Abstract
Background & objectives:
Maternal undernutrition and hyperglycaemia during pregnancy, as well as foetal undernutrition affecting the development of foetal endocrine pancreas structure and function, especially that of β-cells is well known. This study was undertaken to look into the changes in pancreatic islets morphology of aborted normal human foetuses (16-20 wk old) of undernourished and adequately nourished mothers.
Methods:
Foetuses were collected over a 24 month period from medically terminated pregnancies of six undernourished mothers (BMI <18.5 kg/m2) and eight adequately nourished mothers (BMI >18.5 kg/m2). The sections were stained with haematoxylin & eosin as well as Masson trichrome for morphometric estimates such as islet count, area, volume, etc. and immunohistochemistry analysis of β-cells for insulin presence was done.
Results:
Significant correlations between maternal and foetal parameters were seen. However, there were no statistically significant differences in the number, size or density and beta cell counts of the pancreas among foetal pancreas of mothers with BMI <18.5 and >18.5 kg/m2.
Interpretation & conclusions:
Our findings indicate that nutritional status of the mother may not have profound influence on the morphology of beta cells of foetal pancreas in second trimester of pregnancy. Further studies need to be done to confirm these findings.
Keywords: Foetus, islets, maternal undernutrition, pancreas, pregnancy
Apart from genetically determined factors, environmental factors also influence foetal growth and development. For supply of nutrients, the foetus is dependent on the nutrition from mother and the capacity of the placenta to transport nutrients1. Maternal undernutrition (UN) and hyperglycaemia during pregnancy is reported to affect the development of foetus as a whole and foetal pancreas (endocrine) in particular. A reduction in maternal food intake or UN is known to cause elevations in glucocorticoid concentrations, which is critical for ensuring pancreatic architecture and survival, as well as for beta cell mass expansion2.
Foetal UN in utero and low birth weight/IUGR (Intra uterine growth retardation) have been linked to development of type-2 diabetes later in life3. By adapting to a limited supply of nutrients, it has been suggested that the human foetus trades off the development of organs such as kidney (nephron mass) and pancreas (beta cell mass), in favour of more essential organs such as the brain. These developmental adaptations have been suggested to be the origins of a number of diseases in later life, and are thought to be consequences of foetal programming4. The adaptations invoked in response to deficient maternal-placental nutrient supply have permanent effects on structure, physiology, and metabolism of organs, in general. Poor foetal nutrition leads to impaired pancreatic beta-cell function, which persists into adult life5. It has been reported that regardless of the type of diet administered to the mother, calorie deficient/protein deficient/high fat diet, β-cell population was less in progeny and more vulnerable to oxidative stress6.
In humans, insulin secretion by β-cells of pancreas is seen by 18 wk of gestation7. As per Barkers hypothesis of ‘foetal origins of adult diseases’, insulin resistance is also a long term eventuality due to altered intrauterine environment8. Earlier studies on human foetal pancreas of IUGR at 34 wk showed reduced amount of total endocrine tissue as well as the number of insulin secreting β-cells9. However, in another study, no such abnormalities were reported in IUGR foetuses where percentage of β-cells in islets was observed to be normal10. Islet hypertrophy and hyperplasia of β-cells are typical features of foetuses born to diabetic mothers7, but undernutrition leading to the same is controversial.
Therefore, we hypothesized that human maternal UN, if accompanied by impaired glucose tolerance, would affect the foetal pancreatic development in utero and thus compromise the morphology as well as function. Hence, in the present study, an attempt was made to determine the association of maternal body mass index (BMI) as a function of nutritional status on foetal pancreatic development, islets and their insulin content in normal aborted human foetuses at 16-20 wk of pregnancy.
Material & Methods
The study was carried out during 2006-2008 at the National Institute of Nutrition, Hyderabad, and aborted foetuses were collected from the Government Institute of Women and Child Health, Hyderabad, India, catering to low and middle class population. Ethical clearance was obtained from Institutional Human Ethics Committee of the hospital and National Institute of Nutrition, Hyderabad. Human foetuses of gestational age between 16-20 wk were collected from uncomplicated MTPs (medically terminated pregnancies). All the requisite procedures as per the MTP Act, 200211 were followed (including informed consent).
Exclusion criteria for the study were (i) mothers with bad obstetric history, diabetes or any other chronic disease, and (ii) MTPs conducted for congenital/genetic abnormalities in foetus.
On admission to the hospital, maternal weight and height were recorded as per standard anthropometric procedures using calibrated height and weighing instruments. Body mass index (BMI) of mothers were calculated12 and categorized as undernourished (UN) (BMI <18.5 kg/m2) and adequately nourished (AN) (BMI >18.5 kg/m2), respectively. A total of 14 foetal samples were obtained during the 24 month study period, of which 6 were from UN and other eight belonged to AN group.
Three ml of venous blood was collected from ante-cubital vein prior to conducting MTP, for haemoglobin (Hb) and fasting blood sugar (FBS) estimations, to assess the general health and glycaemic status of the mother. Hb was estimated by cyanmethhaemoglobin method13 and FBS by glucose oxidase peroxidase method using a kit (Biosystems, USA).
MTP was done by a qualified obstetrician and extra-amniotic instillation of ethacridine lactate method of evacuation was employed for second trimester. All the necessary pre- and post-operative precautions were followed during the procedure as per the protocol14.
The foetus obtained after MTP was immediately washed to remove blood clots/mucus. Bouins fluid (10-20 ml containing picric acid, formalin and glacial acetic acid)15 was injected intra-abdominally depending on the size of the foetus. The entire foetus was put into a container having 10 per cent neutral buffered formalin for fixation and transported immediately.
On the following day after overnight fixation, the foetus was retrieved and the pancreas was dissected out measured (length, width and thickness) and subsequently segregated into head, body and tail regions. Each part was fixed in Bouins fluid further for 6 h and processed separately after washing in 70 per cent isopropylalcohol (twice) to remove excess of fixative/till yellow colour was removed. Further processing was done as per standard protocol to obtain paraffin sections.
From each region (i.e. head, body & tail) of individual pancreas, an average of 150 serial paraffin sections of 4μ thickness were obtained and thus a total of 450 sections were obtained from each pancreatic specimen. After standardization of various islet parameters in each section, it was found that evaluation of every fifth section would be adequate for histopathology analysis without any compromise on the quality of data obtained. Thus, at the end, an average of 30 paraffin sections were studied for each region (head, body & tail) of an individual pancreas. i.e. total 90 sections per pancreas.
Haematoxylin and eosin (H&E Fig. 1) as well as Masson trichrome (Fig. 2) stained sections of each region were studied and morphometric estimates were done at 10X magnification using an ocular grid. (LEITZ DIAPLAN, USA).
Fig. 1.
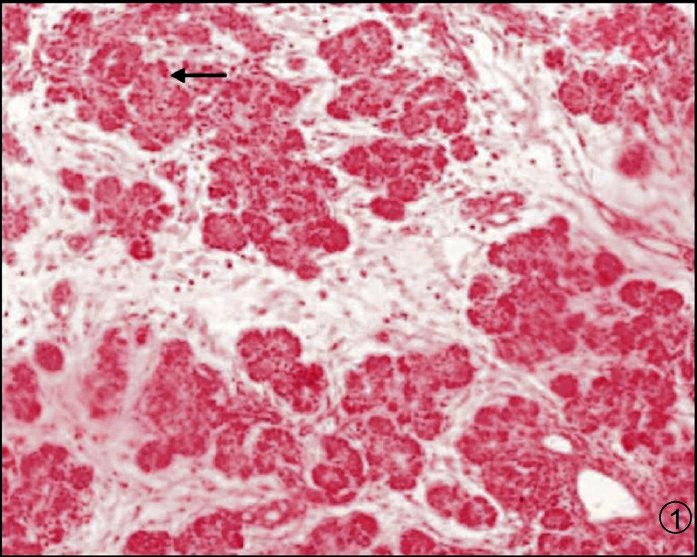
Photomicrograph of pancreas showing acini, ducts and islets (Arrow). H & E X 100.
Fig. 2.
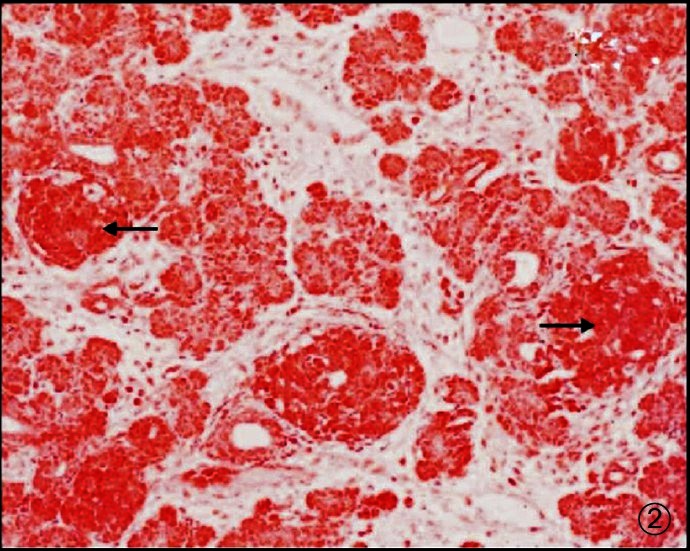
Photomicrograph of pancreas showing acini, ducts and islets (darkly stained - Arrow). Masson trichrome X 100.
Mean area of each of the 30 sections from head, body and tail regions was taken and the pancreatic volume was estimated by multiplying with total thickness. The number of acinar cells (exocrine pancreas) per unit area was also determined. The number of islets was counted for each pancreas sample and total numbers noted. Each islet was then marked in all the 30 sections and followed through. Each individual islet length, width and thickness were counted to obtain the area and volume. Islets with more than 10 cells were considered as fully formed islets and counted. Smaller islets were also noticed (Image-Pro Plus Version 6.2, USA).
Immunohistochemistry (IHC) study: This was done for insulin presence and β-cell estimates on 4 μ paraffin sections, taken on gelatin coated slides. From each region of a pancreatic sample (head, body and tail), three sections were studied. The staining was done with anti-insulin monoclonal antibodies (Sigma Aldrich LifeSciences, USA). Briefly, the sections were deparaffinized and hydrated following which proteinase, 3 per cent hydrogen peroxide and bovine serum albumin were applied before monoclonal primary antibody was applied at 1:800 dilution and secondary antibody (from goat) at 1:20 dilution. This was followed by addition of substrate DAB (di-amino benzidine), counterstaining with haematoxylin, clearing and mounting the slides with DPX for analysis. It was observed that apart from fully formed islets (>10 cells per islet) there were numerous others also, i.e. micro/mini islets having <10 cells (Fig. 3).
Fig. 3.
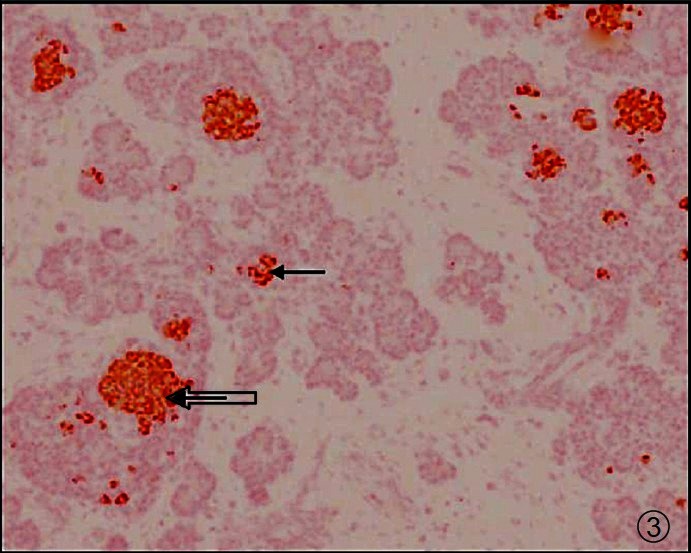
Low BMI subject - Photomicrograph of pancreas showing fully formed islets (Thick arrow) and micro islets with insulin (dark brown stain - Thin arrow). IHC × 100.
Statistical analysis: Descriptive statistics such as mean and standard deviations were calculated for all the variables studied. Comparison of mean values of all the variables was studied between UN and AN mothers using parametric statistics (Student's ‘t’ test) and nonparametric statistics (Mann- Whitney “U” test). Pearson correlations were calculated between all the variables.
Results
The mean (± standard deviation) BMI in all the 14 subjects of the study (AN -8, UN -6) was 20.2 ± 3.4 kg/m2 while the mean gestational age was 17.8 ± 1.6 wk. The mean haemoglobin and fasting blood sugar values were 9.98 ± 2.2 g/dl and 76.0 ± 28.1 mg/dl, respectively. The mean age of AN mothers was 26.87 ± 6.26 yr while that of UN mothers was 21.66 ± 2.94 yr.
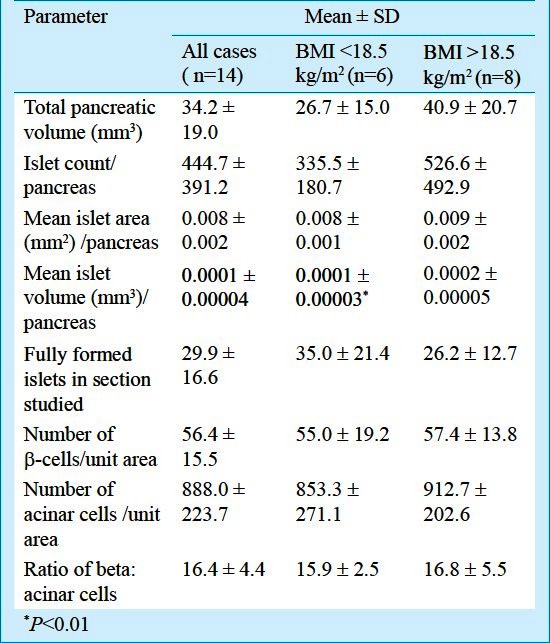
Between UN and AN groups, the pancreatic parameters were comparable and no statistically significant differences were seen except the mean islet volume per pancreas was significantly low (P<0.01) in pancreas of UN mothers as compared to AN mothers (Table I).
Table I.
Foetal exocrine and endocrine pancreas parameters
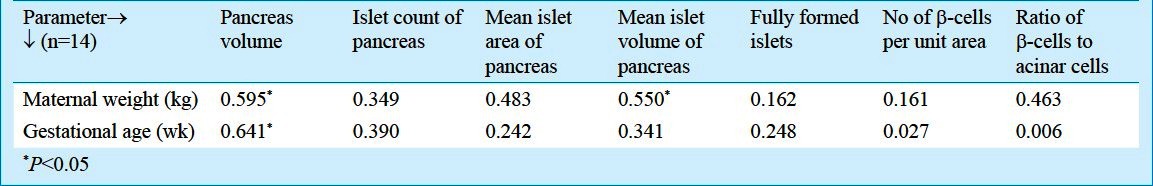
Among the maternal parameters studied, weight of the mothers correlated with pancreatic (r=0.59) and islet volume (r=0.55) significantly (P<0.05) while only gestational age correlated with islet volume significantly (r=0.64; P<0.05) (Table II).
Table II.
Correlation values of maternal parameters with foetal parameters
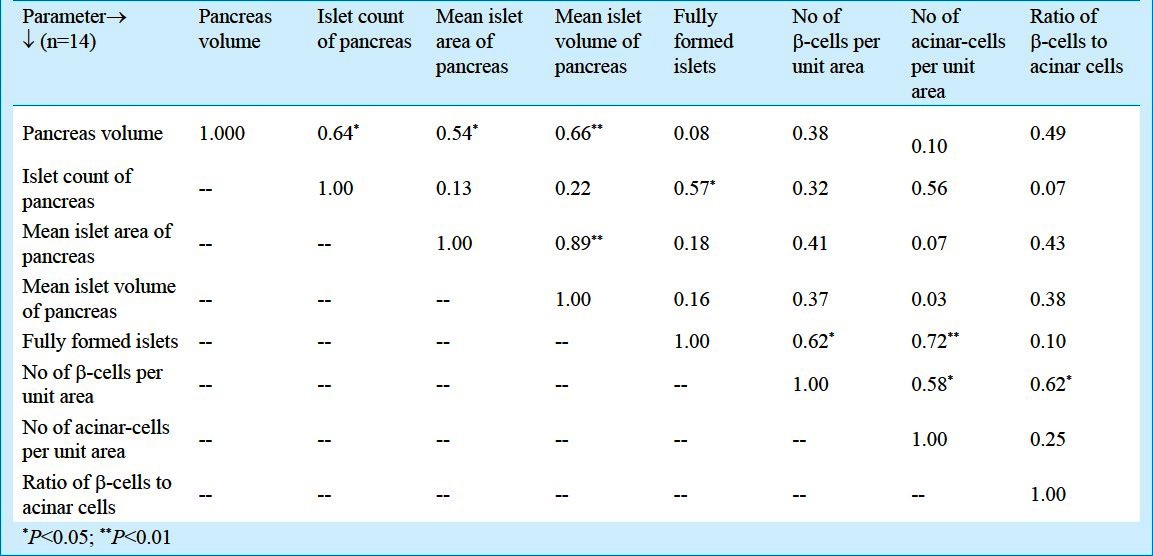
The mean values of pancreatic volume and islet volume were comparable. However, there were significant correlations between total volume of pancreas with islet count (r=0.65; P<0.05), mean islet area (r=0.54; P<0.05) and mean islet volume (r=0.67; P<0.01). Also, islet count of pancreas correlated significantly with number of fully formed islets (r=0.58; P<0.05). Mean islet area showed significant correlation with islet volume (r=0.90; P<0.01) while number of fully formed islets correlated significantly with number of β-cells per unit area (r=0.63; P<0.05) and number of β-cells per unit area with ratio of β-cells to acinar cells (r=0.62; P<0.05), respectively (Table III).
Table III.
Correlation matrix of various foetal pancreatic parameters (pooled data)
Discussion
Nutritional and other factors determining foetal and infant growth influence the size and function of adult pancreatic cell development. It has been reported in an earlier human study that a simple parameter like maternal haemoglobin concentration in the first trimester, has an effect on the foetus and pregnancy outcome and 50 per cent of mothers were anaemic16. In the present study, all mothers were moderately anaemic. The nutritional profile/status of the mother was not looked into specifically by standard scientific means. It has been reported in human studies that BMI directly correlated with β-cell volume and mass in diabetic patients17,18. In the present study, significant correlations were observed between maternal BMI and gestational age and islet volume of foetus. However, there were no significant differences between the mean values of foetal parameters of UN and AN mothers.
Maternal food restriction during pregnancy showed foetal β-cell mass reduction by 70 per cent in rats and subsequent re-nutrition did not help in restoring the same19. In another study in adult rats, by maternal food restriction, there was reduced insulin content of pancreas and β-cell granulation decreased and this was reflected as circulating levels of insulin being 50 per cent of normal values20. A similar observation was made in foetuses born to human diabetic mothers7.
Significant correlations observed between total pancreatic volume with islet count, area and volume have been reported earlier in rats and hamsters21 and agree with the report that physiological growth of islet mass is mainly caused by proportional growth of existing islets21. The number of fully formed islets as an indicator of the functional maturity and their correlation to number of β-cells per unit area as well as islet cell count were also documented earlier in animal studies22. It has been reported that mice fed on low protein diet had smaller islet size and impaired insulin release23. It has been shown in diabetic pregnant rats, that islet cell mass increase was due to increase in number of very small islets, suggesting β-cell neogenesis rather than replication24. In the present study, small islets, as detected by IHC were indicative of possible neogenesis and differentiation, as reported earlier in hamsters25.
The insulin content in human pancreas (in diabetes) was earlier reported to be reduced as compared to normals while in our study, β-cell counts (and insulin content) were comparable between UN and AN groups. Another study looked into quantitative morphology of all different endocrine cells only (ά, β, and δ) in the head and tail parts by immunohistochemistry and not the entire pancreas26.
The total pancreatic volume, islet count per pancreas, number of acinar cells and ratio of β to acinar cells were lesser in UN group compared to AN group. Specific islet parameters like mean area, volume, density and number of β-cells were comparable. The number of fully formed islets was surprisingly more in UN group compared to AN group. This could indicate that the foetal pancreas was probably spared during nutritional insult and would maintain insulin homeostasis.
The limitations of this preliminary study include the small sample size and the inability to match the gestational ages of mothers in UN and AN groups, homogeneity of the groups, anaemia and micronutrient deficiencies which are prevalent in the low socio-economic groups in India. Even though the mean values of parameters studied were comparable between the groups, positive correlations were seen with respect to some pancreatic parameters. No significant differences were observed in our study and this could be due to the small sample size. A larger well designed study would perhaps throw light and provide better understanding of the same.
The findings in our study are in consonance with an earlier study done wherein underfeeding of rat mothers during the first trimester of gestation did not alter insulin action and insulin secretion in the progeny27. Even though it is known that most organ development in humans is completed in the first trimester of pregnancy, it appears that the in utero environment has not been affected by maternal undernourishment so as to effect a change in the foetal pancreatic development or its insulin producing capacity. Possibly, improved nutrition in mid-pregnancy may prevent disease burden in later life for the mother and foetus as well.
Acknowledgment
Our sincere thanks to Mrs. G.Sailaja for all the technical help rendered and Drs K.M. Nair, P. Hemalatha and K.V. Radhakrishna for useful suggestions during preparation of manuscript. Shri Giribabu and Ms. Rekha deserve special mention for secretarial assistance.
References
- 1.Holemans K, Aerts L, Van Assche FA. Lifetime consequences of abnormal fetal pancreatic development. J Physiol. 2003;547:11–20. doi: 10.1113/jphysiol.2002.036582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breant B, Gesina E, Blondeau B. Nutrition, gluco-corticoids and pancreas development. Horm Res. 2006;65(Suppl 3):98–104. doi: 10.1159/000091513. Erratum in Horm Res 2006; 65 : 98-104. [DOI] [PubMed] [Google Scholar]
- 3.Creve ME, Williams K, Nkomo XI, Muller CJ, Du Toit DF, Louw J, et al. Islet cell response in the neonatal rat after exposure to a high fat diet during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:1122–8. doi: 10.1152/ajpregu.00335.2004. [DOI] [PubMed] [Google Scholar]
- 4.Hales CN, Barker DJP. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MR, Hughes SJ. The effect of maternal protein deficiency during pregnancy and lactation on glucose tolerance and pancreatic islet function in adult rat offspring. J Endocrinol. 1997;154:177–85. doi: 10.1677/joe.0.1540177. [DOI] [PubMed] [Google Scholar]
- 6.Ruesens B, Theys N, Dumortier O, Goosse K, Remacle C. Maternal malnutrition programmes the endocrine pancreas in progeny. Am J Clin Nutr. 2011;94(Suppl):18245–95. doi: 10.3945/ajcn.110.000729. [DOI] [PubMed] [Google Scholar]
- 7.Van Assche FA, Gepts W, Aerts L. The fetal endocrine pancreas in diabetes (human) Diabetologia. 1976;12:423. [Google Scholar]
- 8.Barker GJ, Hales CN, Falb CH, Osmond C, Phipps K, Clark PM. Type 2 diabetes mellitus, hypertension and hyper-lipidemia - Syndrome X. Diabetelogia. 1993;36:62–7. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 9.Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small for dates infants. Br J Obstet Gynaecol. 1977;84:751–3. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- 10.Beringue F, Blondeau B, Castellotti MC, Breant B, Czernichow P, Polak M. Endocrine pancreas development in growth retarded human fetuses 2002. Diabetes. 51:385–91. doi: 10.2337/diabetes.51.2.385. [DOI] [PubMed] [Google Scholar]
- 11.New Delhi: Govt. of New Delhi; 2002. The Medical Termination of Pregnancy (MTP) Amendment Act of 2002 (no. 64, 18th December, 2002) [Google Scholar]
- 12.James WPT, Anna Ferro-Lyzzi, Waterlow JC. BMI - Definition of chronic energy deficiency in adults. Eur J Clin Nutr. 1988;42:969–81. [PubMed] [Google Scholar]
- 13.Bain BJ, Bates I, Laftan M, Lewis SM, editors. Dacie and Lewis practical haematology. 10th ed. Elsevier: Churchill Livingstone; 2006. Basic haematological techniques. [Google Scholar]
- 14.Bathena RK, Sheriar NK, Walvekar VR, Guilleband J. Second trimester pregnancy termination using extra-amniotic ethacridine lactate. Br J Obstet Gynaecol. 2005;97:1026–9. doi: 10.1111/j.1471-0528.1990.tb02476.x. [DOI] [PubMed] [Google Scholar]
- 15.Manual of histologic staining methods of the Armed Forces Institute of Pathology. New York: McGraw Hill; 1968. p. 5. [Google Scholar]
- 16.Shobeiri F, Begum K, Nazari M. A prospective study of maternal hemoglobin status of Indian women during pregnancy and pregnancy outcome. Nutr Res. 2006;26:209–13. [Google Scholar]
- 17.Yoon KH, Ko SH, Kim SR, Seo SH, Kang MI, Cha BY, et al. Quantification of the pancreatic cell mass in normal and type 2 diabetic subjects in Korea. J Korean Diabetes Assoc. 2000;24:524–32. [Google Scholar]
- 18.Skau M, Pakkenberg B, Buschard K, Bock T. Linear correlation between the total islet mass and the volume weighted mean islet volume. Diabetes. 2001;50:1763–70. doi: 10.2337/diabetes.50.8.1763. [DOI] [PubMed] [Google Scholar]
- 19.Garofano A, Czernichow P, Breant B. Effect of ageing on β-cell mass and function in rats malnourished during the perinatal period. Diabetologia. 1999;42:711–8. doi: 10.1007/s001250051219. [DOI] [PubMed] [Google Scholar]
- 20.Aerts L, Van Assche FA. Rat fetal endocrine pancreas in experimental diabetes. J Endocrinol. 1977;73:339–46. doi: 10.1677/joe.0.0730339. [DOI] [PubMed] [Google Scholar]
- 21.Inuwa IM, El Mardi AS. Correlation between volume fraction and volume weighted mean volume, and between total number and total mass of islets in post weaning and young Wistar rats. J Anat. 2005;206:185–92. doi: 10.1111/j.1469-7580.2005.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrik J, Reusens B, Arany E, Remacle C, Coelhoc, Hoet JJ, et al. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin like growth factor II. Endocrinology. 1990;140:4861–73. doi: 10.1210/endo.140.10.7042. [DOI] [PubMed] [Google Scholar]
- 23.Morley MG, Leiter EH, Ei Senstein AB, Strack I. Dietary modulation of α-cell volume and function in 129/J mice. Am J Physiol. 1982;242:G354–9. doi: 10.1152/ajpgi.1982.242.4.G354. [DOI] [PubMed] [Google Scholar]
- 24.Aerts L, Vercriusse L, Van Assche FA. The endocrine pancreas in virgin and pregnant offspring of diabetic pregnant rats. Diabetes Res Clin Pract. 1997;38:9–19. doi: 10.1016/s0168-8227(97)00080-6. [DOI] [PubMed] [Google Scholar]
- 25.Del Zotto H, Gomez Dumm CL, Drago A, Fortino A, Luna GC, Garliardino JJ. Mechanisms involved in β-cell mass increase induced by chronic sucrose feeding to normal rats. J Endocrinol. 2002;174:225–31. doi: 10.1677/joe.0.1740225. [DOI] [PubMed] [Google Scholar]
- 26.Clark A, Grant AM. Quantitative morphology of endocrine cells in human fetal pancreas. Diabetologia. 1983;25:31–5. doi: 10.1007/BF00251893. [DOI] [PubMed] [Google Scholar]
- 27.Portha B, Kergoat M, Blondel O, Bailbe D. Underfeeding of rat mothers during the first trimester of gestation does not alter insulin action and insulin secretion in the progeny. Eur J Endocrinol. 1995;133:475–82. doi: 10.1530/eje.0.1330475. [DOI] [PubMed] [Google Scholar]


