to vestibular mechanisms. The lateral geniculate nucleus is the thalamic relay for vision which passes the visual messages on to the primary visual cortex, area 17 or V1. For primates there may be some retinal ganglion cells (the parvocellular component) that send no or only few branches to the midbrain, but there can still be argument about this point (see Guillery, 2003). The other major retinogeniculate components are undoubtedly branches of retinal axons that go to the midbrain.
The visual connections, thus, resemble the other ascending driver inputs to the thalamus in having one branch going to the thalamus for relay to cortex and another branch going to the midbrain for motor actions. The former carries the sensory messages about the outside world and this message must, again, be seen as also representing copies of ongoing motor instructions that are currently being issued for gaze control, accommodation or pupillary control.
In addition to the driver afferents considered so far, which come from subcortical pathways and innervate thalamic relays called first order (Guillery, 1995), drivers from layer 5 of the cortex3 innervate thalamic relays that were earlier classified as “association” nuclei, and that can be considered to be higher order thalamic relays because they receive messages that have already passed through the cerebral cortex (see Fig. 5B and Guillery 1995).
Figs. 8 to 10 show several cortical layer 5 axons. Each has a motor branch going to the superior colliculus or pretectal areas (Figs. 8 and 9C for visual cortex) or descending towards the spinal cord (Fig. 9B for somatosensory, Fig. 10 for motor cortex). These layer 5 axons resemble the ascending sensory afferents not only in their branching pattern, but also in their appearance, in the fine structural characteristics of their thalamic terminals, in their synaptic properties, and in the transmitters and receptors active at these thalamic synapses (Rockland, 1996, 1998; Sherman and Guillery, 1998; Rouiller and Welker, 2000; Sherman, 2001; Reichova and Sherman, 2004). The functional connections established by cortical driver inputs to the thalamus have also been studied in slice preparations from mice, clearly demonstrating that these corticothalamic axons are drivers, sending inputs to the thalamus for relay to higher cortical areas (Reichova and Sherman 2004; Theyel et al., 2010).
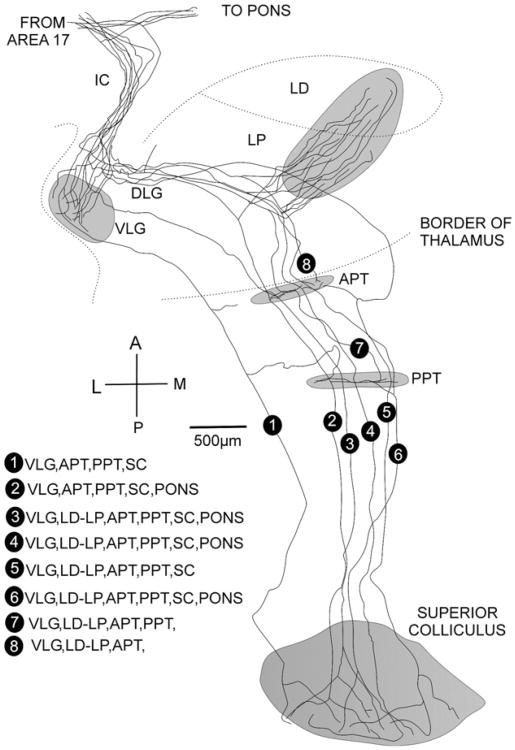
Fig. 8.
Eight labeled axons from layer 5 of the visual cortex of a rat are shown passing through the internal capsule (IC) and sending branches to the pons, the ventral lateral geniculate nucleus (VLG), the lateral posterior (LP) and lateral dorsal thalamic (LD) nuclei and continuing to the anterior pretectal nucleus (APT), posterior pretectal nucleus (PPT and the superior colliculus (SC), but not to the dorsal lateral geniculate nucleus (DLG). The distribution of each axon to these terminal stations is listed in the lower left part of the figure.
Redrawn from Bourassa and Deschênes (1995), with permission.
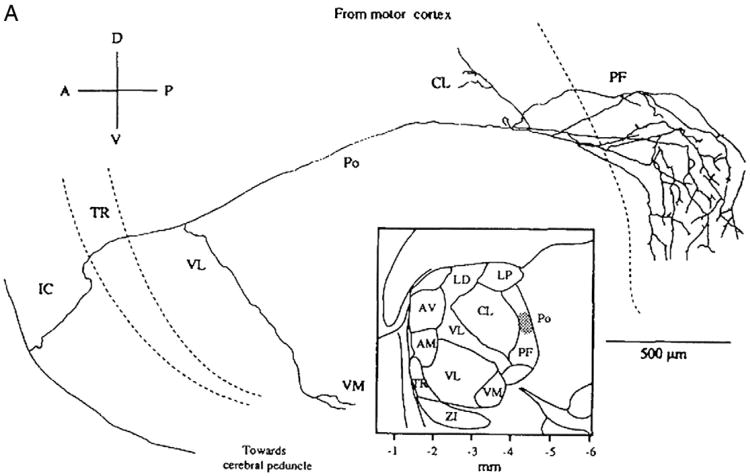
Fig. 10.
An axon from the motor cortex of a rat is shown at the lower left part of the figure. It sends a branch that distributes mainly to the parafascicular nucleus of the thalamus (PF) and continues towards the cerebral peduncle.
From Deschênes et al. (1994) with permission.
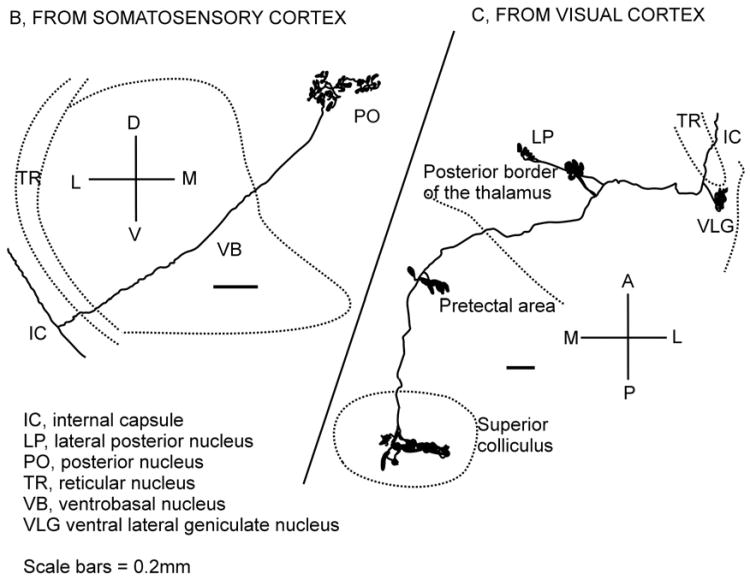
Fig. 9.
Two axons from the cortex of a rat are shown. One comes from the somatosensory cortex (left, B); it sends a branch with terminals in the posterior nucleus of the thalamus and then continues to the cerebral peduncle, the other (right C) comes from the visual cortex and sends branches to the ventral lateral geniculate nucleus (VLG), the lateral posterior thalamic nucleus (LP) and continues to the midbrain with terminals in the pretectal nuclei and the superior colliculus. Redrawn from Deschênes et al. (1994) with permission.
Corticofugal motor outputs with thalamic branches such as those shown in Figs. 8 to 10 have been demonstrated in monkey, cat, rat and mouse and have been described for the visual, somatosensory, motor and other cortical areas (Deschênês et al., 1994; Bourassa and Deschênes, 1995; Bourassa et al., 1995; Rockland, 1996, 1998; Rouiller and Welker, 2000; Guillery et al., 2001). The branching pattern indicates that they resemble the first order ascending afferents from the spinal cord and the retina (Fig. 5) and carry an efference copy through the thalamus to the cortex. The figures show that the thalamic branch of the corticofugal somatosensory axon goes to the posterior nucleus of the thalamus for relay to higher cortical areas in the parietal cortex and that the thalamic branch of the visual axon goes to the lateral posterior nucleus for relay to higher cortical areas in the occipital lobe. These are efference copies since the nonthalamic branch goes to motor centers in brainstem or cord; the message that reaches the higher cortical areas is about a motor message. However, if we look, for example, at the cells in layer 5 of the primary visual cortex, which have the properties of complex receptive fields, then it becomes apparent that these corticothalamic axons are also sending messages that can be read as signals either about visual events or about efference copies. As for the first order efference copies, these are copies of sensorimotor messages relayed through the thalamus to higher cortical areas for information that, in turn, can allow the higher order cortex to compute the contribution being made to action, if any.
Not long ago one of us (RWG) had the opportunity to look at a number of preparations of tracer injections made into several different cortical areas in monkeys that Kathy Rock-land had in her lab in the Riken Institute in Tokyo. These all included many axons going to the thalamus, which were branches of longer descending axons. They were identifiable as layer 5 axons on the basis of the appearance of their thalamic terminals and the absence of any branches in the thalamic reticular nucleus (see Rockland, 1996, 1998). Guillery et al. (2001) had earlier traced 48 axons from either cortical area 17 or 18 of cats through serial sections to the thalamus, and concluded that all of them continued into the midbrain.
In contrast to the first order efference copies shown in Figs. 6 and 7, the thalamic branches of these axons are higher order efference copies. The nonthalamic branch goes to motor centers in brainstem or spinal cord; the transthalamic message that reaches the higher cortical areas is, again, a copy of a motor message. It is to be stressed that each motor branch is likely to make a partial contribution to the execution of the next movement. Where an axon has many branches it will have many distinct (but related) actions, and where, several distinct branching axons contribute inputs to the motor action each will make a separate contribution to the movement. These are not messages about movements; they are messages about distinct identifiable instructions for movements, and the copies of these messages sent to thalamus for relay to cortex are what we refer to as efference copies.
We know very little about the messages layer 5 cells with thalamic and brain stem branches are sending to either the thalamus or the brainstem. Effectively the messages relayed through the thalamus to higher cortical areas are copies of ongoing sensorimotor events that are being passed from one level to the next higher level of cortex. Our knowledge about the nature of the sensory content will depend largely on the cortical origin of the axons and our knowledge of the motor instructions will depend on the motor circuits that the axon innervates. The details of the post-thalamic distribution of the corticofugal axons that have thalamic branches are generally not known, and where, as in the superior colliculus, we have some information about their termination (see Fig. 11) we know almost nothing about their function and this is one of the reasons for writing this essay, to stress how much is still not known about where these motor branches terminate or what may be their function.
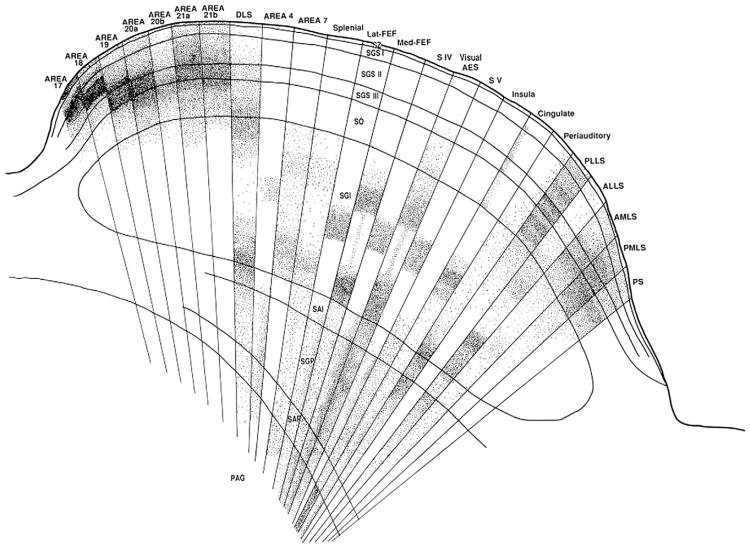
Fig. 11.
Drawing to show the terminal distribution of radioactively labeled corticothalamic axons from 24 different cortical areas in the layers of the superior colliculus.
From Harting et al., 1992 (with permission).
Essentially all cortical areas have layer 5 axons that project to brain stem or spinal cord (e.g. Fries, 1984; Kawamuram and Konno, 1979; Akintunde and Buxton, 1992). Fig. 11 shows that in a cat 25 different cortical areas send axons to the superior colliculus. Area 17, a cortical area with strong claims to be treated as a “primary sensory area”, sends its axons to the most superficial layers and the frontal eye fields, which play a crucial role in the cortical control of gaze, project to the deeper layers of the superior colliculus. The motor outputs of the colliculus arise from the deep layers, suggesting that each single axon from area 17 has a relatively distant contribution to the motor outputs whereas the frontal eye fields have a relatively direct one. That is, the figure suggests a hierarchy of visual areas progressing from area 17 through occipital and parietal areas to the frontal eye fields. Comparably, for the somatosensory pathways, the somatosensory cortex (areas 3, 2 and 1) projects further from the ventral horn motor neurons than does the motor cortex (area 4); (Nyberg-Hansen, 1966; Coulter and Jones, 1977). That is, as the message is passed from sensory receiving areas through higher cortical areas, the motor action appears to become more direct, and correspondingly, probably plays a more significant role in the movement control. Where these corticofugal axons have a thalamic branch the efference copy that goes through the thalamus for relay to the next higher cortical area will then be a copy of a stronger motor action.
Although much more information is needed about the distribution of such corticothalamic driver inputs, there is evidence that all of the higher order thalamic nuclei receive such cortical inputs and that large areas of frontal, parietal, occipital and temporal cortex give origin to them. Many of these axons provide a transthalamic pathway for corticocortical communication that may parallel the direct corticocortical connections described by Van Essen et al. (1992; and see Young, 2000). However, although it is probable that the direct and the indirect transthalamic pathways have a comparable hierarchical organization, there is not sufficient detailed evidence about either pathway for a clear conclusion about such parallelism.
5. What happens when these messages reach the cortex?
This question clearly requires an answer. The following represents a suggestion that needs significant experimental study before it can be taken seriously. It is an attempt to follow through the implications of the observations reported so far. If the observations reported in Sections 1–4 are accepted then the need to find an answer to this question is clear. It would be a mistake to think of the mechanisms proposed in this essay as providing a single clear answer to the motor control issues related to the use of efference copies in the pathways to the cortex. We have stressed that our poikilothermic ancestors may have lacked efficient stable efference copies. They may well have had other ways of dealing with motor control in relation to the changes in outputs of receptors produced by movements. Some of these mechanisms still play a role in mammals. Grillner et al. (2008) have demonstrated the extent to which the spinal mechanisms of a cat resemble those of a lamprey. What follows is an attempt to understand how we can trace the dual message that characterizes the driver inputs to the thalamus. It is not an attempt to explain the complex motor control systems of the limbs or the eyes. This would involve a great many different circuits including the basal ganglia and the cerebellum. The discussion will be limited to the puzzle of the single inputs that represent both sensory events and motor instructions.
A good way to start thinking about this question is to recognize that there is only one message in the afferent pathway to the thalamus at any one time, but that this message can be read as a sensory message or as an efference copy when it first appears. Once the input to a muscle has moved a receptor, then the changed output from the receptor will also represent the reafference signal; it can be used for comparison with its immediate predecessor. That is, there is an important time element to be considered. At time 1 the message can be read as either the afferent (sensory) signal or as the efference copy. At time 2,the message represents the next afferent signal together with another efference copy. However, now the afferent signal at time 2 also represents the reafference signal that needs to be compared with the efference copy at time 1. We stress that at any one time there is only one message. What matters is how each message is processed. Functionally each message plays three roles, at first as sensory input and efference copy, and immediately after this as both of these (current sensory input and efference copy) as well as the reafference signal for the preceding message. In this sense the single message can be functionally tripartite.
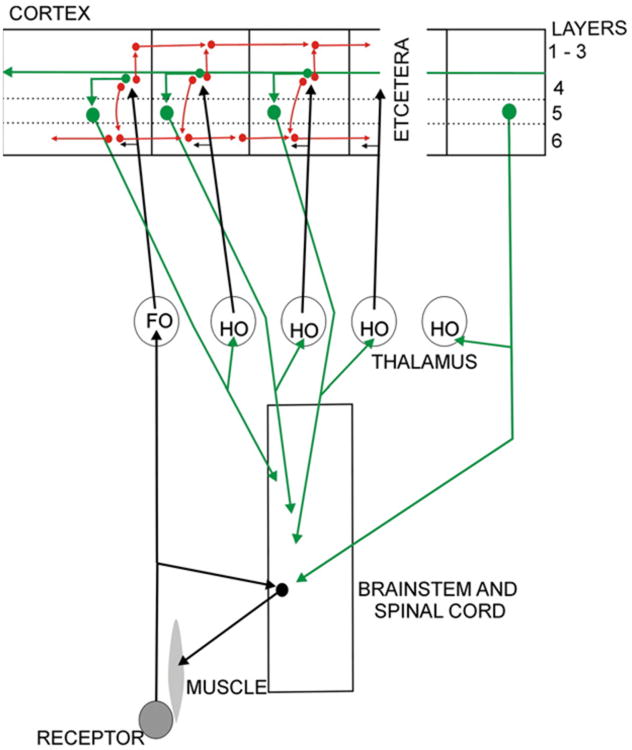
One way in which the cortex handles the incoming sensory message has been well documented for the visual pathways. It processes the message as sensory information, turning simple cells into complex cells and then passes the information by direct corticocortical pathways through a hierarchical series of visual areas for further separation of functions and higher order processing. Van Essen et al. (1992) have described the relevant pathways for the hierarchy of cortical processing, and almost any neuroscience textbook can show well documented details about the sensory visual processing in the occipital, temporal and parietal lobes of cortex. This sensory corticocortical circuitry is represented schematically in red in Fig. 12. A second way in which the cortex must also be handling the incoming messages is to compare each message with its predecessor and then to pass this to the layer 5 cells that have access to motor centers of the brain stem and spinal cord (green in Fig. 12). This comparison is effectively a comparison between the earlier efference copy and the current reafference signal and if there is a discrepancy it has to lead to a compensatory motor action.
Fig. 12.
Schema to illustrate the possible splitting of the sensory pathways within the cortex. Sensory analysis (red) passes through the direct corticocortical connections and the efference copy related analysis (green) passes to the layer 5 output which then innervates the motor system (with a weak message). This layer 5 output, in turn passes another efference copy through the transthalamic pathways to higher cortical areas each of which in its turn may have the capacity to act more directly on the motor apparatus. Abbreviations: FO first order, HO higher order.
This second process (shown in red in the figure) is the aspect of cortical processing that has received almost no attention. If, as our previous arguments indicate, each cortical area receives what is essentially a replica or a model of the sensorimotor activity at the lower level then it is in a strong position to anticipate events, and to produce appropriate outputs. Each area has not only information about the sensory events in the outside world, but in addition each has some information about actions being taken in response to external events and is in a position to assess the extent to which appropriate changes are being made.
Even the primary receiving areas of the cortex have layer 5 outputs with brainstem or spinal connections: V1 (area 17) to the superior colliculus and somatosensory cortex to the dorsal horn of the spinal cord. There is some evidence that these layer 5 cells in visual cortex have motor actions. This is based on experiments in which the geniculate pathway to the visual cortex was silenced or the visual cortex itself was stimulated, indicating that the visual cortex acts through the superior colliculus in the control of eye movements, unlike more anterior cortical regions which act through subtectal oculomotor centers of the midbrain (Schiller and Tehovnik, 2005; Schiller et al., 2008). The motor actions of V1 are subtle and not easily demonstrated for the visual cortex, but it looks as though they are present even in V1.4 Action and perception arrive together at the cortex, but are then separated, the perceptual process continuing in the cortex and the action linking to the body. However, this separation is never complete. The two are brought together again and again at each higher cortical level, because the layer 5 outputs at higher levels also branch and a further copy of this higher layer 5 motor instruction is sent back to the higher order thalamic nucleus as another efference copy, again combined with the sensory content that was necessarily a part of the message coming from the primary sensory or higher cortical area. We emphasize the speculative nature of this proposal for comparing the efference copy with the reafference signal, and acknowledge that relevant direct data are lacking. Probably such results have not been found because nothing like this was expected. These issues have not been considered before. We argue that it is plausible that something like this process must be happening in cortex.
One important question to ask about the cortex is whether it can receive an input and read it first as a message about the sensoriumand immediately there after use the same message to compare the relevant efference copy with the reafference in order to arrive at an output. Is this how we can abstract sensory information from what appears to be a mixed message? And can the process be experimentally demonstrated? Experiments that have demonstrated cells in the lateral intraparietal cortex that appear to anticipate a future receptive field (Duhamel et al.,1992 Berman and Colby, 2009; and see Sommer and Wurtz, 2008) suggest that there is a temporal sequence in the cortical analysis, with the outcome of the comparison between the efference copy signal and the reafference signal being recorded as a “future”, new receptive field. The possibility that a comparable anticipation of a future eye position may exist in other cortical areas (V4, and V1) is suggested by the studies of Tolias et al. (2001) and Trotter and Celebrini(1999). These results indicate that the cortex may be making comparisons across time, and anticipating events on the basis of information about forthcoming movements.
If we now compare Figs. 4 and 12 there are several points that are striking. One is that in the von Holst and Mittelstaedt figure there is a region on the left which we have indicated by a small gray circle, where the three different functions appear together. The “message” here includes a contribution from the receptor marked reafference, the efference copy and, necessarily, though not shown as a separate line in the figure, the actual sensory message. If we treat each line as an axon then the tripartite function of the pathway labeled “message” is clear. The possibility of treating these separate functions as shared within one axon is not a necessary interpretation of Fig. 4, but it is a necessary interpretation of Cajal's figures (Figs. 1 and 2), and of any of the pathways discussed above and identified as single axons.
One major difference between Figs. 1, 2 and 4 is that the message that is being sent to the effector does not come from a hierarchy of the motor centers in receipt of sensory inputs and able to initiate more or less independent motor actions. Figs. 1 and 2 show axons that come from the primary sensory roots that first pass the information to the brain. This may be a crucial difference, because it forces the tripartite functions all into a single axon. It also forces us to recognize that significant parts of the movements necessary for a sensory experience are directly determined by the sensory inputs. They are reflex. They may be relatively weak, as are the collicular inputs from the retina. Even those from area 17 or V1 appear to play a limited role in the complex circuitry of gaze control.
There are several important differences between the functional organization of efference copies that travel in afferents to the thalamus and cortex and those that are generated as copies of motor instructions from higher motor centers as in Fig. 4. The most obvious difference between these circuits is their origin. An efference copy that arises as an immediate response to the environment is significantly different from one arising from a neural center that initiates a new movement. We will call the former a tripartite message and the latter a unitary message. The latter can be thought of as arising from a “voluntary” event, but the former cannot; they are based on reflex events. Another difference is that the tripartite message is using a single axon to carry a message with three interpretations (sensory, efference copy and reafference) whereas the latter appears to be using different pathways for each function. Topographical differences generally serve to separate the circuits concerned with perceptual processing from those concerned with the necessary motor control, but for the tripartite pathways the topographical separation does not start until the cortex is reached (see red and green pathways in Fig. 12) and then is likely to be incomplete.
In the tripartite connections sensorimotor contingencies are present everywhere, but the balance between the sensory and the motor component changes as the sensory messages are processed by the cortex, apparently moving from strongly sensory and weakly motor to the opposite. Can the two functions be separated in the brain, or are they inextricably linked? Having bound the three functions together in a single axon, can the brain separate them or should we recognize that there is no such thing as “pure” sensation (see Churchland et al., 1994, on pure vision). Must our view of the external world be one of “sensorimotor contingencies” as proposed by, for instance, O'Regan and Noë (2001), Noë (2004), Clark (1998), Pfeifer and Bongard (2007) and many earlier investigators who saw that there are aspects of perceptual processing that cannot be understood in terms of the brain (or the mind) receiving sensory signals passively and who understood that the actions related to perception form an integral part of the perceptual process, a view of perception that is sometimes referred to as “embodied perception”.
6. Conclusions
The main point of our review is that, although there are many efference copy messages in the lower motor mechanisms involved in stabilizing the sensory world of a moving organism, the efferent copies that represent most, possibly all,of the driver afferents to the thalamus function primarily to convey to the cortex a model of what the lower levels are doing. They connect to these mechanisms but do not repeat the functions of the efferent copies that characterize lower levels in brainstem and spinal cord. They can be seen as a way for the cortex to be kept up to date on the functional changes at lower levels.
Another major conclusion concerns the tripartite nature of pathways that have long been accepted as purely sensory. A further conclusion concerns the possibility that cortex processes its inputs by making comparisons across time. To our knowledge, apart from the demonstration of “future receptive fields”, this is not an area that has received significant attention. What is the sequence of actions one should expect in the cortex, and to what extent will they involve distinct groups of neurons? In terms of the pathways involved, the differences between those that are tripartite and those that separate the sensory components, the efference copies and the reafference signals may prove important. Investigations of the distribution of the two types of circuit, demonstrating the anatomical and, more importantly, the functional characteristics of each may prove valuable.
The functional organization of the corticothalamic driver axons raises many important issues as well. We currently know all too little about the exact details of the transthalamic corticocortical connections. These connections have received scant attention compared to the details available for the direct corticocortical pathways (see Van Essen et al., 1992; Young, 2000). The extent to which the direct and the transthalamic pathways have parallel hierarchies, and make the same pattern of connections is not known, nor do we have any idea about how the two pathways interact. We lack information about the nature of the messages that each is carrying, knowing only that the layer 5 outputs carry motor messages with branches to the thalamus and that they look as though they are likely to have a tripartite driver action.
When Cajal speculated about the functional significance of the branching sensory axons, he recognized an issue that raises some fundamental problems and that is relevant from the lowest to the highest parts of the central nervous system. Although more than a century has passed since Cajal recognized the importance of these issues we are still relatively ignorant about them. Our knowledge of action potentials and modern methods of studying pathways allow us to reinterpret Cajal's view of how the function of spinal branches might relate to the sensory messages that reach the cortex, but this is still a largely unexplored area. Today theoretical constructs often lead the way and details of the functional activity in anatomically defined pathways are still missing to a large extent.We need the anatomical details and the functional records.
Acknowledgments
We thank Michele Basso, Jeff Hawkins, Donata Oertel, Leslie Osborne, Luis Populin, Fritz Sommer and Brian Theyel for many helpful comments on an earlier draft. SMS was supported by USPHS Grant DC008794.
Footnotes
We have used “efference copy” in preference to “collateral discharge”, because the latter can, in terms of strict meaning, relate to copies of afferent messages, whereas the former is clearly a copy of an effector, motor output. Thus by “efference copy” we explicitly mean a copy of a motor instruction.
A driver input to the thalamus is one that carries a message that is relayed to cortex (see Sherman and Guillery (1998).
This is contrasted to the feedback projection from layer 6 to thalamus that serves a modulatory function and plays no part in the contents of this essay.
The output from layer 5 of area 17 is but one of 24 or more cortical inputs to the superior colliculus, so it is not surprising that its contribution to the control of gaze is small and hard to demonstrate. Merleau-Ponty (2002) wrote: “The object which presents itself to the gaze or the touch arouses a certain motor intention which aims not at the movements of one's own body, but at the thing itself from which they are, as it were, suspended.”, neatly expressing the weak and elusive motor action a subtle observer can link to the visual experience.
References
- Adrian ED. The Basis of Sensation: The Action of the Sense Organs. W.W. Norton; New York: 1928. [Google Scholar]
- Akintunde A, Buxton DF. Origins and collaterization of corticospinal, corticopontine, corticorubral and corticostriatal tracts: a multiple retrograde fluorescent tracing study. Brain Res. 1992;586:208–218. doi: 10.1016/0006-8993(92)91629-s. [DOI] [PubMed] [Google Scholar]
- Berman R, Colby C. Attention and active vision. Vision Res. 2009:1233–1248. doi: 10.1016/j.visres.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa J, Deschênes M. Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an anterograde tracer. Neuroscience. 1995;66:253–263. doi: 10.1016/0306-4522(95)00009-8. [DOI] [PubMed] [Google Scholar]
- Bourassa J, Pinault D, Deschênes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Bridgeman B. Efference copy and its limitations. Comput Biol Med. 2007;37:924–929. doi: 10.1016/j.compbiomed.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Ramachandran VS, Sejnowski TJ. A critique of pure vision. In: Koch C, Davis JL, editors. Large Scale Neuronal Theories in the Brain. MIT Press; Cambridge, Mass: 1994. pp. 23–65. [Google Scholar]
- Clark A. Being There: Putting brain, Body, and World Together Again. MIT Press; Cambridge MA: 1998. [Google Scholar]
- Coulter JD, Jones EG. Differential distribution of corticospinal projections from individual cytoarchitectonic fields in the monkey. Brain Res. 1977;129:335–340. doi: 10.1016/0006-8993(77)90012-9. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Cox CL, Denk W, Tank DW, Svoboda K. Action potentials reliably invade axonal arbors of rat neocortical neurons. Proc Natl Acad Sci U S A. 2000;97:9724–9728. doi: 10.1073/pnas.170278697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênês M, Bourassa J, Pinault D. Corticothalamic projections from layer 5 cells in the rat are collaterals of long-range corticofugal axons. Brain Res. 1994;664:215–219. doi: 10.1016/0006-8993(94)91974-7. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol. 1984;230:55–76. doi: 10.1002/cne.902300106. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallén P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates—an overview. Brain Res Rev. 2008;57:2–12. doi: 10.1016/j.brainresrev.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Grüsser OJ. On the history of the ideas of efference copy and reafference. Clio Med. 1995;33:35–55. [PubMed] [Google Scholar]
- Guillery RW. Anatomical evidence concerning the role of the thalamus in cortico-cortical communication. A Brief Review. J Anat. 1995;187:583–592. [PMC free article] [PubMed] [Google Scholar]
- Guillery RW. Branching thalamic axons link action to perception. J Neurophysiol. 2003;90:539–548. doi: 10.1152/jn.00337.2003. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Feig SL, Van Lieshout D. Connections of higher order visual relays in the thalamus: a study of corticothalamic pathways in cats. J Comp Neurol. 2001;438:66–85. doi: 10.1002/cne.1302. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Harding BN. An ultrastructural study of the termination of afferent fibres within the ventrolateral and centre median nuclei of the monkey thalamus. Brain Res. 1973;54:341–346. doi: 10.1016/0006-8993(73)90058-9. [DOI] [PubMed] [Google Scholar]
- Harting JK, Updyke BV, Van Lieshout DP. Corticotectal projections in the cat: anterograde transport studies of twenty-five cortical areas. J Comp Neurol. 1992;324:379–414. doi: 10.1002/cne.903240308. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL. The Conduction of the Nervous Impulse. Liverpool University Press; Liverpool: 1964. pp. 1–188. [Google Scholar]
- Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science. Third. Elsevier; New York: 1991. pp. 626–646. [Google Scholar]
- Kawamuram K, Konno T. Various types of corticotectal neurons of cats as demonstrated by means of retrograde axonal transport of horseradish peroxidase. Exp Brain Res. 1979;35:161–175. doi: 10.1007/BF00236792. [DOI] [PubMed] [Google Scholar]
- Klier EM, Angelaki DE. Spatial updating and the maintenance of visual constancy. Neuroscience. 2008;156:801–818. doi: 10.1016/j.neuroscience.2008.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kultas-Ilinsky K, Ilinsky I. Fine structure of the ventral lateral nucleus (VL) of the Macaca mulatta thalamus: cell types and synaptology. J Comp Neurol. 1991;314:319–349. doi: 10.1002/cne.903140209. [DOI] [PubMed] [Google Scholar]
- Lu GW, Willis WD. Branching and/or collateral projections of spinal dorsal horn neurons. Brain Res Rev. 1999;29:50–82. doi: 10.1016/s0165-0173(98)00048-4. [DOI] [PubMed] [Google Scholar]
- Merleau-Ponty M. In: Phenomenology of Perception. Smith Colin., translator. Routledge and Kegan Paul; London: 2002. [Google Scholar]
- Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Noë A. Action in Perception. The MIT Press; Cambridge MA: 2004. [Google Scholar]
- Nyberg-Hansen R. Functional organization of descending supraspinal fibre systems to the spinal cord. Anatomical observations and physiological correlations. Ergebn Anat Entwicklungsgesch. 1966;39:1–48. [PubMed] [Google Scholar]
- O'Regan JK, Noë A. A sensorimotor account of vision and visual consciousness. Behav Brain Sci. 2001;24:939–973. doi: 10.1017/s0140525x01000115. [DOI] [PubMed] [Google Scholar]
- Perrone JA, Krauzlis RJ. Vector subtraction using visual and extraretinal motion signals: a new look at efference copy and corollary discharge theories. J Vis. 2008;8:1–14. doi: 10.1167/8.14.24. [DOI] [PubMed] [Google Scholar]
- Petrof I, Sherman SM. Synaptic properties of the mammillary and cortical afferents to the anterodorsal thalamic nucleus in the mouse. J Neurosci. 2009;29:7815–7819. doi: 10.1523/JNEUROSCI.1564-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer R, Bongard J. How the Body Shapes the Way We Think: A New View of Intelligence. The MIT Press; Cambridge MA: 2007. [Google Scholar]
- Raastad M, Shepherd GMG. Single-axon action potentials in the rat hippocampal cortex. J Physiol. 2003;548:745–752. doi: 10.1113/jphysiol.2002.032706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. Histologie du Système Nerveux de l'homme et des Vertébrés. Maloine; Paris: 1911. [Google Scholar]
- Ramon y Cajal S. Azoulay L. Histologie du Système Nerveux del'homme et des Vertébrés. Paris: Maloine; 1952. reprint of 1911 edition. [Google Scholar]
- Ramon y Cajal S. In: Histology of the Nervous System of Man and Vertebrates. Swanson N, Swanson LW, editors. Oxford: Oxford University Press; 1995. [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Rinvik E, Grofova I. Light and electron microscopical studies of the normal nuclei ventralis lateralis and ventralis anterior thalami in the cat. Anat Embryol. 1974;146:57–93. doi: 10.1007/BF00341383. [DOI] [PubMed] [Google Scholar]
- Rockland KS. Two types of corticopulvianr terminations: round (type 2) and elongate (type 1) J Comp Neurol. 1996;368:57–87. doi: 10.1002/(SICI)1096-9861(19960422)368:1<57::AID-CNE5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rockland KS. Convergence and branching patterns of round, type 2 corticopulvinar axons. J Comp Neurol. 1998;390:515–536. doi: 10.1002/(sici)1096-9861(19980126)390:4<515::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. A comparative analysis of the morphology of the corticothalamic projections in mammals. Brain Res Bull. 2000;53:727–741. doi: 10.1016/s0361-9230(00)00364-6. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Neural mechanisms underlying target selection with saccadic eye movements. Prog Brain Res. 2005;149:157–171. doi: 10.1016/S0079-6123(05)49012-3. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Kendall GL, Slocum WM, Tehovnik EJ. Conditions that alter saccadic eye movement latencies and affect target choice to visual stimuli and to electrical stimulation of area V1 in the monkey. Vis Neurosci. 2008;25:661–673. doi: 10.1017/S0952523808080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001;24:122–126. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C. The Integrative Action of the Nervous System. Yale University Press; New Haven: 1906. [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol. 2004a;91:1381–1402. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. II. Role of the SC–MD–FEF pathway in corollary discharge. J Neurophysiol. 2004b;91:1403–1423. doi: 10.1152/jn.00740.2003. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Ann Rev Neurosci. 2008;31:317–338. doi: 10.1146/annurev.neuro.31.060407.125627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi G, Hajdu F, Tömböl T. Ultrastructure of the anterior ventral and anterior medial nuclei of the cat thalamus. Exp Brain Res. 1978;31:417–431. doi: 10.1007/BF00237299. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias AS, Moore T, Smirnakis SM, Tehovnik EJ, Siapas AG, Schiller PH. Eye movements modulate visual receptive fields of V4 neurons. Neuron. 2001;29:757–767. doi: 10.1016/s0896-6273(01)00250-1. [DOI] [PubMed] [Google Scholar]
- Trotter Y, Celebrini S. Gaze direction controls response gain in primary visual–cortex neurons. Nature. 1999;398:239–242. doi: 10.1038/18444. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: an integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. Martin Robert. The Collected Papers of Erich von Holst. Vol. 1. University of Miami Press; Coral Gables Florida: 1950. The reafference principle (interaction between the central nervous system and the periphery) pp. 139–173. [Google Scholar]
- Young MP. The architecture of visual cortex and inferential processes in vision. Spat Vis. 2000;13:137–146. doi: 10.1163/156856800741162. [DOI] [PubMed] [Google Scholar]