Abstract
Background & objectives:
This study was undertaken to evaluate a community based programme of antenatal screening for hepatitis B surface antigen (HBsAg) and selective immunization of children commencing at birth, at a secondary care hospital in south India. The primary objective was to assess immunization coverage among children born to HBsAg positive women; secondary objectives were to study the prevalence of HBsAg among antenatal women, prevalence of HBsAg among immunized children (to estimate vaccine efficacy), seroconversion rate and relationship of maternal hepatitis B e antigen (HBeAg) to hepatitis infection.
Methods:
The prevalence of hepatitis B antigen among antenatal women and immunization coverage achieved with hepatitis B vaccine in a rural block in Vellore, Tamil Nadu were assessed through examination of records. Children born between May 2002 and December 2007 to hepatitis B positive women were followed up for a serological evaluation, based on which vaccine efficacy and the effect of maternal hepatitis B e antigen (HBeAg) on breakthrough infection was estimated.
Results:
The prevalence of hepatitis B surface antigen among antenatal women was 1.58 % (95% CI: 1.35-1.81%). Vaccine coverage for three doses as per a recommended schedule (including a birth dose) was 70 per cent, while 82.4 per cent eventually received three doses (including a birth dose). Estimated vaccine efficacy was 68 per cent and seroconversion 92.4 per cent in children aged 6-24 months. Maternal HBeAg was significantly associated with either anti-HBc or HBsAg in immunized children, RR=5.89 (95% CI: 1.21-28.52%).
Interpretation & conclusions:
The prevalence of hepatitis B among antenatal women in this region was low and a programme of selective immunization was found to be feasible, achieving a high coverage for three doses of the vaccine including a birth dose.
Keywords: Anti-HBc, birth dose, evaluation, HbsAg, hepatitis B, immunization, seroconversion
Perinatal transmission of hepatitis B which is an important route of infection, is believed to be responsible for a third of adult chronic carriers of hepatitis B in India1,2. The hepatitis B vaccine given alone as post exposure prophylaxis soon after birth has a high efficacy ranging from 72-100 per cent, among infants born to hepatitis B surface antigen (HBsAg) positive mothers3–5.
Although universal immunization at birth may be the most cost effective method of preventing horizontal and vertical transmission6, it is not considered feasible in India where the rate of institutional deliveries is low. A recent meta-analysis also showed that the point prevalence of HBsAg in India is 3.70 per cent (95% CI: 3.17-4.24%) corresponding to a chronic carrier rate of 2.96 per cent7, far less than countries like China where it is 7 per cent8.
In India, a hepatitis B immunization programme was introduced as a pilot project, with three doses of vaccine at 6, 10 and 14 wk, with the aim to prevent early horizontal transmission9. The coverage achieved by 2007 was 80.97 per cent in the 33 districts and 58.79 per cent in the 13 cities where this pilot project was implemented10. However, the effectiveness of this strategy in preventing transmission of hepatitis B has not yet been studied11.
Routine antenatal counselling, screening and provision of a birth dose of hepatitis B vaccine when indicated is considered to be difficult. The Community Health Department of Christian Medical College (CMC) in Vellore, south India, has been providing such a screening and immunization programme for antenatal women through mobile services in its project area as well as at its rural secondary care centre since 2002, in an effort to prevent vertical transmission of hepatitis B. The secondary care hospital serves as the referral centre for the primary care services. The present study was an evaluation of the effectiveness of this immunization programme, with the primary objective being estimation of the coverage of the planned immunization schedule, including a birth dose, among children born to HBsAg positive women. The secondary objectives were to study the prevalence of HBsAg among antenatal women, seroconversion to the three doses of vaccine, the vaccine efficacy achieved (by measuring the prevalence of markers of hepatitis B infection among the study children) and the relationship between maternal hepatitis B e antigen (HBeAg) and transmission of hepatitis B infection to the offspring despite immunization.
Material & Methods
Antenatal services were provided by CMC, Vellore as a part of primary health care at the village level in the project area Kaniyambadi, a rural block (population 1, 04,832) in Vellore, Tamil Nadu, between 2002 and 2007. From May 2002, antenatal women were offered screening for hepatitis B and HIV, after appropriate counselling and obtaining signed consent for these tests, as well as for use of residual serum for later research. From 2004, a similar program was commenced in the secondary hospital (CHAD hospital) run by the department of Community Health for women from areas other than Kaniyambadi. The kit used for testing for hepatitis B virus was a rapid one step immunoassay (Hepacard, J. Mitra and Co. Pvt. Ltd, New Delhi, India) which is a card test with a sensitivity of 99.8 per cent according to the manufacturer, with an evaluation in a tertiary institution showing a sensitivity of 79 per cent (95% CI: 57.3-92.1%), with a specificity of 99 per cent12.
HBsAg positive women who received post test counselling at the secondary hospital were advised hospital delivery and hepatitis B immunization for their children within 24 h of birth. In case of home delivery, they were also advised to bring the child to the hospital within 24 h. The schedule for immunization was a birth dose of 0.5 ml of recombinant hepatitis B vaccine (Gene Vac-B, Serum Institute of India Ltd., Pune or Shanvac-B, Shantha Biotechniques Ltd. Hyderabad) intramuscularly, followed by two doses at one and two months. All vaccine doses were provided free of charge. Children resident in the project areas received the second and third doses in the mobile clinics while others received it in the secondary care hospital. Women in the project areas received reminders through home visits by health care workers to ensure second and third doses were taken on time.
Method of evaluation: Presence of HBsAg among antenatal women was obtained from hospital records of all pregnancies registered during the study period 2002 to 2007. Follow up for immunization coverage and serological evaluation were done as a historical cohort study. The cohort of children born to hepatitis B positive women from the beginning of the programme (2002 in the project area and 2004 for the women from other areas) till December 2007, were included if they had completed six months of age. Eligibility for serological evaluation of children was taken as age 6 months or more in order to allow sufficient time for completion of the vaccine series. Mothers were contacted either through health workers, letters or home visits for those who did not respond. Data collected for each child included delivery details, immunization details from parent-retained immunization cards and hospital records (for compliance to the immunization protocol) as well as timing of the birth dose (confirmed from in-patient records where available).
Study children were tested for HBsAg, to check for carrier status; antibody to HBsAg (anti-HBs) to check for seroconversion and total antibody to hepatitis B core antigen (anti-HBc) to look for exposure to the virus after obtaining written informed consent from a parent/guardian. Only children above 24 months were tested for anti-HBc to avoid detection of maternally derived antibodies. The test kits used were the DiaSorin HBsAg kit (S.P.A. Saluggia, Italy); AXSYM AUSAB (Wiesbaden, Germany), a micro particle enzyme immunoassay (MEIA) for the quantitative determination of anti-HBs and AXSYM CORE (Wiesbaden, Germany), an MEIA for anti-HBc. The stored serum samples of the mothers were retrieved and HBeAg testing was done using the ETI-EBK PLUS (DiaSorin S.P.A, Saluggia, Italy).
Information regarding delivery and immunization status of the children of non respondents from the project area was obtained from hospital records for a total of 154 children. Similar data were not available for children born to non respondent women from other areas who had registered at the secondary care hospital.
Data analysis: Data were entered using EpiData version 3.1 (Build 270108; The EpiData Association, Odense, Denmark) and analysed using SPSS 12.0 for Windows (SPSS Inc., 1989-2003, USA). The prevalence of HBsAg among antenatal women, percentage of immunization coverage achieved and occurrence of HBsAg among the immunized children were measured.
Vaccine efficacy was calculated as:
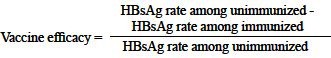
HBsAg rate among unimmunized children was obtained from literature2 whereas the rate among immunized children was calculated from the present evaluation. Subgroup analysis was done according to the type of immunization schedule followed and the mother's HBeAg status. Chi-square and Fisher's exact tests were used to assess significance of different proportions. Secondary outcome measures were occurrence of anti-HBc, seroprotection (presence of anti-HBs of >10 mIU/ml) at the time of testing and geometric mean titres (GMT) of anti-HBs antibody. Geometric mean titres were calculated by finding the anti-log of the mean of log transformed values of anti HBs.
The study was approved by the Institutional Review Board and the Ethics Committee of Christian Medical College, Vellore.
Results
The rate of screening for HBsAg in Kaniyambadi rural block over the five year period (2002-2007) was 93 per cent (12,037 out of the 12,908 pregnancies registered), with 12,977 births occurring during this period. Of the 12,037 pregnancies that had been tested in the peripheral clinics, 190 pregnancies (150 women) were HBsAg positive (Fig. 1). The overall prevalence of HBsAg infection was 1.58 per cent (95% CI: 1.35-1.81%) among all the pregnancies during this period (190 out of 12,037 pregnancies). The prevalence of HBsAg positivity was 1.6 per cent in primigravidas (82 of 5252 pregnancies) and multigravidas (108 of 6771), showing lack of any association between parity and HBsAg status.
Fig. 1.
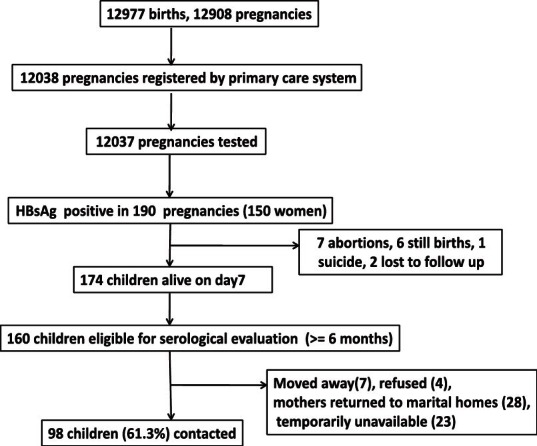
Flow chart of pregnancies in the rural project area (2002-2007) and children eligible for the serological evaluation.
Similarly the overall prevalence of HBsAg among antenatal women attending secondary care hospital was 1.62 per cent (95% CI: 1.36-1.88%), 148 out of 9129 pregnancies (Fig. 2).
Fig. 2.
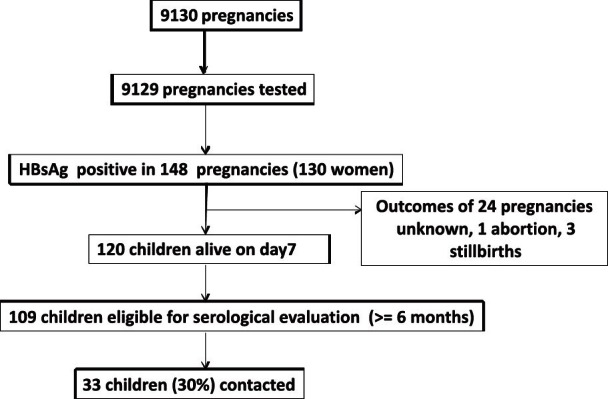
Flow chart of pregnancies registered at the secondary hospital (2004-2007) and children eligible for the serological evaluation.
The proportion of antenatal women registered with our services who consented to screening for HBsAg was 99.9 per cent in both the project area as well as at the secondary care hospital, with only one woman in each group who refused consent.
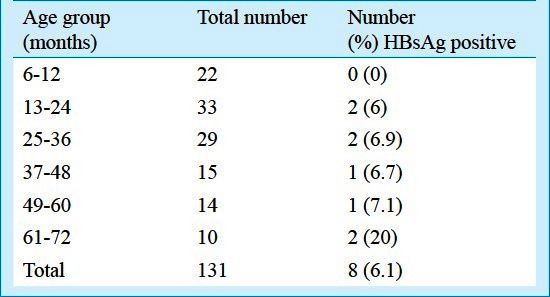
Immunization coverage achieved: Of all the eligible children born to HBsAg positive mothers, 131 were available for our evaluation. Reasons for not being able to contact all eligible children included families having moved away or were temporarily absent, wrong addresses and widely scattered residences within and beyond the district. The median age of the 131 children was 28 months (range: 6-66 months, Table I) and 92.4 per cent of them were born in hospitals.
Table I.
Age groups of the study children and HBsAg results
All the children had received at least one dose of the vaccine (Table II). The programme was able to deliver the first dose of the vaccine within 24 h to 111 children (85%) and within 48 h of birth to 115 children (87.8%, Table II). There was no difference in birth dose coverage (within 48 h) for the 98 children from the project area (87.8%) and the 33 children from the population attending the secondary hospital (87.9%). However, the coverage for the three doses of vaccine including a birth dose, as per a standard schedule was 75.5 per cent for children from the project area, while it was 54.5 per cent for children from non project areas (P<0.05).
Table II.
Immunization schedules followed by the study children and HBsAg results
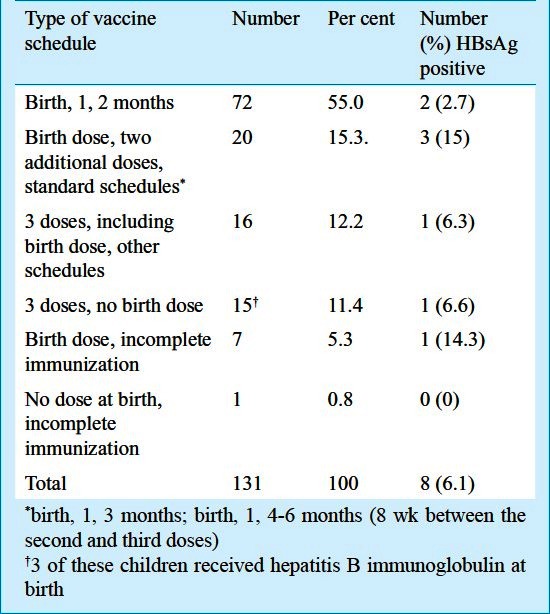
Of the 16 children who had not received a dose within 48 h of birth, four were born at home; three in health centres that were not in our project area (where vaccine was not available); three received hepatitis B immunoglobulin at birth followed by the vaccine after 48 h and six born in the secondary care hospital failed to receive the first dose of the vaccine at birth.
Information regarding 56 non respondents from the project area was also collected from hospital records. The immunization schedules followed by 154 of the 190 children born to the HBsAg positive mothers from the primary care area were thus obtained, showing a birth dose coverage (within 48 h) of 77.2 per cent and overall coverage of 94.6 per cent for three doses of the vaccine.
Serological evaluation: Of the 131 children, eight (6.1%) were found to be HBsAg positive (Table I). As there was no child who was completely unimmunized, the rate of HBsAg among unimmunized children was estimated with previously existing data. Assuming 18.6 per cent as the rate of vertical transmission2 and 90 per cent as the rate of chronicity of infection5, the rate of chronic HBsAg infection among unimmunized children was estimated to be 17 per cent, with a 95% CI: 10.4-23.6 per cent for a sample size of 131. The point estimate of 6.1 per cent HBsAg positive children obtained from our evaluation was clearly different from what would have been expected without immunization. Using these values, the effectiveness of the vaccine in our programme was estimated to be 64 per cent in preventing transmission of hepatitis B virus from mothers who were HBsAg positive.
Immunization schedules of birth, 1, 2 months; birth 1, 3 months and birth, 1, 4-6 months were considered as standard schedules. Among the 92 children who had received three doses of the vaccine, including the birth dose, HBsAg was positive for 5 children (5.4%, 95% CI: 0.69-10.1%) as compared to 3 of 39 children (7.69%) who received incomplete immunization (less than three doses) or non-standard schedules (Table II). The vaccine efficacy for prevention of HBsAg carriage was found to be 68 per cent, assuming HBsAg rate among fully immunized to be 5.4 per cent from our study and 17 per cent for unimmunized children from previous literature.
Among the 115 children who had received the first dose of vaccine within 48 h, HBsAg was detected in seven children (6%) as compared to one out of 16 children (6.25%) who did not (Table II). The difference in immunization failure was not significant even when the definition for birth dose was taken as immunization within 24 h.
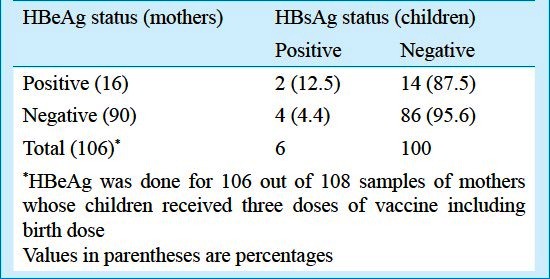
Of the 112 women whose HBeAg status was determined, 18 were found to be positive (16.1%, 95% CI: 9.2-23.0%). The transmission of hepatitis B virus from HBeAg positive mothers, to their fully immunized (including birth dose) children was 12.5% (2 of 16) as compared to 4.4 per cent (4 of 90) from HBeAg negative mothers (RR: 2.77, 95% CI: 0.56- 14.09) (Table III).
Table III.
Maternal HBeAg vs. HBsAg prevalence among immunized children
All 74 children who had completed two years of age underwent additional testing for anti-HBc. Vaccine efficacy and effectiveness of the programme in preventing infection (either anti-HBc or HBsAg positive) was calculated with the assumption of transmission of infection to 29.4 per cent of unimmunized children as shown in an earlier study13. As overall seven of 74 children (9.5%) were either anti-HBc or HBsAg positive, the effectiveness of the programme in preventing infection was 67.7 per cent. As six of 60 children (10%) who received three doses of the vaccine commencing at birth were anti-HBc or HBsAg positive, vaccine efficacy for preventing infection was 66 per cent.
The presence of either HBsAg or anti-HBc in immunized children aged above two years, born to HBeAg positive mothers was 33.3 per cent (two of six children) as compared to 5.7 per cent (three of 53 children) for children of HBeAg negative mothers, RR= 5.9 (95% CI:1.21-28.52%).
Of the 123 children who received three doses of the vaccine, the seroconversion rate (percent with detectable anti-HBs levels) was 91 per cent (112 children). Among the children between 6-24 months who received three doses, the seroprotection rate (anti-HBs >10 mIU/ml) was 92.4 per cent (49 of 53 children). The geometric mean titre of anti-HBs for children between 6-24 months who received three doses of the vaccine and had seroconverted (51 children), was 137.5 mIU/ml (95% CI: 87.4-216.3 mIU/ml).
Discussion
Testing for HBsAg in the antenatal period was found to be acceptable in rural Tamil Nadu with a high rate of consent being obtained from the women. The prevalence of HBsAg was obtained by a high rate of screening (93%) of all the pregnancies in one rural primary care block over five years. This high rate of antenatal registration and screening is not surprising in Tamil Nadu where the proportion of women who received three antenatal care visits was 96.5 per cent as compared to 50.7 per cent in India in 2005-200614.
Although a second HBsAg test must be positive after a 6 month period to diagnose carrier state, in our programme antenatal women were tested only once. The test was done as early in pregnancy as possible, so that even if the women did not come to the secondary care hospital for delivery, they would be aware of the need for hepatitis B vaccine for their children at birth.
The prevalence estimate of HBsAg in our study (1.58%) was lower than the finding of 3.7 per cent from the meta analysis by Batham et al7 and much lower than another community based study in Tamil Nadu15. However, the validity of this estimate arises from the fact that our data represent 93 per cent of antenatal women from a defined area screened over five years, which is a better representation of the community prevalence than groups like voluntary and replacement blood donors, which were included in the meta analysis7. Accounting for the possibility of false negatives (since the test had a sensitivity of 79%)12, the true prevalence could possibly be 2% (95% CI: 1.7-2.29%).
The estimated vaccine efficacy of 68 per cent for prevention of hepatitis B infection among children born to HBsAg positive women under programme conditions was comparable to the efficacy of 72 per cent from a meta analysis of controlled trials4. The absolute risk reduction was 11.6 per cent and the number needed to treat (NNT) was 8.6, implying that 8.6 children would have to be vaccinated to prevent one child from becoming a carrier. The number needed to screen was calculated as described by Rembold (NNT/prevalence of HBsAg among antenatal women) and found to be 54416. In order to prevent one child from acquiring hepatitis B infection from an infected mother, 544 antenatal women would have to be screened in a programme of selective immunization for children born to HBsAg positive mothers.
However, the children who were found to be HBsAg positive in our evaluation could have got the infection either perinatally from their mothers or through horizontal transmission later on in life. The vaccine efficacy calculated in our study is thus not for vertical transmission alone. In order to determine the vertical transmission alone, the presence of IgM anti-HBc in cord blood or the presence of HBsAg in the early post natal period could have been measured, which was not done in our study. Transmission of hepatitis B infection to unimmunized children also could not be assessed from our study as none of the 131 tested children were completely unimmunized.
The presence of maternal HBeAg being a significant risk factor for perinatal transmission was confirmed in our study and 16.1 per cent of HBsAg positive women were positive for HBeAg, which was similar to a review of previous Indian studies where HBeAg was positive in 7.8-47.8 per cent of HBsAg positive individuals17. The seroprotection rate (anti-HBs >10 mIU/ml) of 92.4 per cent among children between 6-24 months of age and the geometric mean titre of 137.5 mIU/ml (95% CI: 87.4-216.3) were comparable to another Indian study on children born to hepatitis B positive mothers, which showed 100% seroprotection, with a GMT of 173.6 mIU/ml a year after immunization18.
Although a limitation of the study was that it involved retrospective record analysis for assessing immunization coverage and timing of vaccine administration, the availability of meticulously maintained secondary data are scarce in India and when evaluated provide valuable information.
In conclusion, evaluation of our programme showed that antenatal screening of hepatitis B achieved 85 per cent coverage for Hepatitis B vaccine within 24 h to children born to hepatitis B positive women. Each State would need to analyse its strengths and weaknesses and find a locally appropriate strategy for control. Cost effectiveness studies of all the models based on Indian data would provide further information for decision making.
References
- 1.Toteja T, Satyamala C, Chowdhary S, Lata S, Puliyel J. Point prevalence of hepatitis B in mother-child dyads in a stratified random sample in an urban resettlement community in Delhi. Indian J Gastroenterol. 2007;26:193–4. [PubMed] [Google Scholar]
- 2.Nayak NC, Panda SK, Zuckerman AJ, Bhan MK, Guha DK. Dynamics and impact of perinatal transmission of hepatitis B virus in North India. J Med Virol. 1987;21:137–45. doi: 10.1002/jmv.1890210205. [DOI] [PubMed] [Google Scholar]
- 3.André FE, Zuckerman AJ. Protective efficacy of hepatitis B vaccines in neonates. J Med Virol. 1994;44:144–51. doi: 10.1002/jmv.1890440206. [DOI] [PubMed] [Google Scholar]
- 4.Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Hepatitis B immunisation for newborn infants of hepatitis B surface antigen-positive mothers. Cochrane Database Syst Rev. 2006;(2):CD004790. doi: 10.1002/14651858.CD004790.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112–25. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal R, Ghoshal UC, Naik SR. Assessment of cost-effectiveness of universal hepatitis B immunization in a low-income country with intermediate endemicity using a Markov model. J Hepatol. 2003;38:215–22. doi: 10.1016/s0168-8278(02)00382-3. [DOI] [PubMed] [Google Scholar]
- 7.Batham A, Gupta MA, Rastogi P, Garg S, Sreenivas V, Puliyel JM. Calculating prevalence of hepatitis B in India: using population weights to look for publication bias in conventional meta-analysis. Indian J Pediatr. 2009;76:1247–57. doi: 10.1007/s12098-009-0246-3. [DOI] [PubMed] [Google Scholar]
- 8.Lu FM, Li T, Liu S, Zhuang H. Epidemiology and prevention of hepatitis B virus infection in China. J Viral Hepat. 2010;17:4–9. doi: 10.1111/j.1365-2893.2010.01266.x. [DOI] [PubMed] [Google Scholar]
- 9.Introduction of Hepatitis B Vaccine in the Universal Immunization Programme. Operations Guide for Program Managers. Child Health Division. Department of Family Welfare. 2002. [accessed on February 1, 2010]. Available from: http://www.whoindia.org/LinkFiles/Hepatitis_B_Operations_Guide.pdf .
- 10.New Delhi: Ministry of Health and Family Welfare, Government of India; 2009. [accessed on April 6, 2010]. Background paper. Ninth Editors’ Conference on Social Sector Issues. Press Information Bureau, Shastri Bhavan. Available from: http://www.pib.nic.in/archieve/ecssi/ecssi2009/hlth&fmly.pdf . [Google Scholar]
- 11.Puliyel JM, Rastogi P, Mathew JL. Hepatitis B in India: Systematic review & report of the ‘IMA sub-committee on immunization’. Indian J Med Res. 2008;127:494–7. [PubMed] [Google Scholar]
- 12.Raj AA, Subramaniam T, Raghuraman S, Abraham P. Evaluation of an indigenously manufactured rapid immunochromatographic test for detection of HBsAg. Indian J Pathol Microbiol. 2001;44:413–4. [PubMed] [Google Scholar]
- 13.Chakravarti A, Rawat D, Jain M. A study on the perinatal transmission of the hepatitis B virus. Indian J Med Microbiol. 2005;23:128–30. doi: 10.4103/0255-0857.16055. [DOI] [PubMed] [Google Scholar]
- 14.National Family Health Survey (NFHS-3),International Institute for Population Sciences. National Family Health Survey India. 2005. [accessed on February 2, 2010]. Available from: http://www.nfhsindia.org/factsheet.html .
- 15.Kurien T, Thyagarajan SP, Jeyaseelan L, Peedicayil A, Rajendran P, Sivaram S, et al. Community prevalence of hepatitis B infection and modes of transmission in Tamil Nadu, India. Indian J Med Res. 2005;121:670–5. [PubMed] [Google Scholar]
- 16.Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 1998;317:307–12. doi: 10.1136/bmj.317.7154.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodha R, Jain Y, Anand K, Kabra SK, Pandav CS. Hepatitis B in India: a review of disease epidemiology. Indian Pediatr. 2001;38:349–71. [PubMed] [Google Scholar]
- 18.Velu V, Nandakumar S, Shanmugam S, Jadhav S, Kulkarni P, Thyagarajan S. Comparison of three different recombinant hepatitis B vaccines: GeneVac-B, Engerix B and Shanvac B in high risk infants born to HBsAg positive mothers in India. World J Gastroenterol. 2007;13:3084–9. doi: 10.3748/wjg.v13.i22.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]


