Abstract
Rationale
Kv1.5 (KCNA5) is expressed in the heart, where it underlies the IKur current that controls atrial repolarization, and in the pulmonary vasculature, where it regulates vessel contractility in response to changes in oxygen tension. Atrial fibrillation and hypoxic pulmonary hypertension are characterized by down-regulation of Kv1.5 protein expression, as well as with oxidative stress. Formation of sulfenic acid (SOH) on cysteine residues of proteins is an important, dynamic mechanism for protein regulation under oxidative stress. Kv1.5 is widely reported to be redox-sensitive, and the channel possesses six potentially redox-sensitive intracellular cysteines. We therefore hypothesized that sulfenic acid modification of the channel itself may regulate Kv1.5 in response to oxidative stress.
Objective
To investigate how oxidative stress, via redox-sensitive modification of the channel with sulfenic acid, regulates trafficking and expression of Kv1.5.
Methods and Results
Labeling studies with the sulfenic acid-specific probe, DAz, and HRP-streptavidin western blotting demonstrated a global increase in sulfenic acid modified proteins in human patients with atrial fibrillation, as well as sulfenic acid modification to Kv1.5 in the heart. Further studies showed that Kv1.5 is modified with sulfenic acid on a single COOH-terminal cysteine (C581), and the level of sulfenic acid increases in response to oxidant exposure. Using live-cell immunofluorescence and whole-cell voltage clamping, we found that modification of this cysteine is necessary and sufficient to reduce channel surface expression, promote its internalization, and block channel recycling back to the cell surface. Moreover, western blotting demonstrated that sulfenic acid modification is a trigger for channel degradation under prolonged oxidative stress.
Conclusions
Sulfenic acid modification to proteins, which is elevated in diseased human heart, regulates Kv1.5 channel surface expression and stability under oxidative stress, and diverts channel from a recycling pathway to degradation. This provides a molecular mechanism linking oxidative stress and down-regulation of channel expression observed in cardiovascular diseases.
Keywords: Oxidative stress, Kv1.5, sulfenic acid, atrial fibrillation, trafficking, voltage-gated potassium channels, Post-translational modification
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is predicted to rise dramatically over the next several years. Treatment includes the use of antiarrhythmic drugs and/or electrical cardioversion. These treatments, although initially successful in managing AF, often become ineffective over time. One likely contributing factor to this resistance is the electrical and structural remodeling that occurs in the heart with the persistence of AF. Chronic AF-induced electrophysiological remodeling of the atria includes changes to action potential duration and refractory period that result from alterations in the function and expression of ion channels in cardiac myocytes.
In the atria, the balance of inward Ca2+ and outward K+ currents determines action potential duration and refractoriness. Potassium channel blocking drugs have been used as antiarrhythmics to treat patients for many years; however, these agents are most effective in treating recent onset AF and are less effective in patients with chronic AF. One of the predominant repolarizing K+ currents in the atria is the ultrarapid delayed-rectifier current, IKur. This current is encoded by the Kv1.5 channel subunit, which in the human heart is selectively expressed in the atria and is considered an important therapeutic target for treatment of AF1, 2. Paradoxically, IKur and Kv1.5 protein are reduced in chronic human AF which may limit its potential therapeutic efficacy in patients with persistent or permanent arrhythmias3. This pathology-specific decrease in Kv1.5 protein level is also observed in the pulmonary vasculature during chronic hypoxic pulmonary hypertension (HPH)4. While an important therapeutic consideration, the precise mechanisms underlying this ion channel remodeling and decrease in channel protein remain unknown.
One element common to both HPH and chronic AF is a metabolic imbalance leading to a pro-oxidant shift in cellular redox state, which causes cellular oxidative stress. Oxidative stress, defined as excessive production of reactive oxygen species (ROS), and/or diminished antioxidant capacity, is a newly recognized hallmark of cardiovascular disease. Rapid activation of the atrium, which occurs during AF, leads to increased ROS and a decrease in tissue levels of antioxidants 5, 6. Multiple K+ channels and currents, including the Kv1.5-encoded IKur, are regulated by oxidizing agents4, 7-9. Therefore, it is likely that the altered oxidative state of myocytes contributes to electrical remodeling. However, in spite of significant evidence linking conditions of oxidative stress to reduced Kv1.5 expression, the molecular link(s) between these observations is unclear.
One potential direct mechanism for Kv1.5 regulation is via redox-sensitive post-translational modifications to the channel. The thiol (-SH) group of the amino acid cysteine is a principal target for ROS in many proteins, including enzymes, signaling proteins, and transcription factors10. Oxidation of key cysteine residues is an important mechanism whereby changes in cellular redox balance can lead to modification of protein function. Several different oxidative cysteine modifications (oxoforms) have been identified in vivo that play a crucial role in protein stability and function11. Of these cysteine oxoforms, reversible formation of cysteine sulfenic acid (Cys-SOH) is emerging as an important mechanism for dynamic regulation of protein function in response to changes in cellular redox state10, 11. Cys-SOH can form during conditions of cellular oxidative stress and, depending on the protein microenvironment, afford a metastable modification or represent a transient species leading to a more stable disulfide or sulfinic acid form11. Kv1.5 possesses six intracellular cysteines divided among the NH2 and COOH termini (Figure 2A). This, combined with the widely reported redox sensitivity of the channel, led to the hypothesis that sulfenic acid modification to the channel may regulate its function and expression. Here we show that there is a global increase in the level of sulfenic acid-modified proteins in the atria of human patients with chronic atrial fibrillation, compared to healthy controls. We further demonstrate that Kv1.5 is a substrate for sulfenic acid modification that, under conditions of oxidative stress, functions as a fate switch to divert the channel from a recycling to a degradation pathway in myocytes.
Figure 2. Sulfenic acid modification of Kv1.5.
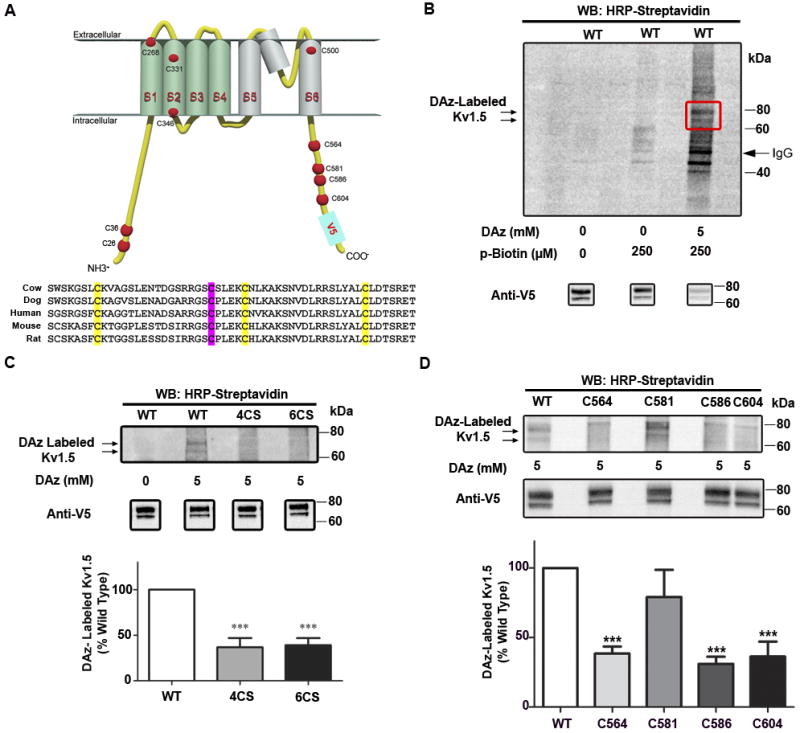
A, Topology of a single alpha subunit of wild type, human Kv1.5, showing all ten cysteine residues. Aligned vertebrate Kv1.5 sequences centered on human C-terminal cysteines. Conserved cysteines are highlighted, with C581 in magenta. B, LTK cells stably expressing V5-tagged wild-type (WT) were labeled with DAz-1 and conjugated to p-biotin (lane 3). Sulfenic acid modification was detected by HRP-streptavidin western blot. C, Top: LTK cells stably expressing V5-tagged wild-type (WT) Kv1.5, Kv1.5-4CS, or Kv1.5-6CS were labeled with DAz-1. Bottom: averaged quantified densitometry data from three experiments normalized to WT Kv1.5, and analyzed using 1-way ANOVA, followed by Tukey’s post-hoc comparison. *** indicates p<0.0001 relative to WT D, Top: COOH-terminal cysteines were individually re-introduced into the null background of Kv1.5-6CS. Sulfenic acid modifications were detected by labeling with DAz-1. Bottom: Summary of three experiments quantified via densitometry, normalized to WT Kv1.5, and analyzed using 1-way ANOVA, followed by Tukey’s post-hoc comparison. *** indicates p<0.0001 relative to WT.
METHODS
Human heart tissue procurement
Atrial myocardial tissue from patients with chronic atrial fibrillation was collected at the time of transplantation at the University of Michigan or the University of California, San Francisco. Atrial myocardial tissue from non-failing hearts was collected from unmatched donors from the University of Michigan under approval from the Gift of Life: Michigan Organ and Tissue Donation Program. Prior to tissue retrieval, all hearts were perfused with ice-cold cardioplegia. Samples from each heart were snap frozen in liquid N2 in the operating room and stored at -80°C. Tissue collection from human hearts used in this study has the approval of the University of Michigan Institutional Review Board (IRB) and subjects gave informed consent.
Stable cell lines
Stable cell lines expressing Kv1.5 wild type and cysteine mutant constructs were created in LTK cells (a mouse fibroblast cell line) using the Retro-X Universal Packaging System from Clontech (Mountain View, CA), according to the manufacturer’s instructions. HL-1 cells were a gift from William Claycomb.
DAz labeling of sulfenic acid, Staudinger ligation, and immunoprecipitation of Kv1.5
Rodent or human tissue samples, LTK or HL-1 cells were harvested in non-denaturing lysis buffer containing DAz, followed by Staudinger ligation and HRP-streptavidin western blotting12 (see online supplement). Blots were stripped and re-probed with anti-V5 or anti-Kv1.5 antibody to assess immunoprecipitation efficiency.
Perfusion of isolated rat heart
Rat hearts were excised and Langendorff perfused with Krebs-Henseleit buffer (KHB) with or without 200μM diamide for 55 min.13, followed by isolation of membranes14 and labeling with DAz12 (see online supplement).
Immunocytochemistry and confocal imaging
For surface, internalization and recycling assays, immunocytochemistry was performed as described previously, with minor modifications15 (see online supplement).
Electrophysiology
Ionic currents were recorded at room temperature in HL-1 cells transiently expressing Kv1.5 using the whole cell configuration of the patch clamp technique, as described previously, with minor modifications16 (see online supplement). For measurement of IKur, rat cardiac myocytes were isolated sterilely, and currents were recorded as outlined in the online supplement17.
Proteasome activity assay
An optimized method was used for determining heart tissue chymotrypsin-like activity18. Reported values are without ATP and were averaged from 3 independent experiments. Values for dimedone-treated cell lysates were expressed as a percentage of values for untreated cells for each experiment.
Statistics
Statistics were performed using Prism software Version 5 from Graphpad Prism Software (San Diego, CA). All data are expressed as mean +/- SEM. Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test, or unpaired, two-tailed t-test. A p value of <0.05 was considered significant.
RESULTS
Human chronic atrial fibrillation is accompanied by a global increase in sulfenic acid-modified proteins
Significant evidence indicates that atrial fibrillation is associated with oxidative stress5, 6, 19, and that this may play an important role in the pathological remodeling that occurs in chronic disease5; however, a role for sulfenic acid modification in this process has not been investigated. We therefore hypothesized that atrial fibrillation in human patients is accompanied by an increase in sulfenic acid-modified proteins. To test this hypothesis, we utilized the novel chemical probe, DAz. When coupled to a biotinylated secondary agent, DAz enables detection of sulfenic acid-modified protein in cells and tissue12 (Online Figure IA). We first used this technique in oxidized mouse heart and detected a robust increase in sulfenic acid, confirming that DAz labels sulfenic acid modified proteins in tissue (Online Figure IB). Consistent with our hypothesis, the level of sulfenic acid modification in the human atria from patients with chronic atrial fibrillation was substantially higher than that of healthy control patients (Figure 1A-B and Online Figure II). This finding, combined with the evidence that Kv1.5 current is redox-sensitive, led us to explore a mechanistic role for sulfenic acid regulation of Kv1.5 in atrial fibrillation.
Figure 1. Increase in global sulfenic acid modification in human heart tissue with AF.
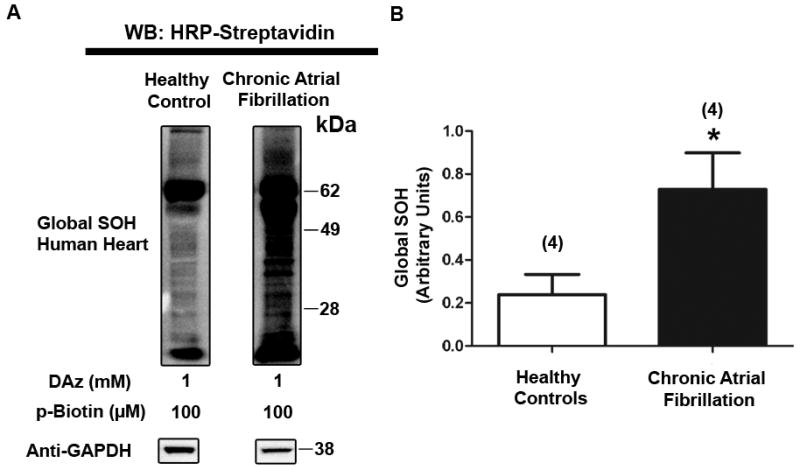
A, HRP-streptavidin western blot depicting DAz-labeled sulfenic acid in atrial tissue lysate from healthy and diseased human hearts. B, Global sulfenic acid modification (HRP-streptavidin signal) from patient samples was quantified via densitometry and normalized to GAPDH levels. p=.04, significant.
Kv1.5 is modified by sulfenic acid on a single COOH-terminal cysteine
Kv1.5 possesses six intracellular cysteines: two on the NH2 terminus, and four on the COOH terminus, which may be candidates for oxidation to sulfenic acid (Figure 2A). Sequence comparison revealed that all four COOH-terminal sites are conserved in mammalian sequences (Figure 2A). Therefore, we hypothesized that Kv1.5 may be a substrate for sulfenic acid modification, and that this may account for its reported redox sensitivity. Kv1.5 appears as a doublet comprised as a nascent, non-glycosylated form and a mature, glycosylated form16. In LTK cells stably expressing Kv1.5 wild-type (WT), we found that Kv1.5 is modified with sulfenic acid, as indicated by a doublet appearing at approximately 80 kDa on the HRP-streptavidin Western blot (Figure 2B, lane 3). Omitting DAz or p-biotin resulted in few nonspecific bands, indicating that DAz is specific for sulfenic acid-modified proteins, and that p-biotin is specific for proteins labeled with DAz (Figure 2B, lanes 1-2). To confirm that the observed bands were indeed Kv1.5, we performed DAz labeling and immunoprecipitation, followed by deglycosylation of the channel with PNGase. Consistent with literature indicating that Kv1.5 undergoes glycosylation16, PNGase treatment eliminated the upper, glycosylated band in both the HRP-streptavidin Western blot and the anti-V5 Western blots, further indicating that the sulfenic acid-modified protein is Kv1.5 (Online Figure IIIA). To test the specificity of this labeling, we utilized the parent compound and competitive inhibitor of DAz, 5,5-dimethyl-1,3-cyclohexanedione (dimedone)12. Including dimedone with DAz in the lysis buffer blocked labeling of sulfenic acid-modified Kv1.5 (Online Figure IIIB), demonstrating the specificity of DAz for sulfenic acid-modified Kv1.5.
To determine the specific sulfenic acid modification profile of Kv1.5 we first utilized LTK cells stably expressing Kv1.5-WT, as well as two additional cysteine mutant proteins: (i) Kv1.5-4CS, in which all four COOH-terminal cysteines were mutated to serine, and (ii) Kv1.5-6CS, in which all six intracellular cysteines were mutated to serine. Importantly, all cysteine mutant channels undergo proper folding, are able to traffic to the cell surface, and are fully functional 16. As expected, mutating all six intracellular cysteines substantially reduced sulfenic acid modification of Kv1.5. However, sulfenic acid modification of Kv1.5-4CS was reduced to the same extent (Figure 2C), indicating that sulfenic acid modification occurs primarily on the COOH-terminus of the channel. To resolve the specific locus of this modification, we developed four additional stable cell lines, each expressing Kv1.5 with a different COOH-terminal cysteine re-introduced individually into the null background of the 6-cysteine mutant (Kv1.5-S564C, S581C, S586C, and S604C). As shown in Figure 2D, re-introducing C581 restored sulfenic acid modification of Kv1.5 to a level that was similar to wild-type. Taken together, these studies show that C581, located on the cytoplasmic portion of Kv1.5 near the COOH-terminus, is the principal site for oxidation to sulfenic acid.
Oxidative stress induces formation of sulfenic acid on Kv1.5
To explore the redox sensitivity of sulfenic acid modification, we induced oxidative stress in cells stably expressing Kv1.5-WT using the organic hydroperoxide, tertiary butyl hydroperoxide (tBOOH). Using DAz labeling, immunoprecipitation of Kv1.5 and HRP-streptavidin Western blotting, we found that 30 min. treatment with 200μM tBOOH caused a significant increase in the fraction of sulfenic acid modified-Kv1.5 (p<.01, Figure 3A). Importantly, concurrent treatment with the thiol-specific antioxidant, N-acetylcysteine (NAC), abolished the tBOOH-induced increase in sulfenic acid, while having no significant effect alone. We also observed an increase in sulfenic acid modification to Kv1.5 after treatment with the more physiologically relevant oxidizing agent, hydrogen peroxide, or by depletion of reduced intracellular glutathione with diamide20 (Figure 3B-C), indicating that the modification is not specific to a particular oxidant. We also examined the oxidant-induced modification of Kv1.5 in HL-1 atrial myocytes, which maintain a differentiated, contractile phenotype in vitro21. Importantly, we observed a similar increase in sulfenic acid modification to Kv1.5 in myocytes after treatment with either tBOOH or diamide (Online Figure IVA-B) indicating that this modification was not cell type-specific. Together, these data show that sulfenic acid modification of Kv1.5 reflects changes in cellular redox state induced by multiple oxidants in varying cell types, including HL-1 cardiac myocytes.
Figure 3. Redox sensitivity of Kv1.5 sulfenic acid modification.
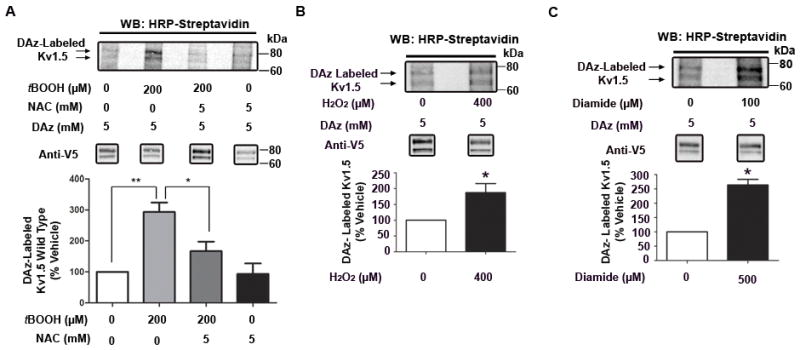
A, Top: HRP-streptavidin western blot depicting LTK cells stably expressing Kv1.5-WT, treated for 30 min with tBOOH or vehicle in the presence or absence of N-acetylcysteine (NAC), followed by labeling with DAz-1. Bottom: Data from three separate experiments were quantified via densitometry. HRP-streptavidin signal was normalized to V5 (Kv1.5) signal, converted to ratios (relative to vehicle) and analyzed using 1-way ANOVA, followed by Tukey’s post-hoc comparison. ** indicates p<0.01, * indicates p<0.05. B-C, Top: LTK cells stably expressing Kv1.5-WT were treated for 30 min with H2O2 (B), diamide (C) or vehicle, followed by labeling with DAz-1, and HRP-streptavidin western blot. Bottom: Data from three separate experiments were quantified via densitometry and analyzed via unpaired t-test with Welch’s correction. * indicates p<0.05.
Endogenous Kv1.5 is modified with sulfenic acid, which regulates channel current and surface levels in cardiac myocytes
To further explore a role for sulfenic acid modification in regulating Kv1.5 in vivo, we perfused freshly isolated, intact rat hearts with normal KHB solution, or with KHB solution containing 200μM diamide, which increases intracellular peroxide levels22, induces sustained arrhythmia13, inhibits other Kv currents in vivo 23, and induced sulfenic acid modification of Kv1.5 in our atrial myocyte cell system (Online Figure IVB). We immunoprecipitated endogenous Kv1.5 channel and detected sulfenic acid using DAz labeling. Importantly, we validated this antibody in experiments that demonstrated it recognizes both endogenous and overexpressed channel (Figure 4A and Online Figure VA). Diamide treatment produced a robust, global increase in sulfenic acid-modified proteins in the heart (Online Figure VB), and importantly, increased sulfenic acid modification to Kv1.5 (Figure 4A). Significant evidence indicates that oxidative stress attenuates potassium current in the cardiovascular system. Kv1.5 underlies IKur, an important component of repolarizing current in the heart. Given our discovery that endogenous Kv1.5 undergoes sulfenic acid modification in the heart, we next investigated the effect of this modification on IKur in native cells, using patch-clamp recordings from acutely dissociated rat cardiac myocytes. Online Figure VI shows that increased oxidative stress with diamide treatment was accompanied by a significant decrease in IKur current, indicating that perturbation of cellular redox equilibrium decreases IKur current in native cardiac myocytes. To determine a specific role for sulfenic acid modification in modulation of IKur currents, we used the sulfenic acid-specific alkylating agent, dimedone24. Our rationale for using dimedone was based on the fact that this covalent modification of sulfenic acid precludes its reduction back to the thiol form and prevents further oxidation to sulfinic or sulfonic acid (Figure 4B, top). As shown in Figure 4B, treatment with dimedone significantly reduced IKur currents in dissociated cardiac myocytes. These results collectively show that Kv1.5 is a substrate for sulfenic acid in the heart, which regulates endogenous Kv1.5 mediated IKur currents.
Figure 4. Redox sensitivity of endogenous Kv1.5 and IKur curents.
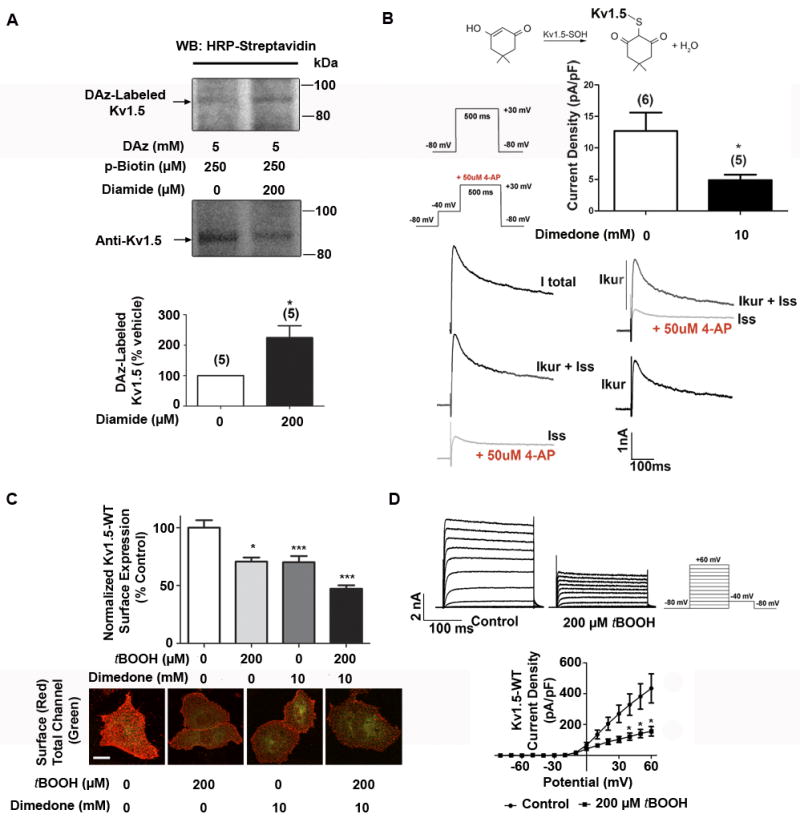
A, Representative Western blot images depicting sulfenic acid modification on Kv1.5 immunoprecipitated from isolated, perfused rat hearts. Data from 5 experiments were quantified using densitometry and analyzed via unpaired t test with Welch’s correction. p=.03, significant B Rat ventricular myocytes were acutely isolated and currents were recorded following 1 hr. treatment with dimedone, which is specific for sulfenic acid-modified proteins (schematic at top). The protocol used to isolate IKslow (IKur + Iss) and Iss are shown with representative traces. Bar graph depicts quantification of Kv1.5-specific (IKur) current density upon treatment with dimedone. * indicates p < 0.05 as determined by t test. C, HL-1 cells transiently expressing Kv1.5-GFP were treated with vehicle, tBOOH and/or dimedone for 60 min. Cells were then stained live to label surface populations of Kv1.5 (red, collapsed Z-stacks). Surface channel was quantified using NIH ImageJ software, normalized to total GFP fluorescence (green, i.e. total Kv1.5), and analyzed via Kruskal-Wallis test, followed by Dunn’s post test, n=100+ cells per condition, three experiments. * indicates p<0.05, relative to vehicle. Scale bar: 30 microns. D, Results of whole cell voltage clamping experiments in HL-1 cells transiently transfected with Kv1.5-GFP-WT and treated with vehicle (n=6) or tBOOH (n=4) for 60 min. Top: Sample outward Kv channel current traces for vehicle and peroxide-treated cells. Bottom: Current density. Results were analyzed via unpaired t-test. *indicates p<0.05 relative to control.
Our previous work demonstrated that changes in current density can result from acute regulation of channel trafficking, leading to altered Kv1.5 levels on the cell surface15, 25. This evidence, combined with the findings shown in Online Figure VI and 4A-B, led us to explore the effects of sulfenic acid modification on channel trafficking. To this end, we used HL-1 cells transiently expressing Kv1.5 with an extracellular GFP tag inserted between the S1 and S2 segments (Kv1.5-GFP, Online Figure VIIA), and measured changes in cell surface expression of Kv1.5 using live-cell immunofluorescent labeling15. This technique utilizes an antibody against the external GFP tag in live, non-permeabilized cells, therefore allowing the discrimination between surface and total cellular populations of channel (total GFP florescence). Our previous work demonstrated that the GFP tag has no effect on the electrophysiological properties or glycosylation of the channel15. In control experiments, we found that Kv1.5-WT-GFP was modified with sulfenic acid to the same extent as untagged Kv1.5-WT, suggesting that potential sulfenic acid modification of the GFP tag itself does not contribute significantly to the signal (Online Figure VIIB). In live-cell surface labeling experiments we found that oxidant treatment resulted in a significant decrease in the surface levels of Kv1.5 in HL-1 myocytes (Figure 4C). This reduction was time-dependent (Online Figure VIIC), with no difference in the level of GFP fluorescence in oxidant- treated cells compared to vehicle (Online Figure VIID).
To determine a specific role for sulfenic acid modification of Kv1.5 in modulation of channel surface expression, we again used dimedone24. Treatment with dimedone decreased surface Kv1.5 (Figure 4C), analogous to treatment with tBOOH, and combining both agents potentiated this effect. This is consistent with our biochemical and in vivo data (Figure 2B-D and 4A-B) showing that there is a basal level of sulfenic acid modification in non-oxidant treated cells, and suggests that hyperoxidation of cysteine to sulfinic or sulfonic acid does not trigger the decrease in channel surface levels. This reduction in channel surface expression was also observed in cells treated with either hydrogen peroxide or diamide, indicating that the effect is not oxidant-specific (Online Figure VIIIA-B). As a functional correlate to our immunostaining results, we performed whole cell voltage clamp measurements in HL-1 myocytes expressing Kv1.5-GFP. Treatment with tBOOH caused a significant reduction in Kv1.5 steady-state current density (Figure 4D), which paralleled the effects observed in our immunofluorescence assay. Importantly, we found that oxidant exposure does not alter the biophysical properties of Kv1.5 (Online Table I), suggesting that a reduction in channel surface expression underlies the reduction in current. This is the first report to show that oxidative stress regulates the surface density of ion channel proteins, providing insight into the molecular mechanisms underlying the effects of ROS on channel current.
Oxidation of Kv1.5 C581 is necessary and sufficient for reduction in channel current and surface density
Our data show that C581 is necessary for sulfenic acid modification of Kv1.5 (Figure 2D). Therefore, we tested the requirement of C581 for oxidant-induced changes in channel function. Mutation of C581 on Kv1.5-GFP (Kv1.5-GFP-C581S) was sufficient to abrogate the reduction in channel surface expression and prevent the decrease in Kv1.5 channel current induced by tBOOH or dimedone in HL-1 atrial myocytes (Figure 5A-B). Significantly, re-introduction of this cysteine alone to the Kv1.5 6-cysteine mutant (Kv1.5-GFP-S581C) restored the effect on surface levels and channel current (Figure 5C-D). Importantly, there was no significant change in total channel protein expression in these experiments, as indicated by western blotting for WT Kv1.5-GFP, as well as total GFP fluorescence in all experiments (Online Figure IXA-E). Because the Kv1.5-S581C mutant possesses a single intracellular cysteine, this finding further implicates sulfenic acid modification of C581, rather than an intra-molecular disulfide bond or other modification, in modulation of channel surface expression. Thus, inducing sulfenic acid modification on C581 of Kv1.5 with oxidizing agents, or trapping Kv1.5 in the sulfenic acid-modified state, causes a reduction in channel surface density and current. Collectively, these results demonstrate a general mechanism for redox modulation of channel surface expression, in which acute oxidant exposure induces sulfenic acid modification of Kv1.5 in atrial myocytes and significantly decreases the levels of the channel at the plasma membrane.
Figure 5. Functional effect of oxidant induced sulfenic acid modification of Kv1.5 at C581.
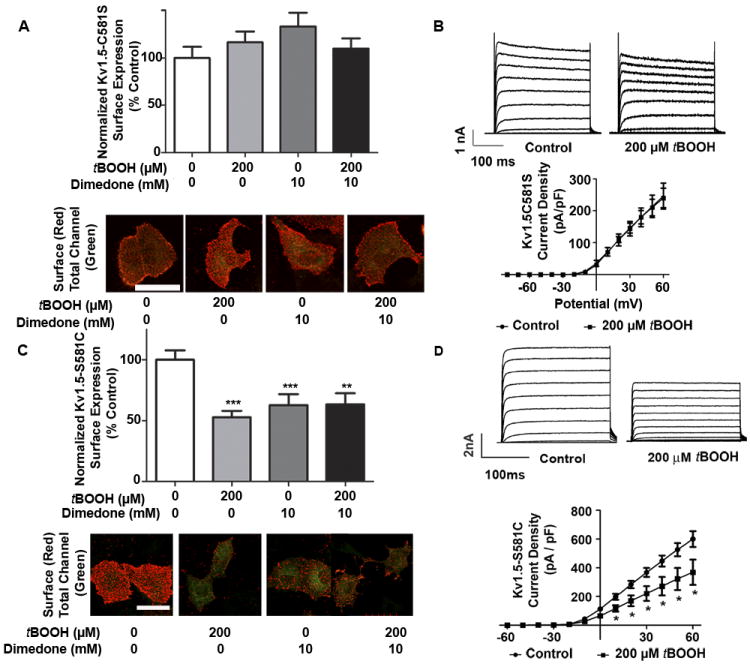
A, HL-1 cells transiently expressing Kv1.5-C581S-GFP were treated with vehicle, tBOOH, and/or dimedone for 60 min. Cells were then analyzed as described in Figure 3B (n=60+ cells per condition, three experiments). B, Results of whole cell voltage clamping experiments in HL-1 cells transiently transfected with Kv1.5-GFP-C581S and treated with vehicle (n=5) or tBOOH (n=4) for 60 min. Top: Sample outward Kv channel current traces for vehicle and tBOOH treated cells. Bottom: Current density. Results were analyzed via unpaired t-test. C, HL-1 cells transiently expressing Kv1.5-GFP-S581C were treated and analyzed as outlined in (A) (n=60+ cells per condition, three experiments). D, Results of whole cell voltage clamping experiments in HL-1 cells transiently transfected with Kv1.5-GFP-S581C and treated with vehicle (n=5) or tBOOH (n=4) for 60 min. and analyzed as in (B) ** indicates p<0.001. *** indicates p<0.0001.
Oxidation triggers internalization of Kv1.5 and diverts channel protein from a recycling pathway
Several acute mechanisms exist for reduction of Kv1.5 surface expression, including increased channel internalization, impaired recycling of internalized channel back to the cell surface, and enhanced channel degradation. Our western blotting and immunofluorescence data showed no channel degradation with short-term oxidative stress (Online Figure IXA-B). Therefore, using a live cell immunofluorescence-based internalization assay (Online Figure XA)15, we first measured the effects of tBOOH or dimedone on channel internalization. To directly measure Kv1.5 internalization, surface channels were initially labeled at low temperature with a primary antibody directed against the extracellular GFP epitope tag, followed by treatment at 37°C with tBOOH, dimedone, or vehicle. Cells were then removed from the treatment, and channels remaining at the plasma membrane were labeled at 4°C with a saturated concentration of AlexaFluor 405-conjugated secondary antibody. Cells were subsequently fixed, permeabilized, and incubated with a second biotinylated secondary antibody, which was detected with streptavidin Cy5. Using this strategy, only those channels originally present at the cell surface can be detected. Of these, only the fraction that remained at the plasma membrane was labeled with the first AlexaFluor secondary antibody, whereas the fraction that was internalized was detected with the second Cy5-labeled secondary antibody. At t=0, prior to oxidant treatment at 37°C, we observed no internalized channel, indicating complete saturation of all surface sites, consistent with our previously published work15. As expected, increasing sulfenic acid with tBOOH, or trapping this modification with dimedone, significantly increased internalization of Kv1.5 (Figure 6A). These effects were not due to differences in overall channel protein expression, as indicated by total GFP fluorescence (Online Figure IXF).
Figure 6. Effect of sulfenic acid on Kv1.5 internalization and recycling.
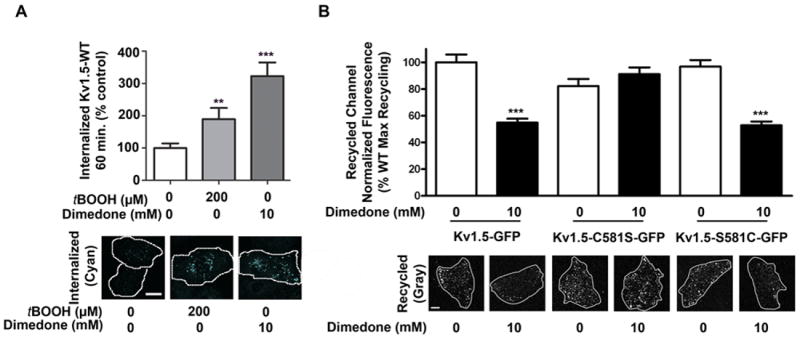
A, HL-1 cells transiently expressing Kv1.5-WT-GFP were treated with vehicle, tBOOH, or dimedone for 60 min. Internalized channel (cyan) was visualized using confocal microscopy, quantified using NIH ImageJ software, normalized to GFP fluorescence (i.e. total Kv1.5), and analyzed via Kruskal-Wallis test, followed by Dunn’s post test (n= 85+ cells per condition, three experiments). ** indicates p<0.001, *** p<.0001 relative to vehicle. Scale bar: 30 microns. B, Left: In HL-1 cells, recycled channel (gray) at 60 min was measured after vehicle or dimedone treatment for 90 min. Data were quantified using NIH ImageJ software and analyzed via 1-way ANOVA, followed by Tukey’s post-hoc comparison (n= at least 70 cells per condition, three experiments). *** indicates p<0.0001 relative to vehicle control.
We previously reported that a population of constitutive internalized Kv1.5 recycles back to the cell surface15. Therefore, we determined whether sulfenic acid modification of Kv1.5 interferes with this dynamic recycling process. Surface populations of Kv1.5 were first tagged with an anti-GFP antibody at 4°C. Cells were treated at 37°C for 60 min. with dimedone or vehicle, during which time a population of surface channel was allowed to internalize (Online Figure XB). Any remaining cell surface channels were then labeled at 4°C with saturating concentrations of AlexaFluor 405-conjugated secondary antibody. At t=0 min., cells were returned to 37°C for 60 min. to allow for recycling back to the plasma membrane. The previously tagged and internalized Kv1.5 channels, which returned to the surface, were then labeled with a biotin-conjugated anti-rabbit secondary antibody and detected with Cy5-conjugated streptavidin. The internalized channel population originally labeled on the cell surface is therefore protected against detection with the AlexaFluor 405 secondary antibody whereas Kv1.5 that has returned to the plasma membrane, but not newly synthesized channel, is detected with Cy5-conjugated streptavidin15. At t=0, prior to returning the cells to 37°C to permit channel recycling, we observed no recycled channel, indicating complete saturation of all surface sites (Online Figure XC). Notably, we observed that trapping sulfenic acid modification significantly reduced recycling of internalized Kv1.5 back to the cell surface (Figure 6B). This effect was not due to changes in total channel protein expression, as total GFP levels for the treatment groups were not significantly different (Online Figure IXG). Further, C581 is necessary and sufficient to mediate this effect (Figure 6B, Kv1.5-C581S and Kv1.5-S581C). Therefore, under acute oxidative stress, sulfenic acid modification triggers internalization of Kv1.5 and significantly reduces channel recycling back to the cell surface.
Sulfenic acid modification of Kv1.5 in response to oxidative stress results in channel degradation
On the basis of the preceding results, we sought to determine the intracellular fate of internalized channel following exposure to oxidant. Consistent with our previous report15, intracellularly-retained channel colocalized with EEA1, a marker for early endosomes (Figure 7A). This interaction was more pronounced after a 60 min. treatment with tBOOH, an observation that likely reflects the larger pool of internalized channel (Figure 7A). After performing co-immunostaining experiments with a number of intracellular markers, we uncovered a previously unreported, oxidative stress-induced colocalization of Kv1.5 with the molecular chaperone, HSP70 (Figure 7B). HSP70, which is highly inducible under conditions of cellular stress, plays a pivotal role in stabilizing proteins, including ion channels, and in targeting misfolded proteins for ubiquitination and proteasomal degradation26. Consistent with a role for this protein in cell stress, HSP70 acquires a punctate pattern after tBOOH treatment and colocalizes with a substantial population of internalized Kv1.5 (Figure 7B). Colocalization of Kv1.5 with HSP70, combined with data suggesting that over-oxidation of proteins may target them for degradation 27-29, led us to hypothesize that prolonged oxidative stress diverts the channel to a degradation pathway. To investigate this possibility, we treated HL-1 cells stably expressing WT Kv1.5 with tBOOH, alone or in the presence of the proteasomal inhibitor, MG132. To prevent oxidant-induced changes in Kv1.5 transcription30, we performed all treatments in the presence of the protein synthesis inhibitor, cycloheximide. Treatment with tBOOH caused degradation of Kv1.5 in as little as 15 hours, which was rescued by inhibition of the proteasome (Figure 8A). Importantly, oxidant-induced channel degradation was not observed in the Kv1.5-C581S mutant protein (Figure 8B), implicating oxidation of this cysteine on the channel in the degradation. Since sulfenic acid can oxidize further to sulfinic (Cys-SO2H) or sulfonic (Cys-SO3H) acid11, we hypothesized that irreversible hyperoxidation of Kv1.5 might promote degradation. To test this possibility, we used dimedone to trap the sulfenic acid modification on Kv1.5, thereby preventing further oxidation. Figure 8C shows that dimedone attenuates Kv1.5 degradation under oxidative stress conditions, as predicted. Although dimedone blocked channel degradation, it remained possible that these effects were mediated by depletion of the intracellular pool of ubiquitin, or direct inhibition of the proteasome. To rule out direct effects of dimedone and oxidant treatments on the activity of the proteasome, we first treated HL-1 cells with dimedone and tBOOH, alone and in the presence of the proteasomal inhibitor MG132, followed by western blotting with an anti-ubiquitin antibody. In contrast with the positive control samples treated with MG132, treatment of cells with tBOOH or dimedone alone caused no accumulation of ubiquitinated proteins in the cells (Online Figure XIA). To directly assess the effects of dimedone on proteasomal activity, we measured the chymotrypsin-like activity of the proteasome using a previously-published procedure18 after 15 hour treatments with dimedone. Dimedone treatment alone did not affect activity of the proteasome (Online Figure XIB). These results indicate that oxidant and dimedone treatments do not alter proteasomal activity. Taken together, these findings support the hypothesis that sulfenic acid diverts Kv1.5 from a recycling to a degradation pathway (Figure 8D).
Figure 7. Sub-cellular localization of internalized Kv1.5 following oxidative stress.
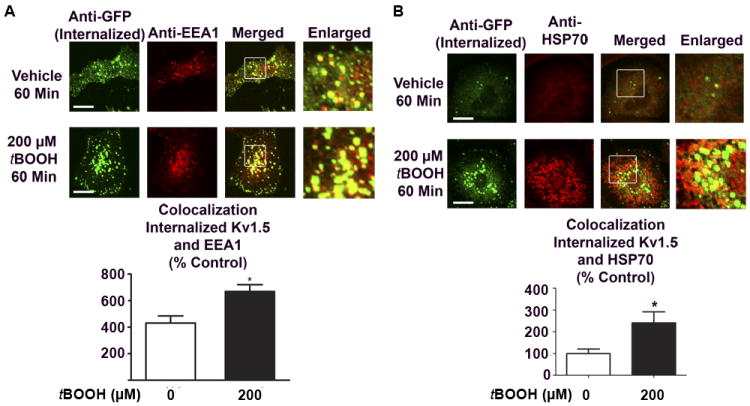
A, HL-1 cells transiently expressing Kv1.5-GFP were treated for 60 min with tBOOH, followed by staining with EEA1 antibody. Figure depicts a cross sectional image. Yellow puncta indicate colocalization between internalized Kv1.5 and EEA1. Bottom: Quantified data (n=25 cells, 3 separate experiments), * indicates p<.05 relative to vehicle control. B, HL-1 cells transiently expressing Kv1.5-GFP were treated for 60 min with tBOOH, followed by staining with HSP70 antibody. Figure depicts a cross sectional image. Yellow puncta indicate colocalization between internalized Kv1.5 and HSP70. Bottom: Quantified data (n=30 cells, 3 separate experiments), * indicates p<.05 relative to vehicle control.
Figure 8. Effect of sulfenic acid on Kv1.5 degradation.
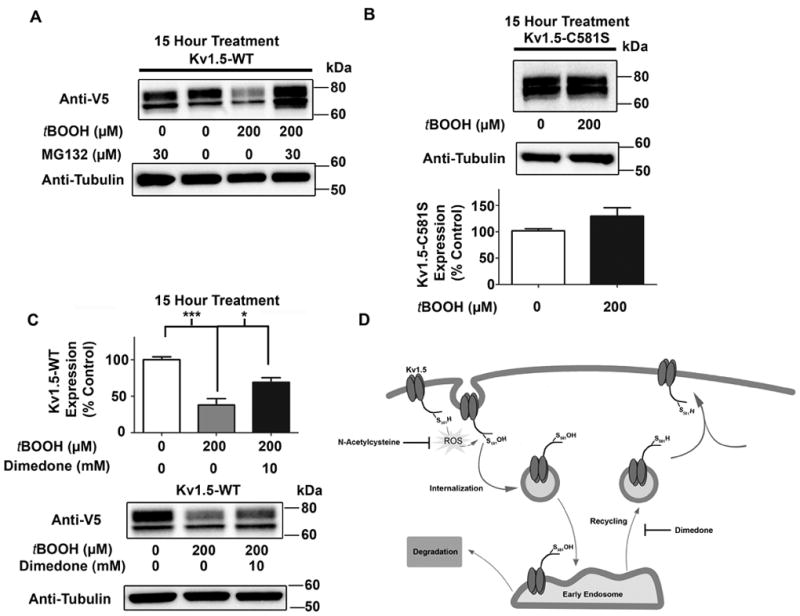
A, HL-1 cells stably expressing Kv1.5-WT were treated with vehicle or tBOOH, alone or concurrently with the proteasome inhibitor, MG132 in the presence of cycloheximide (5 μM) to block the synthesis of new proteins. Cell lysates were generated and analyzed by western blotting with anti-V5 antibody. B, HL-1 cells stably expressing Kv1.5-C581S were treated and analyzed as in (A) (quantified data based on three separate experiments). C, HL-1 cells stably expressing Kv1.5-WT were treated with tBOOH as outlined in (A), alone or concurrently with dimedone. Cell lysates were generated and analyzed by western blotting with anti-V5 antibody (quantified data based on three separate experiments). D, Hypothetical model for sulfenic acid modulation of Kv1.5 trafficking and stability.
DISCUSSION
Numerous reports suggest that Kv1.5, which comprises an important repolarizing current in the atrium of the human heart, is sensitive to cellular redox state. Furthermore, disease states associated with oxidative stress, such as atrial fibrillation and chronic pulmonary hypertension, are characterized by down-regulation of channel protein expression, with no change in channel transcription3, 4. Despite these observations, the molecular mechanism(s) underlying this pathological remodeling have remained elusive. In the present study, we show that Kv1.5 is modified with sulfenic acid in the heart, and that this increases under oxidative stress. We further show that this modification occurs on a single, COOH-terminal cysteine, and that it regulates IKur currents in native myocytes. To our knowledge, this is the first report demonstrating sulfenic acid modification to an ion channel. We demonstrate that peroxide-induced stress leads to increased internalization and impaired recycling of the channel in cardiac myocytes, and that oxidation of COOH-terminal C581 is sufficient to mediate these effects. Importantly, sulfenic acid modification ultimately leads to channel degradation. These results therefore provide a molecular mechanism by which oxidative stress can lead to Kv1.5 degradation in cardiac myocytes via oxidation of the channel itself.
The finding that sulfenic acid modification occurs on a single COOH-terminal cysteine emphasizes the fact that, although formation of sulfenic acid occurs via a non-enzymatic mechanism, it is not a random occurrence. Recent functional site profiling has revealed that sulfenic acid-modified proteins are flanked by polar amino acid residues capable of forming hydrogen bonds31. It is interesting that the modified cysteine, C581, (Figure 2A, magenta) is preceded by a polar serine residue, which is also conserved. Thus, the protein microenvironment flanking reactive cysteines may explain the propensity for particular cysteines to form sulfenic acid. The conservation of this cysteine may provide insight into the unique role for Kv1.5 in oxygen sensing in the heart and likely the pulmonary vasculature. Indeed, our results show that oxidation of this cysteine to sulfenic acid enables cells to translate acute changes in redox state into altered cellular excitability.
A link between oxidative stress and down-regulation of Kv channels is currently the subject of intense research4, 9, 32. However, studies of the redox sensitivity of Kv channels thus far have not explored redox-sensitive changes in channel surface levels. Surface levels of several Kv channels, including HERG1 and Kv1.5, are sensitive to other factors, such as intracellular signaling molecules33 and pharmacological agents25. Importantly, our work provides the first evidence that oxidative stress, via sulfenic acid modification to the channel itself, can acutely regulate the level of channel at the cell surface. Indeed, we observe a significant reduction in channel surface expression and current after accumulation of sulfenic acid via two distinct means: induction of oxidative stress with tBOOH, or trapping of the modification with dimedone, effects which are more pronounced when both agents are combined (Figure 4B-C). The presence of cysteine 581 is necessary and sufficient to mediate these effects (Figure 5). We observe similar increases in sulfenic acid modification (Figure 3) and decreased Kv1.5 surface expression (Online Figure VIII) with multiple oxidants, demonstrating that the effects are not specific to one particular oxidant and likely represent a general cellular mechanism for redox-regulation of channel expression and trafficking. Our whole cell voltage clamping results provide confirmation of our immunofluorescence data via a second, parallel method. Importantly, we localized these functional effects using both approaches to a single COOH-terminal cysteine (Figure 5).
In accordance with our surface labeling experiments, we find a similar increase in channel internalization after treatment with tBOOH or dimedone. The finding that dimedone treatment alone causes internalization suggests that there is a basal level of sulfenic acid-modified channel in cardiac myocytes. This is supported by data in Figures 2-4 and Online Figure IV, showing a basal level of sulfenic acid-modified channel in cells and myocytes that have not been challenged with oxidant. Accordingly, the increase in internalization is accompanied by impairment in channel recycling (Figure 6B), indicating that sulfenic acid modification diverts channel away from the normal recycling pathway. These results are in agreement with other studies showing that oxidative stress can interfere with trafficking of other membrane proteins, such as the transferrin receptor34, or alter the molecular machinery involved in this trafficking process35. As the molecular mechanisms governing Kv1.5 endocytosis are elucidated, this may lead to further insight into how sulfenic acid triggers internalization.
Our results show that human atrial fibrillation is accompanied by a global increase in sulfenic acid-modified protein in the atrium and that endogenous Kv1.5 is modified with sulfenic acid. The finding that Kv1.5 is a substrate for sulfenic acid modification and that chronic oxidative stress results in channel degradation, suggests that this is at least one mechanism linking ion channel remodeling to persistent AF. The loss of channel protein associated with AF precludes the direct measurement of sulfenic acid modified channel in the pathophysiological state. However, our results are consistent with reports showing that chronic heart failure is a substrate for atrial fibrillation resulting in the oxidative-stress induced loss of Kv1.5 channel protein 19. Importantly, it was shown that this ion channel remodeling was sensitive to the antioxidant, NAC indicating that redox-senstive remodeling in the atria may be prevented or reversed. Targeting sulfenic acid modification to ion channel proteins may provide a novel therapeutic approach to treat persistent AF.
In summary, we demonstrate that human chronic atrial fibrillation is associated with a global increase in sulfenic acid. We further show that oxidative stress can lead directly to internalization and protein degradation of Kv1.5, and that sulfenic acid modification of COOH-terminal C581 alone is sufficient to trigger these events. Redox modulation of Kv1.5 current is associated with numerous pathophysiological states that involve loss of channel protein, including atrial fibrillation and pulmonary hypertension3, 4. We show, for the first time, that oxidative stress acutely regulates Kv1.5 surface expression. This is the first report of sulfenic acid modification to an ion channel, which provides a potential molecular explanation for the pathological remodeling that occurs in cardiovascular diseases. While the roles of ROS and modulation of Kv1.5 in the pathogenesis of disease continue to be intensely studied, it is clear that other factors peripheral to the channel itself, including expression of modulatory Kv beta subunits, cannot fully account for channel redox sensitivity36. Indeed, our results indicate that oxidative modification of the Kv1.5 channel itself has a profound effect on stability and functional density. By extension, sulfenic acid modification may also regulate surface levels of other ion channels, thereby linking oxidative stress to altered cellular excitability and disease.
Supplementary Material
Novelty and Significance.
What Is Known?
Multiple potassium channels, which play an essential role in maintaining normal cardiac rhythm and blood vessel contractility, are regulated by oxidative stress.
Modification of cysteine residues of proteins to sulfenic acids alters the expression and function of many proteins under oxidative stress.
Diseases such as chronic atrial fibrillation (AF) and hypoxic pulmonary hypertension (HPH) are characterized by both oxidative stress and reduced expression of the oxygen-sensitive, voltage-gated potassium (Kv) channel, Kv1.5.
What New Information Does This Article Contribute?
Chronic AF in human patients is accompanied by a global increase in sulfenic acid-modified proteins in the heart.
Sulfenic acid containing Kv1.5 protein is present in normal heart, and the level of this modification increases under oxidative stress.
Sulfenic acid formation in Kv1.5 reduces the level of the channel protein on the cell surface, disrupts its normal trafficking, and promotes its degradation in cardiac myocytes.
Oxidative stress, characterized by excessive formation of reactive oxygen species or insufficient antioxidant capacity, is a common denominator in a vast array of cardiovascular diseases. Another common hallmark of cardiovascular disease is a pathological change in the expression level of ion channel proteins, leading to impaired vessel contractility and cardiac rhythm. Chronic AF and HPH are characterized by both oxidative stress and a reduction in the level of Kv1.5 channel protein, although the molecular mechanism(s) linking these observations is unclear. Here, we demonstrate a global increase in the level of sulfenic acid on proteins in the atria of human patients with chronic AF, and that oxidative stress results in formation of sulfenic acid in Kv1.5 in the rat heart. This modification interrupts the normal, dynamic trafficking of the channel, and promotes its degradation. This is the first report demonstrating sulfenic acid modification of an ion channel, and provides a molecular mechanism linking oxidative stress to reduced channel expression levels observed in AF and HPH. Targeting sulfenic acid modification of ion channel proteins may provide a novel therapeutic approach for treating cardiovascular diseases.
Acknowledgments
We would like to thank Dr. William Pratt for his advice and critical review of this manuscript. We thank Dr. Yoichi Osawa for providing the anti-HSP70 antibody, and Dr. Stephen Ragsdale for providing diamide.
SOURCES OF FUNDING
This work was funded by an American Heart Association Predoctoral Fellowship 0910001G (to L.K.S.), NIH grant HL0270973 (to J.R.M.), the NIEHS Toxicology Training Grant T32ES07062, and an AHA Scientist Development Grant 0835419N (to K.S.C.).
Non-Standard Abbreviations
- AF
atrial fibrillation
- DTT
Dithiothreitol
- EEA1
early endosomal antigen 1
- GFP
green fluorescent protein
- HPH
hypoxic pulmonary hypertension
- HRP
horseradish peroxidase
- HSP70
heat shock protein 70
- IK
delayed rectifier potassium current
- KHB
Krebs-Henseleit buffer
- MG132
N-(benzyloxycarbonyl)leucinylleucinylleucinal protease inhibitor
- NAC
N-acetylcysteine
- ROS
reactive oxygen species
- SOH
sulfenic acid
- tBOOH
tertiary butyl hydroxperoxide
Footnotes
DISCLOSURES
None
References
- 1.Wang Z, Fermini B, Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res. 1993;73:276–85. doi: 10.1161/01.res.73.2.276. [DOI] [PubMed] [Google Scholar]
- 2.Ford JW, Milnes JT. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (I Kur): rationale, pharmacology and evidence for potential therapeutic value. J Cardiovasc Pharmacol. 2008;52:105–20. doi: 10.1097/FJC.0b013e3181719b0c. [DOI] [PubMed] [Google Scholar]
- 3.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–81. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 4.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–8. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 5.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM, Bauer JA, Van Wagoner DR. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–8. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 6.Giannitsis E, Tettenborn I, Wiegand U, Potratz J, Sheikhzadeh A, Stierle U. Neutrophil-derived oxidative stress after myocardial ischemia induced by incremental atrial pacing. Pacing Clin Electrophysiol. 1998;21:157–62. doi: 10.1111/j.1540-8159.1998.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 7.Cai SQ, Sesti F. Oxidation of a potassium channel causes progressive sensory function loss during aging. Nat Neurosci. 2009;12:611–7. doi: 10.1038/nn.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciorba MA, Heinemann SH, Weissbach H, Brot N, Hoshi T. Modulation of potassium channel function by methionine oxidation and reduction. Proc Natl Acad Sci U S A. 1997;94:9932–7. doi: 10.1073/pnas.94.18.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schach C, Xu M, Platoshyn O, Keller SH, Yuan JX. Thiol oxidation causes pulmonary vasodilation by activating K+ channels and inhibiting store-operated Ca2+ channels. Am J Physiol Lung Cell Mol Physiol. 2007;292:L685–98. doi: 10.1152/ajplung.00276.2006. [DOI] [PubMed] [Google Scholar]
- 10.Poole LB, Karplus PA, Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol. 2004;44:325–47. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- 11.Reddie KG, Carroll KS. Expanding the functional diversity of proteins through cysteine oxidation. Curr Opin Chem Biol. 2008;12:746–54. doi: 10.1016/j.cbpa.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 12.Reddie KG, Seo YH, Muse WB, Iii, Leonard SE, Carroll KS. A chemical approach for detecting sulfenic acid-modified proteins in living cells. Mol Biosyst. 2008;4:521–31. doi: 10.1039/b719986d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frasier CR, Sloan RC, Bostian PA, Gonzon MD, Kurowicki J, Lopresto SJ, Anderson EJ, Brown DA. Short-term exercise preserves myocardial glutathione and decreases arrhythmias after thiol oxidation and ischemia in isolated rat hearts. J Appl Physiol. 2011;111:1751–9. doi: 10.1152/japplphysiol.01214.2010. [DOI] [PubMed] [Google Scholar]
- 14.Brackenbury WJ, Davis TH, Chen C, Slat EA, Detrow MJ, Dickendesher TL, Ranscht B, Isom LL. Voltage-gated Na+ channel beta1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo. J Neurosci. 2008;28:3246–56. doi: 10.1523/JNEUROSCI.5446-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen DP, Schumacher SM, Li Q, Benson MD, Iniguez-Lluhi JA, Van Genderen KM, Martens JR. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. J Biol Chem. 2007;282:29612–20. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Foster K, Li Q, Martens JR. S-acylation regulates Kv1.5 channel surface expression. Am J Physiol Cell Physiol. 2007;293:C152–61. doi: 10.1152/ajpcell.00480.2006. [DOI] [PubMed] [Google Scholar]
- 17.Dhamoon AS, Pandit SV, Sarmast F, Parisian KR, Guha P, Li Y, Bagwe S, Taffet SM, Anumonwo JM. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004;94:1332–9. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- 18.Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR, Day SM. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation. 121:997–1004. doi: 10.1161/CIRCULATIONAHA.109.904557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sridhar A, Nishijima Y, Terentyev D, Khan M, Terentyeva R, Hamlin RL, Nakayama T, Gyorke S, Cardounel AJ, Carnes CA. Chronic heart failure and the substrate for atrial fibrillation. Cardiovasc Res. 2009;84:227–36. doi: 10.1093/cvr/cvp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosower NS, Kosower EM. Diamide: an oxidant probe for thiols. Methods Enzymol. 1995;251:123–33. doi: 10.1016/0076-6879(95)51116-4. [DOI] [PubMed] [Google Scholar]
- 21.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mawatari K, Yasui S, Morizumi R, Hamamoto A, Furukawa H, Koyama K, Hattori A, Yoshioka E, Yoshida M, Nakano M, Teshigawara K, Harada N, Hosaka T, Takahashi A, Nakaya Y. Reactive oxygen species induced by diamide inhibit insulin-induced ATP-sensitive potassium channel activation in cultured vascular smooth muscle cells. Asia Pac J Clin Nutr. 2008;17(Suppl 1):162–6. [PubMed] [Google Scholar]
- 23.Liang H, Li X, Li S, Zheng MQ, Rozanski GJ. Oxidoreductase regulation of Kv currents in rat ventricle. J Mol Cell Cardiol. 2008;44:1062–71. doi: 10.1016/j.yjmcc.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison WS. Formation and Reactions of Sulfenic Acids in Proteins. Accounts of Chemical Research. 1976;9:293–299. [Google Scholar]
- 25.Schumacher SM, McEwen DP, Zhang L, Arendt KL, Van Genderen KM, Martens JR. Antiarrhythmic drug-induced internalization of the atrial-specific k+ channel kv1.5. Circ Res. 2009;104:1390–8. doi: 10.1161/CIRCRESAHA.108.192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pratt WB, Morishima Y, Peng HM, Osawa Y. Proposal for a role of the Hsp90/Hsp70-based chaperone machinery in making triage decisions when proteins undergo oxidative and toxic damage. Exp Biol Med (Maywood) 235:278–89. doi: 10.1258/ebm.2009.009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. Faseb J. 1997;11:526–34. [PubMed] [Google Scholar]
- 28.Tasaki T, Kwon YT. The mammalian N-end rule pathway: new insights into its components and physiological roles. Trends Biochem Sci. 2007;32:520–8. doi: 10.1016/j.tibs.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Ying J, Sharov V, Xu S, Jiang B, Gerrity R, Schoneich C, Cohen RA. Cysteine-674 oxidation and degradation of sarcoplasmic reticulum Ca(2+) ATPase in diabetic pig aorta. Free Radic Biol Med. 2008;45:756–62. doi: 10.1016/j.freeradbiomed.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fountain SJ, Cheong A, Li J, Dondas NY, Zeng F, Wood IC, Beech DJ. K(v)1.5 potassium channel gene regulation by Sp1 transcription factor and oxidative stress. Am J Physiol Heart Circ Physiol. 2007;293:H2719–25. doi: 10.1152/ajpheart.00637.2007. [DOI] [PubMed] [Google Scholar]
- 31.Salsbury FR, Jr, Knutson ST, Poole LB, Fetrow JS. Functional site profiling and electrostatic analysis of cysteines modifiable to cysteine sulfenic acid. Protein Sci. 2008;17:299–312. doi: 10.1110/ps.073096508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bubolz AH, Wu Q, Larsen BT, Gutterman DD, Liu Y. Ebselen reduces nitration and restores voltage-gated potassium channel function in small coronary arteries of diabetic rats. Am J Physiol Heart Circ Physiol. 2007;293:H2231–7. doi: 10.1152/ajpheart.00717.2007. [DOI] [PubMed] [Google Scholar]
- 33.Ramstrom C, Chapman H, Viitanen T, Afrasiabi E, Fox H, Kivela J, Soini S, Korhonen L, Lindholm D, Pasternack M, Tornquist K. Regulation of HERG (KCNH2) potassium channel surface expression by diacylglycerol. Cell Mol Life Sci. 67:157–69. doi: 10.1007/s00018-009-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malorni W, Testa U, Rainaldi G, Tritarelli E, Peschle C. Oxidative stress leads to a rapid alteration of transferrin receptor intravesicular trafficking. Exp Cell Res. 1998;241:102–16. doi: 10.1006/excr.1998.4020. [DOI] [PubMed] [Google Scholar]
- 35.Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberg J. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell. 2001;7:421–32. doi: 10.1016/s1097-2765(01)00189-7. [DOI] [PubMed] [Google Scholar]
- 36.Platoshyn O, Brevnova EE, Burg ED, Yu Y, Remillard CV, Yuan JX. Acute hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C907–16. doi: 10.1152/ajpcell.00028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


