Abstract
Background & objectives:
Chronic fluoride intoxication through drinking water is a serious health problem. Patients with diabetes are known to have impaired renal function and elimination of fluoride from the body is mainly done through kidney. Fluoride toxicity in diabetes patients may aggravate complications. In this study, the influence of fluoride was assessed on streptozotocin (STZ) induced diabetes in mice as also the efficacy/protective effective of oral supplementation of ginseng (GE) and banaba leaf extracts (BLE).
Methods:
The efficacy of plant extracts, GE and BLE at doses of 50, 150, 250 mg/kg b.w./day alone and in combination, was tested for a period of 15 days on fluoride treated STZ induced diabetic animals.
Results:
Fluoride exposure to mice with STZ-induced diabetes produced significant changes in OSI (organo-somatic index), fluoride content, blood glucose, urea, serum creatinine and oxidative stress indices in kidney tissues with evident histological alterations. Among the antioxidant treatments, combination therapy of GE and BLE at 150 mg/kg b.w. significantly normalized the impaired biochemical variables in kidney tissues of fluoride toxicated diabetic mice.
Interpretations & conclusions:
High fluoride uptake was found to be diabetogenic and further aggravated the renal oxidative damage and thereby the toxicity in mice with STZ induced diabetes mice. GE and BLE exposure individually or in combination at a dose of 150 mg/kg b.w./day for 15 days exhibited protective effects on fluoride toxicated STZ induced nephrotoxicity in mice.
Keywords: Banaba, diabetes, fluoride, ginseng, mitochondrial oxidative stress
Chronic fluoride (F) intoxication is also a wide spread health problem. World Health Organization has established 1.5 mg F/l drinking water as the safe limit, where the average daily intake of fluoride by adults should not exceed 2 mg/day1. However, in some regions of the world especially in developing countries like India and China where the concentration of fluoride in the groundwater may be exceedingly high, the estimated intake of fluoride in adults may be as high as 27 mg/day1. Elimination of ingested fluoride is mainly done by kidney, and kidney malfunction can impede this excretion, thus increasing body burden of F2. People with diabetes mellitus were found to have impaired renal clearance of fluoride and tend to drink more water than normal3–5. Although adverse effects have been reported on long term low dose levels of fluoride ingestion, but short term high dose fluoride levels could result in serious acute toxicity and may aggravate the diabetic complications to many-fold4. Mitochondria may be the major target of fluoride toxicity in several organs, but the role of kidney mitochondria is least known.
Mitochondria generate ATP through oxidative phosphorylation machinery. Under normal conditions, damage by the toxic free radicals is physiologically counteracted by the intracellular antioxidant systems: antioxidant enzymes and endogenous free radical scavengers. However, when the rate of free radical generation exceeds the capacity of antioxidant defences, oxidative stress ensues with consequential severe damage to DNA, proteins and lipids6. Cumulative free radical damage leads mitochondria to a state of mitochondrial dysfunction.
Ginseng (Panax ginseng; Family Araliaceae) root extract and Banaba (Lagerstroemia speciosa; Family Lythraceae) leaf extract are commonly used herbs in many parts of the world and have long been used for the treatment of hyperglycaemia in traditional Chinese medicine8. The predominant physiological active component in ginseng is a group of triterpinoid, saponin glycosides also known as ginsenosides7. Tropical plant L. speciosa (banaba), found in India, Philippines, southern China, Malaya and tropical Australia, has been used as a folk medicine for the treatment of diabetes and kidney diseases. Hypoglycaemic activity of banaba extract was studied in genetically induced diabetic mice and results indicated the presence of ‘insulin- like principle’, in the leaf extract8. Combined supplementation of mulberry, Korean red ginseng and banaba, and traditional anti-diabetic drugs has been shown to increase insulin insensitivity, improve hyperglycaemia and up-regulation on PPARs (peroxisome proliferator-activated receptors) expression by stimulating mitochondrial oxidation and cellular uptake of free fatty acids9 and thereby suggests the existence of synergistic effect among ginseng, mulberry and banaba. Basha and Madhusudan10 have shown a possibility of eliminating fluoride or suppressing fluoride toxicity in vivo in rat by restoring the perturbed redox status and enhancing the homeostatic antioxidant efficiency. In vitro antioxidant ability and total phenolic content of ginseng and banaba have also been elucidated11. This present study was undertaken to evaluate the effect of ginseng and banaba on the mitochondrial oxidative stress in mice with streptozotocin (STZ) induced diabetes and fluoride toxicity.
Material & Methods
Plant material: Standardized aqueous root and leaf extract of Panax ginseng (Asian Ginseng having 80% ginsenosides) (GE) and leaf extract of L. speciosa (banaba, having 1% Corosolic acid fraction) (BLE) were procured from Changsha Botaniex Inc, China.
Chemicals: Streptozotocin (STZ) was obtained from Sigma Aldrich, USA. 5,5’-Dithio-bis (2-nitrobenzoic-acid) (DTNB) and 1-chloro-2,4-dinitrobenzene (CDNB) were purchased from Merck India Ltd. All other chemicals were of analytical grade and were purchased from SD fine chemicals Ltd, India and SISCO research laboratories (SRL), India.
Animals: Adult albino mice, Mus musculus, three months old, weighing 30 ± 5g were procured from Sri Raghavendra Enterprises, Bangalore and acclimatized to laboratory conditions (12:12 h dark/light, 25°±2 °C) in Zoology Department at Bangalore University, Bangalore, India. Standard rodent pellet diet was given ad libitum. The experimental protocol was approved by Institutional Animal Ethical Committee of Bangalore University, Bangalore.
Experimental design: Each group comprised six mice. Group I comprised control animals given normal tap water and Group II animals were made diabetic by single dose intraperitoneal injection of STZ (50 mg/kg b.w. in 0.1mol/l citrate buffer (pH 4.5), in a volume of 1 ml/kg b.w.) and confirmed diabetes after 7 days12. Group III animals were treated with 600 ppm sodium fluoride (NaF, 270 ppm F)13 in drinking water. Group IV served as positive control and on confirmation of induced diabetes (>200 mg/dl) on 7th day, these animals were treated with 600 ppm NaF through drinking water for 30 days. These were segregated into groups to check the efficacy of plant extracts, GE and BLE, exposed alone and together for a period of 15 days14–16. Groups V-VII had phytoextract supplemented experimental mice (viz., group V A-C GE; group VI A-C BLE; group VII A-C GE +BLE) at a dose of 50, 150, 250 mg/kg b.w./day while maintaining the F exposure. At the end of 52nd day (24 h after the last administration), blood was collected from tail vein puncture. All animals in the test and control groups were sacrificed by spinal dislocation and kidneys were immediately dissected out. Of the two kidneys, one was taken for histopathological examination and for fluoride content estimation; the other was washed in ice-cold saline, homogenized and subjected to subcellular fractionation to obtain mitochondrial fraction for biochemical assays. The body weight of each animal was noted before treatment, periodically every alternative day and also on 15th day. The weight of kidney of respective groups of animals was recorded and organo-somatic index (OSI) was calculated by the following formula:
![]()
Estimation of fluoride: Fluoride was estimated by the modified method of Inkielenicz et al21 using Thermo Orion 720A+ with fluoride ion sensitive electrode 9609BNWP and expressed as μg of fluoride/g dry tissue.
Determination of glucose level: Fasting blood glucose was monitored by One Touch Horizon Glucometer (LifeScan Inc, USA).
Estimation of blood urea and creatinine: Blood urea and serum creatinine levels were estimated using standard methods18,19.
Preparation of mitochondrial fraction: Briefly, tissues were rinsed with saline, weighed, and put into ice-cold isolation buffer containing 0.25 mol/l sucrose, 10mmol/l Tris base, 0.5 mmol/l EDTA, pH 7.4. Tissues were smeared carefully to mince, rinsed to get rid of residual blood, and homogenized in 2.5 volume of isolation buffer. The homogenate was centrifuged at 6500g for 4 min; the supernatant fraction was decanted and saved. The pellet was washed once with two volume of isolation buffer. The supernatant fractions were combined and centrifuged at 12,000 g for 4 min. The mitochondrial pellet was washed twice with isolation buffer. All above operations were carried out at 4°C20.
Estimation of lipid peroxidation and antioxidant enzymes: Lipid peroxidation product (LPO) was estimated by measurement of thiobarbituric acid reactive substances (TBARS) using the method of Nehius and Samuelson21. The pink chromogen produced by the reaction of thiobarbituric acid with malondialdehyde, a secondary product of lipid peroxidation was estimated at 535 nm.
Superoxide dismutase (SOD) Activity was assayed by the method of Kakkar et al22, based on the 50 per cent inhibition of the formation of nicotinamide adenine dinucleotide (NADH)-phenazine methosulphate-nitroblue tetrazolium formazon at 520 nm.
Catalase (CAT) Activity was measured as described by Aebi23 and the decomposition of hydrogen peroxide was monitored by measuring the decrease in absorbance at 240 nm.
The reduced glutathione (GSH) content was determined by the method of Ellman24, based on the development of a yellow colour while adding 5,5-dithio (2-nitrobenzoic acid) (DTNB) to compounds containing sulphhydryl groups.
Glutathione peroxidase (GPX) activity was assessed by the method of Rotruck et al25. A known amount of enzyme preparation was incubated with H2O2 in the presence of GSH for a specified time period. The amount of H2O2 utilized was determined by the method24.
Glutathione-S-transferase (GST) activity was estimated by the method of Habig et al26, by following the increase in absorbance at 340 nm using 1-chloro-2,4-dinitrobenze (CDNB) as substrate. Protein was estimated by the method of Lowry et al27using bovine serum albumin as standard.
Histological studies: Kidney samples were harvested and tissue fragments were immediately transferred into bouin's fluid for paraffin embedding and stained with hematoxylin and eosin (H&E). The preparations were evaluated by means of an image analyser (Leica Image Analyzer, Germany).
Statistical analysis: Results were expressed as Mean ± standard error (SEM) of six observations. Data compilation was carried out using SPSS 15.0 software (User guide, Chicago, Illinois). Data from in vivo biochemical studies were analysed by employing One-way analysis of variance (ANOVA). Multiple pair-wise comparisons among all treatment groups were performed by Bonferroni post hoc.
Results
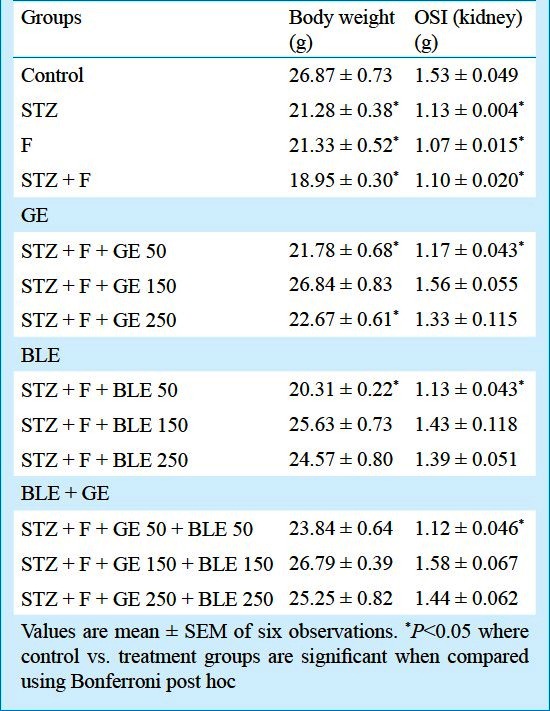
Effect on body weight and organ somatic index (OSI): Body weight and organ (kidney) somatic index showed significant (P<0.05) decrease in fluoride intoxicated diabetic group when compared to control, whereas they were brought to near normal by GE and BLE at dose of 150 mg/kg b.w. treatments alone as well as in combination (Table I).
Table I.
Effect of GE and BLE on body weight (g) and kidney somatic index in fluoride toxicated diabetic mice
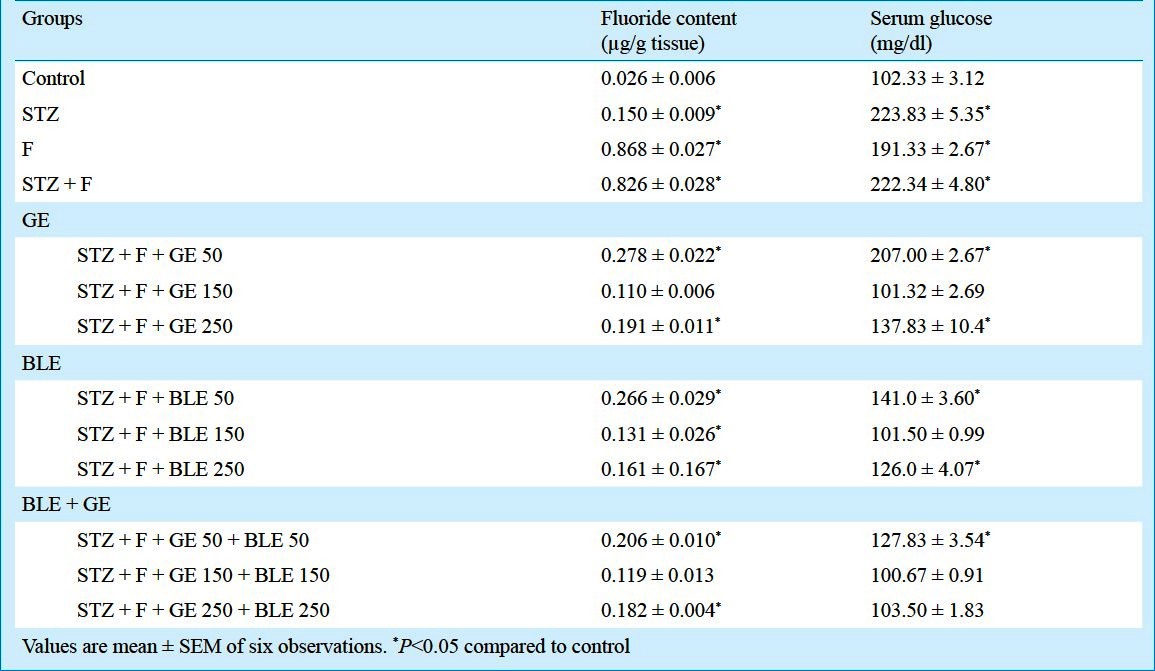
Effect on blood glucose level and fluoride content: Elevated glucose levels were observed in STZ plus F group whereas GE and BLE at doses of 150mg/kg b.w. caused a significant (P<0.05) decrease (54.4% and 54.35%, respectively) and GE and BLE in combination at a dose of 150+150mg/kg b.w. exhibited significant (P<0.05) reduction of 54.64 per cent (Table II). The fluoride content of kidney increased in F treated (32.38%) and STZ plus F (30.76%) treated groups. GE supplementation and combined GE and BLE treatment, especially at a dose of 150 mg/kg b.w., significantly reduced (P<0.05) the fluoride content exhibiting its detoxifying efficacy.
Table II.
Effect of ginseng extract (GE) and banaba leaf extract (BLE) on kidney fluoride content and serum glucose levels in fluoride intoxicated diabetic mice
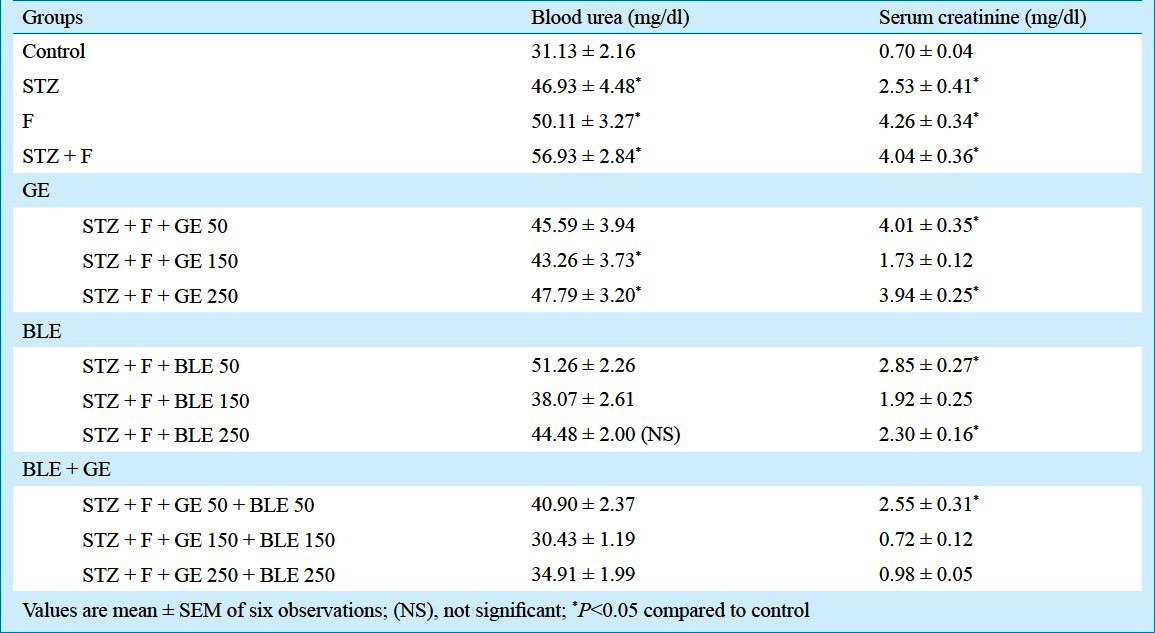
Effect on blood urea and creatinine levels: The blood urea and creatinine levels were found to be significantly (P<0.05) increased and decreased, respectively, in both fluoride exposed and STZ induced diabetic mice and further aggravated in fluoride toxicated diabetic mice. On supplementation of GE 150 mg/kg b.w., the urea levels recovered 23.99 per cent and on BLE 150 mg/kg b.w. treatment 33.11 per cent of recovery was found. Combination therapy with GE and BLE 150 mg/kg b.w. exhibited significant recovery of 46.54 per cent in blood urea level. Blood creatinine levels recovered 57.15 and 52.39 per cent on GE 150 mg/kg b.w. and BLE 150mg/kg b.w. alone treatments, whereas 82.06 per cent recovery (P<0.05) was shown in combination therapy of GE and BLE 150 mg/kg b.w. (Table III).
Table III.
Effect of ginseng root extract (GRE) and banaba leaf extract (BLE) on blood urea and creatinine levels in fluoride intoxicated diabetic mice
Effect on kidney LPO and antioxidant enzymes: STZ and F treatments caused a significant (P<0.05) elevation in kidney MDA levels as well as the STZ plus F group, while the treatments of GE at doses 50, 150, 250 mg/kg b.w. showed recovery of 1.09, 1.2 and 1.0 fold, respectively from that of STZ plus F group (Table IV). Banaba leaf extract of doses 50, 150 and 250mg/kg b.w. recovered 1.09, 1.71 and 1.2 fold of MDA levels, respectively from the positive control, STZ plus F treated group. Among combination therapy of GE and BLE at a dose of 150+150 mg/kg b.w. showed maximum recovery (4.0 fold) in MDA level compared to other doses.
Table IV.
Effect of ginseng extract (GE) and banaba leaf extract (BLE) on oxidative stress indices in fluoride intoxicated diabetic mice
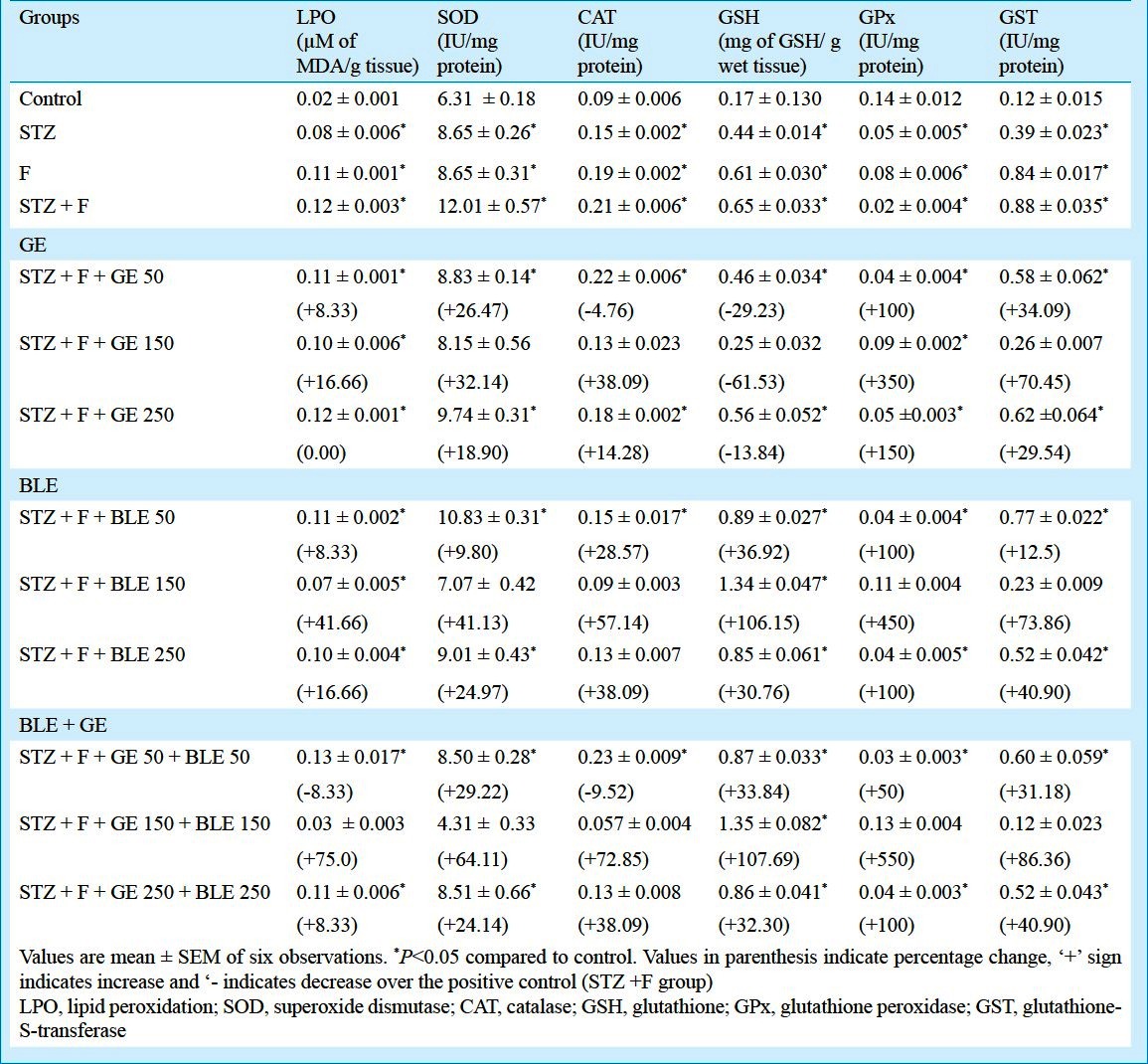
MnSOD, CAT and GST activities and GSH level were significantly elevated (P<0.05) whereas GSH GPx was found suppressed (P<0.05) in STZ plus F group. The Mn-SOD activity levels reduced (1.14 to 1.6 fold) in all the GE and BLE treatments alone and combination treatments but 2.4 fold reduction was shown in GE and BLE of dose 150+150 mg/kg b.w. combination therapy. The treatments with GE at doses of 150 and 250 mg/kg b.w. reduced the CAT activity levels in 0.95, 1.6 and 1.16 fold respectively from that of STZ plus F group and reduction of 1.4, 2.3 and 1.6 fold was found in BLE treatments of doses 50, 150 and 250 mg/kg b.w., respectively. Combination therapy using 150 mg/kg b.w. of GE and BLE showed maximum recovery of 3.6 fold reduction in CAT activity levels when compared to STZ plus F group. The GSH content exhibited significant (P<0.05) reduction of 1.41, 2.6 and 1.16 fold in 50, 150, 250 mg/kg b.w. GE exposure, respectively when compared to STZ plus F group whereas GSH levels significantly (P<0.05) increased (about 1.36, 2.06 and 1.30 fold) in BLE exposed groups at doses 50, 150 and 250mg/kg b.w. and 1.33, 2.07 and 1.32 fold in combination therapy of 50, 150 and 250 mg/kg b.w. treated groups, respectively. Individual GE and BLE treatment showed significant increment in GPx activity of 2.0, 4.5 and 2.5 fold and 2.0, 5.5 and 2.0 fold at 50, 150 and 250 mg/kg b.w. dose treated groups, respectively compared to STZ + F group. Among the combination therapy, 150 mg/kg b.w. dose had the maximum increase of 6.5 fold in GPx activity when compared to other dose combinations. With respect to GST activity, combination therapy (GE and BLE) at dose of 150 mg/kg b.w. was shown the maximum reduction of 7.33 fold among all the other alone and in combination (Table IV).
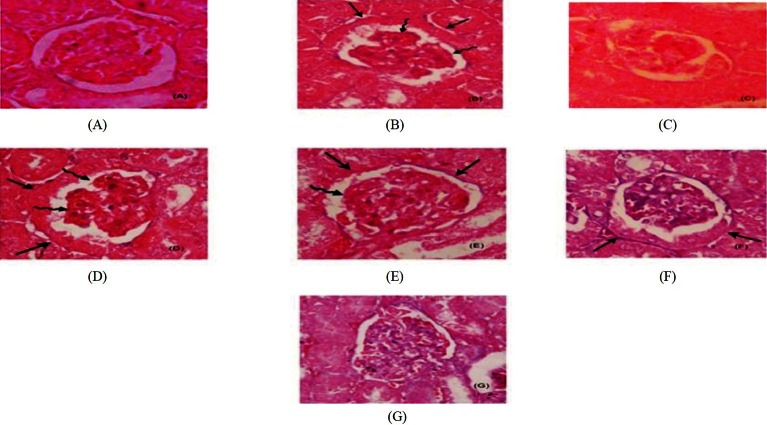
Histopathological changes: The gross histological examinations showed no change in the colour on the external surface of the kidneys taken from the experimental groups (Table V). The kidney of control group showed the normal architecture with no signs of pathology in cortex and medulla. STZ induced kidney exhibited distorted and slightly expanded glomeruli with slightly thickened glomerular basement membranes (GBMs) while least necrosis was also noticed in some sections of convoluted tubules. Tubular necrosis and loss of brush border leading to degeneration of convoluted tubules were seen in fluoride toxicated animals. In STZ plus F treated group, besides the extensive tubular necrosis, presence of lobulated, hypertrophied glomeruli, accelerated mesangial expansion and glomeruli K-W (Kimmelstiel Wilson) nodules were observed. Among the different doses of antioxidant-supplemented groups, significant observations were found only in combined GE and BLE 150 mg/kg b.w. supplemented group (Fig. A–G). Combination treatment of GE and BLE at dose of 150 mg/kg b.w. reduced the glomerular lesions /nodules, reduced thickening of GBM and increased mesangial matrix content and tubular dilatation.
Table V.
Histopathological changes in kidney sections (H&E staining)
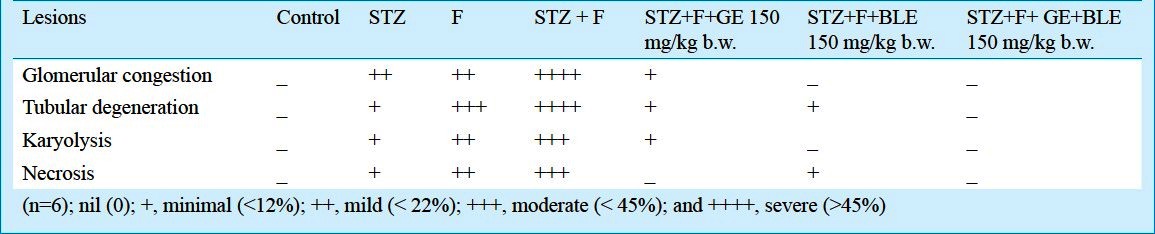
Fig.
Effect of ginseng and banaba leaf extract (GE and BLE) on accelerated mesengial expansion in fluoride toxicated STZ diabetic mice. Paraffin embedded sections of renal cortex were stained with hematoxilin and eosin (H&E). Representative light micrographs (400 X) from each mice groups are shown. (A) Control group (showing normal glomerulus; (B) STZ diabetic control; (C) Fluoride (F) toxicated control; (D) F plus STZ control; (E) G E treated F plus STZ group; (F) BLE treated F plus STZ group; (G) GE and BLE F plus group. Mesangial expansion and thickened GBMs are seen in (B) and (D) (in stright arrows) and lesions/nodules of glomeruli in (B) and (D) (in spiral arrows). Reduction in acelerated mesangial expansions seen in (E), (F) and (G).
Discussion
Although fluoride is considered as an essential trace element, but its exposure at high doses results in fluoride accumulation in body tissues. Besides, the vital organs like liver, kidney and brain are also susceptible to its toxic effects, where the pathological changes occur even before the development of overt clinical signs of fluoride toxicity. Since fluoride is eliminated through kidney, people with renal insufficiency would have impaired renal clearance of fluoride, as commonly seen in diabetic patients2. Studies suggest that the untreated or poorly controlled diabetic state may have substantially higher fluid intakes and thereby enhance the manifestations of adverse effects of long-term ingestion of fluoride via drinking water and less ability to eliminate F due to renal impairments2.
In this study decrease in kidney OSI and body weight in the STZ, F treated and STZ plus F groups showed significant impact of STZ as well as fluoride toxicity on organ and whole body, however, the gain in body weight and OSI were pronounced in plant extract treated groups. Increased fluoride content was observed in F treated, STZ plus F exposed groups and STZ induced group which indicated that the STZ administration and high fluoride exposure posed renal impairment in diabetic mice. Studies have detected high concentrations of fluoride in kidney and in urine during exposure which may be due to elevated body fluid fluoride concentrations and prolonged decrease in glomerular filtration rate2. Increased blood urea nitrogen as well as reduced creatinine levels also result from a reduction in glomerular filtration rate as seen in chronic renal failure2. The present study showed that GE and BLE at dose of 150mg/kg b.w. helped in improving the renal functions (blood urea and creatinine) as well as reduced the F toxic levels. Antioxidant supplementation might have increased the urinary elimination of fluoride thereby reducing the renal fluoride burden in mice, but still the mechanism remains unclear. F treated and STZ plus F groups expressed significant high blood glucose level revealing that fluoride exposure induces a diabetogenic action and elevates the blood glucose level on par with STZ administration, which concomitantly impairs the renal functions.
Free radicals induced impairments and/or altered antioxidant defence capability may be, at least in part, responsible for tissue damage and renal dysfunction in F toxification28 and in diabetes mellitus contributing to the onset, progression and pathological consequences of disease. Amelioration of toxic effects of fluoride in man and animal remained unresolved and controversial till date due to lack of safe effective ameliorative agents that can remove F from the body and can ameliorate toxic effects as well. Basha and Madhusudan10 suggested supplementing antioxidants can restore the perturbed redox status and antioxidant homeostasis of cell, consequently regaining homeostasis which might favour the elimination of F or suppress the fluoride toxicity. In this study, the altered balance of antioxidant enzymes in STZ plus F group caused by a decrease in CAT, MnSOD and GST, and an increase in GPx activities may be responsible for the inadequacy of antioxidant defence in combating reactive oxygen species- mediated damage. The opposing responses of CAT and GPx in STZ plus F group are in agreement with the earlier studies on kidney of diabetic rats29. In present investigation, enhanced GSH synthesis might be an adaptive process against oxidative stress, but could not prevent the progression of renal injury in both fluoride toxicity and in diabetes. Upon GE administration, a reduction in GSH levels was observed followed by enhanced GPx activity in fluoride toxicated diabetic mice which might be due to enhanced utilization of GSH through the mediation of GPx, such utilization is in dire need to protect kidney oxidative damage. Imbalance in antioxidant enzyme system and lipid peroxidation levels in STZ plus F group indicates the synergistic action of fluoride over diabetes models and high doses of fluoride multiply the toxicity to many fold. Supplementation of phytoextracts, GE and BLE, individually and in combination at dose of 150 mg/kg b.w. for 15 days exhibited protective effects by normalizing the lipid peroxidation as well as the mitochondrial antioxidant enzymes and GSH redox status in F treated STZ induced nephrotoxicity in mice.
Purified ginsenosides (the saponin constituents of ginseng root) improve insulin signalling and glucose uptake by stimulating the expression of insulin receptors (IRS-1) and glucose transporter (GLUT4)7, and exert anti-hyperglycemic effect. Though banaba is known to be a potential antioxidant8, no report exists which elicit the efficiency of banaba extract (1% corosolic acid) on fluorosis. Presence of a triterpenoid component in banaba leaf, corosolic acid (CA), is responsible for the glucose transporter activity; translocation of glucose transporter-4 (GLUT4) from intracellular microsomal membrane to plasma membrane in genetically induced diabetic mice and its exposure lowered the blood glucose level30. Glomerular hypertrophy is one of the earliest alterations during the development of diabetic nephropathy2. Increased glomerular volume and hypertrophy observed in STZ induced diabetes and the more pronouncement on fluoride exposure in diabetic mice, lead to decreased ability of glomeruli in eliminating the toxic fluoride through urine and cause premature degeneration of the kidneys3,31. Treatment of GE and BLE at dose 150 mg/kg b.w. reduced the glomerular size, thickening of GBM and mesangial matrix and further absence of KW nodules and tubular dilations were also noticed. Thus the combined treatment of GE and BLE at dose 150 mg/kg b.w. of each slowed down the histopathological alterations and the extent of deterioration in the early stages of diabetic nephropathy.
In conclusion, fluoride uptake in high quantity appeared to be diabetogenic in mice with STZ-induced diabetes and aggrevated the renal oxidative demange. Combination therapy with GE and BLE at dose of 150 mg/kg b.w. each was more effective than GE or BLE alone for the protection against kidney damage and attenuation of lipid peroxidation, thereby normalize the homeostatic antioxidant system.
Acknowledgment
This study was partly supported by University Grants Commission, New Delhi, in form of project grant to first author.
References
- 1.Fluorides. Geneva: WHO; 2002. World Health Organization. [Google Scholar]
- 2.Fluoride in drinking water: A scientific review of EPA's standards. Washington, DC: National Academies Press; 2006. NRC (National Research Council) Report. [Google Scholar]
- 3.Dote T, Kono K, Usuda K, Nishiura H, Tagawa T, Miyata K, et al. Toxicokinetics of intravenous fluoride in rats with renal damage caused by high-dose fluoride exposure. Int Arch Occupy Environ Health. 2000;73:S90–2. doi: 10.1007/pl00014633. [DOI] [PubMed] [Google Scholar]
- 4.Menoyo I, Rigalli A, Puche RC. Effect of fluoride on the secretion of insulin the rat. Drug Res. 2005;55:455–60. doi: 10.1055/s-0031-1296888. [DOI] [PubMed] [Google Scholar]
- 5.Hanhijarvi H, Penttila I, Pekkarinen A, Hakulinen A. The effect of age on free ionized plasma fluoride concentrations in patients from Artificially Fluoridated and Non-fluoridated Drinking Water Communities. Proc Finn Dent Soc. 1974;3:25–34. [Google Scholar]
- 6.Green K, Brand MD, Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 2004;53:110–8. doi: 10.2337/diabetes.53.2007.s110. [DOI] [PubMed] [Google Scholar]
- 7.Huang CK. Ginseng-Panax ginseng. In: Meyer CA, editor. The pharmacology of Chinese herbs. Boca Raton, FL: CRC Press; 1993. pp. 21–45. [Google Scholar]
- 8.Kakuda T, Sakane I, Takihara T, Ozaki Y, Takeuchi H, Kuroyanagi M. Hypoglycemic effect of extracts from Lagerstroemia speciosa L. leaves in genetically diabetic KK-AY mice. Biosci Biotechnol Biochem. 1996;60:204–8. doi: 10.1271/bbb.60.204. [DOI] [PubMed] [Google Scholar]
- 9.Park MY, Lee KS, Sung MK. Effects of dietary mulberry, Korean red ginseng, and banaba on glucose homeostasis in relation to PPAR-alpha, PPAR-gamma, and LPL mRNA expressions. Life Sci. 2005;77:3344–54. doi: 10.1016/j.lfs.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 10.Basha PM, Madhusudhan N. Pre and Post Natal exposure of fluoride induced oxidative macromolecular alterations in developing central nervous system of rat and amelioration by antioxidants. Neurochem Res. 2010;35:1017–28. doi: 10.1007/s11064-010-0150-2. [DOI] [PubMed] [Google Scholar]
- 11.Saumya SM, Basha PM. In vitro evaluation of free radical scavenging activities of panax ginseng and lagerstroemia speciosa: a comparative analysis. Int J Pharm Pharm Sci. 2011;3:165–9. [Google Scholar]
- 12.Katakam AK, Chipitsyna G, Gong Q, Vancha AR, Gabbeta J, Arafat HA. Streptozotocin (STZ) mediates acute upregulation of serum and pancreatic osteopontin (OPN): a novel islet-protective effect of OPN through inhibition of STZ-induced nitric oxide production. J Endocrinol. 2005;87:237–47. doi: 10.1677/joe.1.06411. [DOI] [PubMed] [Google Scholar]
- 13.Sinha M, Manna P, Sil PC. Aqueous extract of the bark of Terminalia arjuna plays a protective role against sodium-fluoride-induced hepatic and renal oxidative stress. J Nat Med. 2007;61:251–60. [Google Scholar]
- 14.OECD Guideline for testing of chemicals. Paris: Organization for Economic Cooperation & Development; 2001. Organization of Economic Co-operation and Development (OECD). Test Guideline 420. Acute oral toxicity-fixed dose method. [Google Scholar]
- 15.Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–8. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 16.Saumya SM, Basha PM. Antioxidant effect of Lagerstroemia speciosa Pers (banaba) leaf extract in streptozotocin-induced diabetic mice. Indian J Exp Biol. 2010;49:125–31. [PubMed] [Google Scholar]
- 17.Inkielewicz I, Czarnowski W, Krechniak J. Determination of fluoride in soft tissues. Fluoride. 2003;36:16–20. [Google Scholar]
- 18.Kanter MW. Clinical chemistry. USA: The Bobber Merill Company Inc; 1975. [Google Scholar]
- 19.Hare RS. Endogenous creatinine in serum and urine. Proc Soc Exp Biol Med. 1950;74:148–51. doi: 10.3181/00379727-74-17837. [DOI] [PubMed] [Google Scholar]
- 20.Katyare SS, Fatterpaker P, Sreenivasan A. Heterogeneity of rat liver mitochondrial fractions and the effect of tri-iodothyronine on their protein turnover. Biochem J. 1970;118:111–21. doi: 10.1042/bj1180111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niehaus WG, Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 22.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 23.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods in enzymatic analysis. New York: Academic Press; 1983. pp. 276–86. [Google Scholar]
- 24.Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 25.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 26.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 27.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 28.Shivarajashankara YM, Shibashamkara AR, Gopalakrishna BP, Hanumanth RS. Effects of fluoride in toxication on lipid peroxidation and antioxidant systems in rats. Fluoride. 2001;34:108–13. [Google Scholar]
- 29.Kakkar R, Mantha SV, Radhi J, Prasad K, Kaira J. Antioxidant defense system in diabetic kidney: A time course study. Life Sci. 1997;60:667–79. doi: 10.1016/s0024-3205(96)00702-3. [DOI] [PubMed] [Google Scholar]
- 30.Judy WV, Hari SP, Stogsdill WW, Judy JS, Naguib YM, Passwater R. Antidiabetic activity of a standardized extract (Glucosol) from Lagerstroemia speciosa leaves in Type II diabetics:A dose-dependence study. J Ethnopharmacol. 2003;87:115–7. doi: 10.1016/s0378-8741(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 31.Zafar M, Naeem-ul-Hassan Naqvi S, Ahmed M, Kaimkhani ZA. altered kidney morphology and enzymes in streptozotocin induced diabetic rats. Int J Morphol. 2009;27:783–90. [Google Scholar]