Abstract
Background & objectives:
Iron deficiency (ID) affects a large number of women in India. An inverse relationship exists between iron (Fe) status and Fe absorption. Dietary inhibitory and enhancing factors exert a profound influence on bioavailability of Fe. Although the current recommended dietary allowance (RDA) for Fe is based on 8 per cent bioavailability, it is not clear if this holds good for the usual highly inhibitory Indian diet matrix. This study was aimed to determine Fe absorption from several habitually consumed south Indian food and to evaluate the interaction of Fe status with absorption.
Methods:
Four Fe absorption studies were performed on 60 apparently healthy young women, aged 18-35 years. Based on blood biochemistry, 45 of them were ID and 15 were iron replete (IR). The habitual meals assessed were rice, millet and wheat based meals in the ID subjects and rice based meal alone in the IR subjects. Each subject received the test meal labelled with 3 mg of 57Fe and Fe absorption was measured based on erythrocyte incorporation of isotope label 14 days following administration.
Results:
Mean fractional Fe absorption from the rice, wheat and millet based meals in the ID subjects were 8.3, 11.2 and 4.6 per cent, respectively. Fe absorption from the rice-based meals was 2.5 per cent in IR subjects.
Interpretation & conclusions:
Fe absorption is dictated by Fe status from low bioavailability meals. Millet based meals have the lowest bioavailability, while the rice and wheat based meals had moderate to good bioavailability. In millet based meals, it is prudent to consider ways to improve Fe absorption.
Keywords: Bioavailability, habitual meal, India, iron deficiency, young women
Nutritional iron (Fe) deficiency and consequent anaemia arises when physiological requirements cannot be met by Fe absorption from diet. Data from India indicate that about 40-60 per cent of women of childbearing age may suffer from anaemia1, mostly due to iron deficiency (ID). Women of childbearing age are at risk for a negative Fe balance due to their increased Fe loss through menstruation, gastrointestinal blood loss due to intestinal parasitic infections, and high parity2,3. Vegetarian diets in these women add to the problem3.
Dietary Fe bioavailability is low in populations consuming predominantly plant-based diets with little meat4. In meat, 30-70 per cent of Fe is heme Fe, of which 15-35 per cent is absorbed. However, in plant-based diets in developing countries, dietary Fe is non-heme, and its absorption is often less than 10 per cent5. Studies of dietary Fe intake in Indian low socio-economic populations have revealed that the intake was lower than the requirement6, as the diets were predominantly plant based (rice, pulses and vegetables) with high phytic acid levels7. The absorption of Fe from these diets is likely to be poor.
The human body tightly regulates the absorption of Fe, such that there is an inverse relationship between Fe status and absorption9. Our previous study, using stable isotopes of Fe in a neutral meal matrix showed mean fractional Fe absorption from a neutral rice meal to be 17.5% in Fe deficient subjects and 7.0 per cent in controls10. This has relevance to the setting of the recommended dietary allowance (RDA) for the daily intake of Fe, which is critically based on the expected bioavailability of Fe from the diet.
At present, the bioavailability of Fe from plant based diets is assumed to be 8 per cent in calculating the RDA for Indians11; this means that the RDA for Fe, which is the daily physiological Fe requirement adjusted for bioavailability, is 21 mg/day. Since the key assumption in this recommendation is the Fe bioavailability, and considering lack of human data on the bioavailability of Fe from typical Indian diet in normal or anaemic states, the present study was aimed to measure and compare Fe absorption in both iron deficiency and iron replete (ID and IR) states, using a stable isotopic tracer technique, in a variety of common south Indian meals.
Material & Methods
The study was conducted in the Division of Nutrition, St. John's Research Institute, Bangalore.
Subjects: Iron deficient (ID) and replete (IR) women were chosen for the study. They were recruited from a garment factory in Bangalore during October-December 2008.The women, who came from a low socio-economic stratum of society, were aged 18-35 yr, were non-pregnant and non-lactating, and were in apparently good health. The ID women were also anaemic, and the criteria for their selection were haemoglobin (Hb) <12.0 g/dl, serum ferritin (SF) <15 μg/l and zinc protoporphysin (ZnPP) >40 μM/mol heme or soluble transferrin receptor (sTfR) >8.5 mg/l. Women with an elevated serum C-reactive protein (CRP >10 mg/l) were excluded. Since it was planned to study different meals in the ID group, and a single meal matrix in the IR group for comparison, 45 ID and 15 IR subjects were finally selected for the study. The sample size was based on previous data on erythrocyte incorporation of Fe stable isotopes10. It was estimated that 10 subjects would be sufficient to detect a nutritionally significant (50%) difference in absorption (α=0.05 and power 80%). Fifteen women were recruited to allow for any dropout. All 60 subjects out of 334 completed the Fe absorption study. Written informed consent was obtained from all women and the experimental protocol was approved by the ethics committee of St. John's Medical College, Bangalore, India.
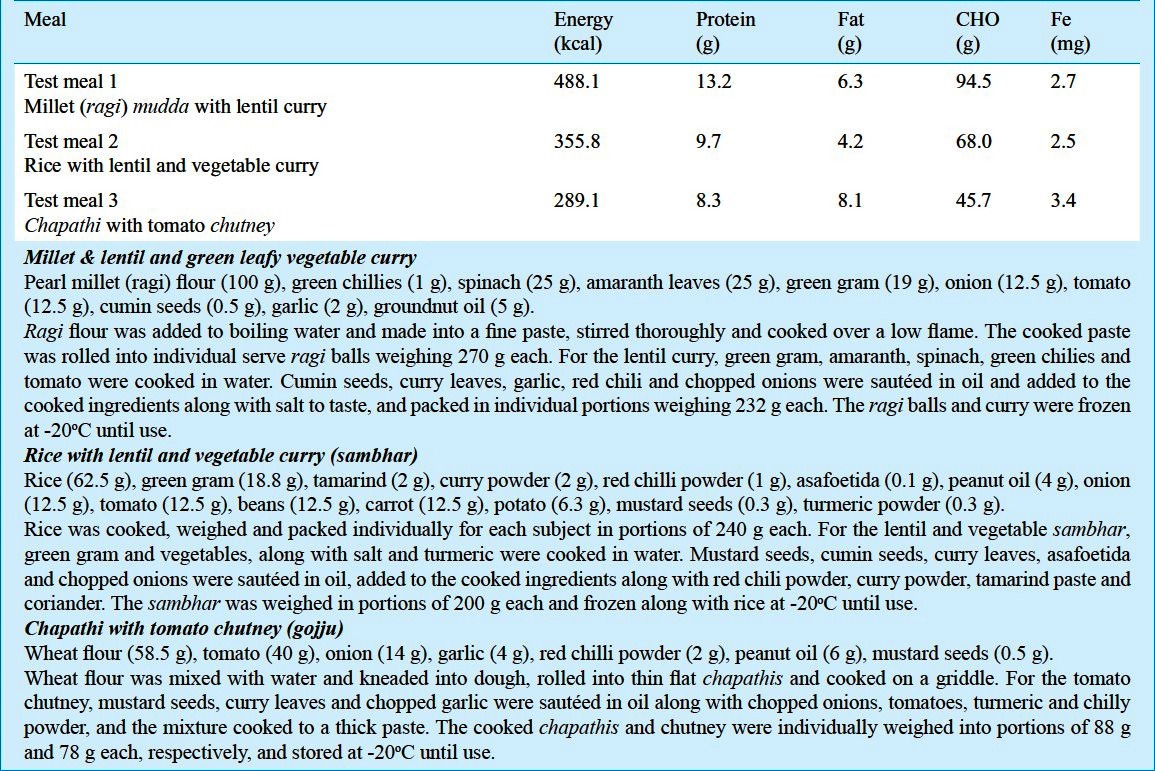
Test meal preparation: Three traditionally consumed south Indian test meals based on millet, rice or wheat were chosen from a dietary survey that was carried out in the local population (Table I). Test meal 1 consisted of millets with lentils and green leafy vegetables [millet (ragi) mudda with lentil curry]; Test meal 2 consisted of rice with lentils and vegetables (rice and sambhar) and Test meal 3 consisted of wheat with tomato chutney (chapathi and tomato gojju). All test meals contained approximately 2-3 mg of native non-heme Fe. The meals were cooked with aluminium or plastic cooking utensils, which were washed with filtered water and dried before use. All the raw foods (in flour form, except for the rice) were procured from the local market and were washed with filtered water. The meals were cooked in bulk, weighed into individual portions in labelled polythene containers, sealed, and frozen at -20°C until use.
Table I.
Composition of the test meals (entire meal consumed by each subject)
Study design: The study was a non-randomized controlled trial. The subjects were divided into groups of 15 each, in which groups A, B, C were ID women and group D consisted of IR women. Subjects reported to the laboratory after overnight fast, in the morning on the study days. On day 1, body weight to the nearest 0.1 kg (Soehnle, Germany) and height to the nearest cm using a stadiometer was measured. The frozen test meal was heated in a microwave oven before serving. Following this, the isotopic labels were loaded on to the test meals just prior to administration. One gram of the solution of isotope label containing 3 mg of 57Fe was dispensed onto the surface of the test meal prior to administration. Groups A, B, C consumed test meal 1, 2, 3 respectively while group D consumed test meal 2. The test meal containers were washed with 10 ml of nano pure water (Millipore, France) three times to ensure complete consumption of the meal and the isotope label. No food or fluids were allowed until 3 hours post test meal administration. A venous blood sample was drawn on day 15 to study Fe absorption. Later the Fe absorption rates were compared among the groups (3ID +1IR).
Measurements of haemoglobin and Fe status: Hb levels in whole blood were determined by a haematology analyzer (ABX Pentra 60 c+, Montpellier, France) with 3 level quality controls (Liquichek, Bio-Rad, USA). Plasma serum ferritin (SF) was measured by an electro-chemiluminescence immunoassay (Roche, Mannheim, Germany), and plasma sTfR was determined by ELISA (Roche, Mannheim, Germany). ZnPP was measured after the cells were washed with normal saline, with a haematofluorometer (Aviv Biomedical, New Jersey, USA) and 3-level control material provided by the manufacturer.
Stable isotopic label preparation for Fe absorption studies: The preparation of the isotopic labels was similar to that described earlier12, which followed the measurement of isotopic composition of the dose using negative thermal ionization mass spectrometry (TIMS, Triton, Thermo, Bremen, Germany). The dose was calculated based on the estimated amount of circulating Fe in the subjects, the expected range of fractional Fe absorption and the attainable precision of the isotopic analysis.
Isotopic analysis of the blood samples: Whole blood samples were ashed and then subjected to acid digestion using a HNO3/H2O2/HCl mixture, followed by separation of the Fe from the sample matrix by anion exchange chromatography (200-400 mesh Ag 1-X8, Biorad, California, USA) and a solvent/solvent extraction step into diethyl ether. Isotopic analysis was carried out by thermal ionization mass spectrometry (TIMS) with a multicollector system for simultaneous ion beam detection12. The precision of the system for isotopic ratios was 0.01 per cent (57/56 Fe ratios). The amount of circulating label was calculated as the product of the shift in the Fe isotopic ratio and the amount of circulating Fe in the blood12. Circulating Fe was calculated from blood volume and haemoglobin(Hb) concentration13. Blood volume calculations were made from height and weight based prediction equations14. For calculations of fractional Fe absorption, it was assumed that there was an 80% incorporation of absorbed Fe into red cells.
Statistical analysis: Statistical analyses were performed using SPSS version 18 (SPSS Inc, Chicago, IL). Serum ferritin and Fe absorption were not normally distributed and were log transformed. Independent t test was used to compare the Fe absorption in rice based meals in ID and IR groups. One way ANOVA was performed to compare the four (3 ID & 1 IR) groups and post- hoc analysis with Bonferroni test was performed to test the significance between the groups. Analysis of covariance was done to compare the Fe absorption among all the four groups controlling for weight and BMI status of the subjects. Probability value less than 5% was considered as statistically significant.
Results
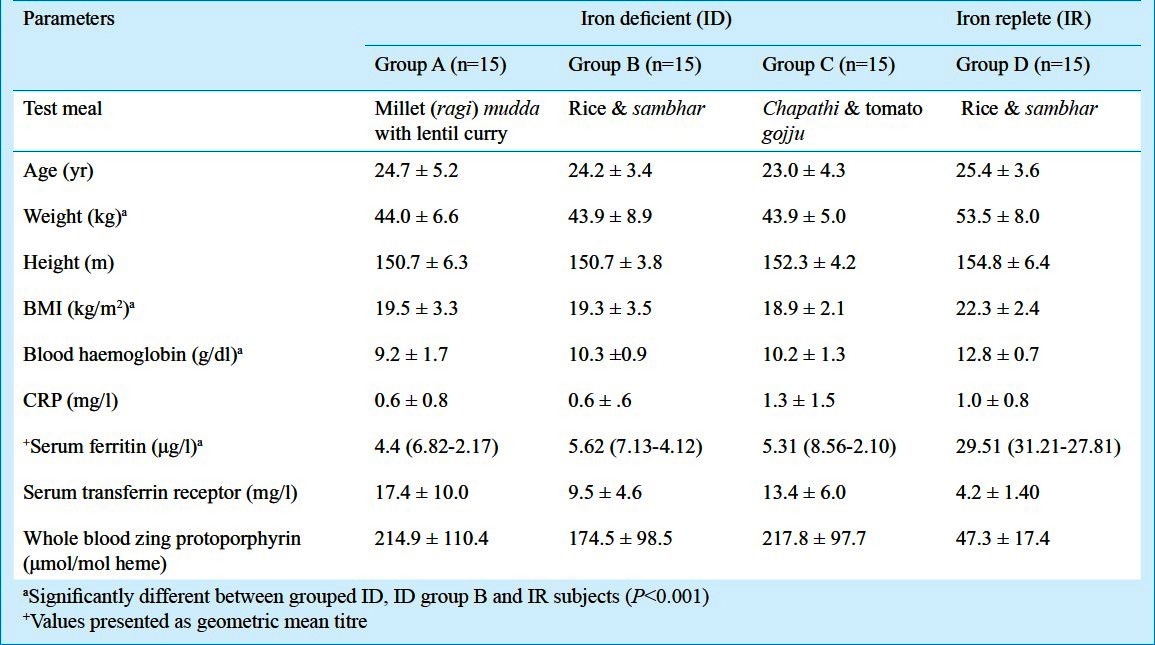
The mean age of the subjects was 24.4 ± 4.2 yr. All anthropometric measures were comparable between the groups (Table II). The mean weight, BMI and Fe status were significantly different between the ID group (3 groups clubbed together) with the IR group (P<0.001).
Table II.
Anthropometry and iron (Fe) status indicators of the study groups
Fe absorption from test meals: Mean fractional Fe absorption was different in all the groups (3ID+1IR). Within the ID group the Fe absorption in the wheat group [11.2 (9.7; 12.8) %] and the rice group [8.3 (6.2; 10.5) %] was significantly higher than in the millet group [4.6 (2.5; 6.6) %] (P<0.05). However, Fe absorption in rice based and wheat based meal groups was not significantly different from each other. Between the groups, Fe absorption from rice based meal in ID group was significantly upregulated and on an average 3 times higher when compared to IR group [2.7 (1.1;4.4)%] (P<0.05). While comparing all the four groups using ANCOVA, Fe absorption was significantly higher in the wheat based and rice based ID groups even after controlling for weight and BMI status of the study subjects (P<0.05).
Discussion
The data from this stable isotope based human study showed that average Fe absorption from three habitually consumed meals varied between 3 to 11 per cent in women with ID anaemia, depending on the type of meal consumed. Millet-based meals had the lowest Fe absorption, followed by rice-based meals while wheat-based meals had the highest Fe absorption. The Fe absorption in IR group women who consumed rice based meal was 2.7 per cent, which was consistent with earlier rice-based studies done in IR adult men and women from other countries and reported a range of Fe absorption values from 0.4 to 9.1 per cent8,15–19. However, the fractional Fe absorption in the IR women in the present study was lower in comparison to an earlier study on habitually consumed Indian diets in IR men, which showed Fe absorption from millet, rice and wheat based diets to be about 1, 5 and 2 per cent, respectively8. This difference may be attributable to differences in methodology and to the precise state of Fe homeostasis between the present and earlier study subjects, but was not unreasonably different. In an earlier neutral (low in Fe absorption inhibitors and enhancers) rice based study using similar stable isotopic methods, Fe absorption was 5.4 per cent in IR subjects and higher (20%) in ID subjects10. In our study subjects there was a significant difference in weight and BMI of the two groups (ID & IR) who consumed rice based meals. This, however, did not affect the differences in Fe absorption between the two groups significantly. Previously it has been demonstrated that greater adiposity is associated with lower fractional iron absorption in humans independent of iron status20. However, as our study subjects were all in a normal BMI range, where this effect is not expected to be very strong, the differences in BMI do not seem to have affected our results.
Although whole wheat flour has abundant phytic acid21, it was surprising to see a high Fe absorption from whole wheat flour based meals in the present study. It is possible that the tomato preparation that was given along with the wheat based test meal may have enhanced Fe absorption. Cooked tomatoes are a rich source of vitamin C22 and several studies have shown vitamin C to enhance Fe absorption10,23–25. For example, Fe absorption was 11 per cent from wheat flour based meals given with vitamin C containing lemonade to ID women, and identical to the Fe absorption in the present study26. As a vegetable, tomato forms a vital component of Indian cooking and is traditionally consumed as chutney, or as a vegetable along with chapathi or rice, and it might be that the habitual consumption of tomatoes has a beneficial effect on Fe absorption from Indian diets.
Millet based diets are predominantly consumed as a staple in the two southern States of India (Karnataka and Andhra Pradesh). Although millet is dense with micronutrients such as Fe, calcium and zinc, these are less bioaccessible due to high levels of tannins and phytate27. Millet-based meals with green leafy vegetable curry, showed the lowest Fe absorption in our study. The addition of the green leafy vegetables to the millet based meal probably made it more inhibitory, and populations that habitually consume such high phytate containing diets may not meet their daily Fe requirements and eventually may lead to a state of ID28. Equally the addition of carrot or amaranth has been shown through the increased ß carotene content to enhance bioaccessibility of Fe from food grains, and the addition of vegetables may counteract the effect of potent inhibitors like phytates on the Fe absorption29.
Several studies have shown that more Fe is absorbed in an ID state than in an IR state9,10. Our earlier stable isotope based studies had shown mean fractional Fe absorption from a neutral rice meal to be upregulated by about 2.5-fold in IDA women10. Similarly, Fe absorption in the present study was 3-fold higher in the iron deficient anemia (IDA) group despite the non-neutral nature of the test meals, which had higher levels of spices and phytic acid. Therefore, the differences in Fe absorption between the ID and IR groups appeared independent of the meal and were most probably dictated by Fe status, even though Fe absorption from different meals can vary markedly and is known to be modulated by enhancers and inhibitors in the meal.
Currently, the RDA for Fe in women is calculated using a bioavailability of 8 per cent (in the presence of vitamin C at 1:2 molar ratio), such that the daily intake is required to be 21 mg Fe/day11. Essentially, this requirement can be met when the Fe density of the diet is about 10 mg Fe/1000 kcal, or higher. As women become more ID, their Fe absorption will upregulate, and due to this it is probable that they will be in Fe balance with the same level of Fe intake. For example, even if the physiological requirement of Fe was double due to ID, an upregulation of Fe absorption by 2-fold, i.e., from 8 to 16 per cent, it would be sufficient to ensure that this greater than normal physiological requirement would be absorbed. However, these are calculations based on single meal absorption, and it remains to be seen if this holds true in the long term, and there is still a tipping point where greater and greater levels of ID and the ensuing upregulation of Fe absorption will not be able to balance chronic losses. In these chronic situations, Fe supplementation or fortification will be inevitably required. A priori, it would appear that a greater degree of caution is required with inhibitory millet based diets, where the Fe density of millet based flours through food fortification, may need consideration along with measures to enhance the vitamin C content of the meal as vitamin C in a diet matrix can increase Fe absorption by 3-3.5 times10. A recent study explored the suitability of millet flour as a vehicle for fortification of Fe along with EDTA and folic acid as co-fortifants and found to have improved the bioaccessibility of Fe30. Polyphenols and phytates in the diet are also important; the habit of ingesting tea with the meal is particularly important, since the former can reduce Fe absorption by 2-2.5 times10. Therefore, dietary practices are critical to ensure optimal Fe absorption from the diet. Overall, it appears that the current bioavailability Figure used in the RDA will hold true for a variety of diets, their inhibitor/enhancer contents, and Fe homeostasis conditions, except in the case of habitual millet based diets.
One of the limitations of this study was that Fe absorption was measured at a single time point and day-to-day variations in Fe absorption were not considered. Further, in the IR subjects, Fe absorption was only measured in rice based meals; therefore, the interaction between meal matrix and Fe status could not be evaluated for the wheat and millet based meals.
In conclusion, the bioavailability of Fe from a standard Indian rice based diet is low in IR women, but upregulated considerably in ID. It is, therefore, likely that the present RDA will be appropriate for normal or mildly anaemic women, given the upregulation in Fe absorption. Diet matrix can play a key role in Fe absorption, and special attention needs to be given to millet based meals.
References
- 1.National Family and Health survey 3. India 2005-06. Mumbai: 11PS; 2007. International Institute of Population Sciences, Mumbai, India and ORC Macro, Calverton, Maryland, USA. [Google Scholar]
- 2.Crompton DWT, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 3.Harvey LJ, Armah CN, Dainty JR, Foxall RJ, Lewis DJ, Langford NJ, et al. Impact of menstrual blood loss and diet on Fe deficiency among women in the UK. Br J Nutr. 2005;94:557–64. doi: 10.1079/bjn20051493. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann MB, Chaouki N, Hurrell RF. Iron deficiency due to consumption of a habitual diet low in bioavailable iron: a longitudinal cohort study in Moroccan children. Am J Clin Nutr. 2005;81:115–21. doi: 10.1093/ajcn/81.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Hurrell RF. How to ensure adequate iron absorption from iron-fortified food. Nutr Rev. 2002;60:S7–15. doi: 10.1301/002966402320285137. [DOI] [PubMed] [Google Scholar]
- 6.Thankachan P, Muthayya S, Walczyk T, Kurpad AV, Hurrell RF. An analysis of the etiology of anemia and iron deficiency in young women of low socioeconomic status in Bangalore, India. Food Nutr Bull. 2007;28:328–36. doi: 10.1177/156482650702800309. [DOI] [PubMed] [Google Scholar]
- 7.Torre M, Rodriguez AR, Saura-Calixto F. Effects of dietary fiber and phytic acid on mineral availability. Crit Rev Food Sci Nutr. 1991;30:1–22. doi: 10.1080/10408399109527539. [DOI] [PubMed] [Google Scholar]
- 8.Rao Narasinga BS, Vijayasarathy C, Prabhavathi T. Iron absorption from habitual diets of Indians studied by the extrinsic tag technique. Indian J Med Res. 1983;77:648–57. [PubMed] [Google Scholar]
- 9.Tomas G. Hepcidin and its role in regulating systemic iron metabolism. Hematology. 2006;1:29–35. doi: 10.1182/asheducation-2006.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Thankachan P, Walczyk T, Muthayya S, Kurpad AV, Hurrell RF. Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. Am J Clin Nutr. 2008;87:881–6. doi: 10.1093/ajcn/87.4.881. [DOI] [PubMed] [Google Scholar]
- 11.Nutrient requirements and recommended dietary allowances for Indians. Hyderabad: National Institute of Nutrition; 2010. A report of the expert group of ICMR 2010; pp. 157–73. [Google Scholar]
- 12.Walczyk T, Davidsson L, Zavaleta N, Hurrell RF. Stable isotope labels as a tool to determine the iron absorption by Peruvian school children from a breakfast meal. Fresenius J Anal Chem. 1997;359:445–9. [Google Scholar]
- 13.Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF. A double stable isotope technique for measuring iron absorption in infants. Br J Nutr. 1994;71:411–24. doi: 10.1079/bjn19940148. [DOI] [PubMed] [Google Scholar]
- 14.Brown E, Hopper J, Jr, Hodges JL, Jr, Bradley B, Wennesland R, Yamauchi H. Red cell, plasma, and blood volume in the healthy women measured by radiochromium cell-labeling and hematocrit. J Clin Invest. 1962;41:2182–90. doi: 10.1172/JCI104677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayers MH, Lynch SR, Charlton RW, Bothwell TH, Walker RB, Mayet F. Iron absorption from rice meals cooked with fortified salt containing ferrous sulphate and ascorbic acid. Br J Nutr. 1974;31:367–75. doi: 10.1079/bjn19740045. [DOI] [PubMed] [Google Scholar]
- 16.Hallberg L, Garby L, Suwanik R, Bjorn-Rasmussen E. Iron absorption from Southeast Asian diets. Am J Clin Nutr. 1974;27:826–36. doi: 10.1093/ajcn/27.8.826. [DOI] [PubMed] [Google Scholar]
- 17.Hallberg L, Bjorn-Rasmussen E, Rossander L, Suwanik R. Iron absorption from Southeast Asian diets. II. Role of various factors that might explain low absorption. Am J Clin Nutr. 1977;30:539–48. doi: 10.1093/ajcn/30.4.539. [DOI] [PubMed] [Google Scholar]
- 18.Tuntawiroon M, Sritongkul N, Rossander-Hulten L, Suwanik R, Brune M, Hallberg L, et al. Rice and iron absorption in man. Eur J Clin Nutr. 1990;44:489–97. [PubMed] [Google Scholar]
- 19.Fidler MC, Davidsson L, Walczyk T, Hurrell RF. Iron absorption from fish sauce and soy sauce fortified with sodium iron EDTA. Am J Clin Nutr. 2003;78:274–8. doi: 10.1093/ajcn/78.2.274. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann MB, Zeder C, Muthayya S, Winichagoon P, Chaouki N, Aeberli I, et al. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int J Obes (London) 2008;32:1098–104. doi: 10.1038/ijo.2008.43. [DOI] [PubMed] [Google Scholar]
- 21.Hurrell RF. Fortification: overcoming technical and practical barriers. J Nutr. 2002;132(Suppl 4):806S–12S. doi: 10.1093/jn/132.4.806S. [DOI] [PubMed] [Google Scholar]
- 22.Rao Narasinga BS, Prabhavathi T. Tannin content of foods commonly consumed in India and its influence on ionisable iron. J Sci Food Agric. 1982;33:89–93. doi: 10.1002/jsfa.2740330116. [DOI] [PubMed] [Google Scholar]
- 23.James DC, Manju BR. Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet. Am J Clin Nutr. 2001;73:93–8. doi: 10.1093/ajcn/73.1.93. [DOI] [PubMed] [Google Scholar]
- 24.Hallberg L, Brune M, Rossander L. Effect of ascorbic acid on iron absorption from different types of meal. Studies with ascorbic-acid-rich foods and synthetic ascorbic acid given in different amounts with different meals. Hum Nutr Appl Nutr. 1986;40:97–113. [PubMed] [Google Scholar]
- 25.Davidsson L, Walczyk T, Zavaleta N, Hurrell RF. Improving iron absorption from a Peruvian school breakfast meal by adding ascorbic acid or NaFeEDTA. Am J Clin Nutr. 2001;73:283–7. doi: 10.1093/ajcn/73.2.283. [DOI] [PubMed] [Google Scholar]
- 26.Olivares M, Pizarro F, Hertrampf E, Fuenmayor G, Estevez E. Iron absorption from wheat flour: effects of lemonade and chamomile infusion. Nutrition. 2007;23:296–300. doi: 10.1016/j.nut.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Shashi BK, Sharan S, Hittalamani S, Shankar AJ, Nagarathna TK. Micronutrient composition, antinutritional factors and bioaccessibility of iron in different finger millet (Eleusine coracana) genotypes. Karnataka J Agric Sci. 2007;20:583–5. [Google Scholar]
- 28.Zimmermann MB, Chaouki N, Hurrell RF. Iron deficiency due to consumption of a habitual diet low in bioavailable iron: a longitudinal cohort study in Moroccan children. Am J Clin Nutr. 2005;81:115–21. doi: 10.1093/ajcn/81.1.115. [DOI] [PubMed] [Google Scholar]
- 29.Smita G, Kalpana P, Krishnapura S. Influence of β-carotene-rich vegetables on the bioaccessibility of zinc and iron from food grains. Food Chem. 2010;122:668–72. [Google Scholar]
- 30.Bhumika T, Kalpana P. Iron fortification of finger millet (Elucene coracana) with EDTA and folic acid as co fortificants. Food Chem. 2011;126:537–42. [Google Scholar]


