Sir,
Natural products are the logical starting point for discovering new drugs as evidenced by the discovery of penicillin1. More than 60 per cent of approved antitumour drugs are derived from natural compounds2,3. Drugs with anticancer properties including anthracycline, bleomycin, actinomycin and mitomycin have been isolated from actinobacteria4,5. We have earlier reported isolation and characterization of bioactive metabolites for the first time from Nocardia levis MK-VL_113 and Streptomyces tendae TK-VL_3336–10. In this study these bioactive metabolites were tested for cytotoxicity which is the pre-requisite assay for testing anticancer activity.
For the extraction and purification of bioactive metabolites produced by N. levis and S. tendae, the pure cultures of the strains grown in seed medium (Yeast extract-malt extract-dextrose broth) were transferred individually to optimized fermentation media (Sucrose-tryptone broth for N. levis6 and galactose-tyrosine broth for S. tendae7 under aseptic conditions. The fermentation process was turned off after 96 h of incubation and the culture broths were collected, extracted with ethyl acetate and evaporated to vacuum. The crude residues thus obtained were subjected to purification by using chromatographic techniques (silica gel column, thin layer and semi-preparative high performance liquid chromatography) and the structure of the pure bioactive compounds was elucidated and confirmed on the basis of Fourier transform infrared (FTIR), Electron impact mass (EIMS)/Electron spray ionization mass (ESIMS) spectrometry and nuclear magnetic resonance (1H NMR and 13C NMR) spectroscopy8–10.
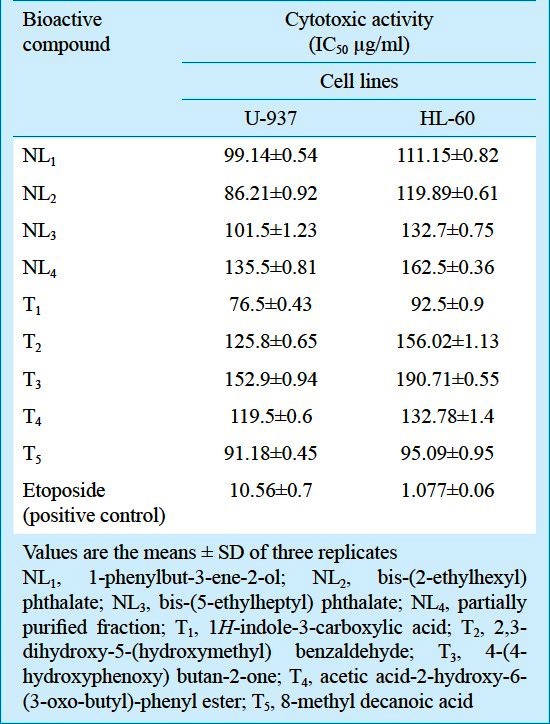
Screening of secondary metabolites obtained from N. levis led to the identification of bioactive compounds namely 1-phenylbut-3-ene-2-ol, bis-(2-ethylhexyl) phthalate, bis-(5-ethylheptyl) phthalate and a partially purified fraction containing phenylethyl alcohol, dibutyl phthalate and 1,2-benzenedicarboxylic acid, 3-nitro8,9. Five bioactive compounds namely 1H-indole-3-carboxylic acid, 2,3-dihydroxy-5-(hydroxymethyl) benzaldehyde, 4-(4-hydroxyphenoxy) butan-2-one, acetic acid-2-hydroxy-6-(3-oxo-butyl)-phenyl ester and 8-methyl decanoic acid were purified from the culture broth of S. tendae10. No information is available on the production of these bioactive metabolites by Streptomyces. Hence, these bioactive metabolites isolated from N. levis MK-VL_113 and S. tendae TK-VL_333 were tested for cytotoxic activity using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay11.
Cell lines viz., U-937 (Human leukemic monocyte lymphoma cell line) and HL-60 (Human promyelocytic leukemia cell line) procured from National Centre for Cell Science, Pune, India were cultured on RPMI-1640 (Hi-media®, Mumbai) medium supplemented with 10 per cent (v/v) foetal bovine serum, 1 mM NaHCO3, 2 mM L-glutamine and penicillin-streptomycin in a humidified atmosphere (95%) with 5 per cent CO2 at 37°C. Cells of U-937 and HL-60 (2 × 104 cells per well) were seeded in each well of 96-well microtiter plate containing 0.1 ml of medium. After overnight incubation, the cells were treated with different test concentrations of bioactive compounds (0-200 μg/ml) of the strains at identical conditions with three replicates of each concentration. After 24 h of incubation, the cell viability was assessed by adding 10 μl of MTT (5 mg/ml) per well and the plates were incubated in a CO2 incubator at 37°C for 4 h. The formazan crystals formed in the cells were dissolved with 100 μl of 0.1 per cent DMSO and the rate of colour development was measured at 570 nm in a spectrophotometer (Spectra MAX Plus, Molecular Devices, supported by SOFTmax PRO-3.0, USA). The inhibition of cell viability (IC50) was determined with reference to that of etoposide (standard).
Bioactive compounds produced by N. levis and S. tendae showed cytotoxic activity against U-937 and HL-60 cell lines. Among these, NL1 and NL2 from N. levis and T1 and T5 from S. tendae exhibited high cytotoxicity when compared to the others (Table). However, their activity was less when compared to etoposide (positive control). Actinobacteria particularly Streptomyces spp. are known to produce a number of potent cytotoxic compounds including anthracyclines, FCE 21424, FCE 24366 and FCE 2436712, saptomycins13, cremeomycin14, clecarmycins15, actinomycins G2-G616, moromycins A and B, saquayamycin B and fridamycin D17 while brasiliquinones A, B and C18, nocardiones A and B19and chemomicin A20 were recorded from Nocardia spp. Keeping the potentiality of the actinobacterial metabolites as anticancer agents, the bioactive metabolites of N. levis and S. tendae showing moderate cytotoxic activity against the cell lines, U-937 and HL-60 may be used as model systems for the preparation of anticancer drugs by testing further anticancer assays.
Table.
Cytotoxic activity of the bioactive compounds produced by N. levis MK-VL_113 and S. tendae TK-VL_333
Acknowledgment
The first author (AK) thanks Indian Council of Medical Research (ICMR), New Delhi, for providing financial assistance in the form of senior research fellowship.
References
- 1.Wedge DE, Camper ND. Connections between agrochemicals and pharmaceuticals. In: Cutler SJ, Cutler HG, editors. Biologically active natural products: pharmaceuticals. Washington DC: CRC Press; 1999. pp. 1–15. [Google Scholar]
- 2.Cragg G, Newman D, Snader K. Natural products in drug discovery and development. J Nat Prod. 1997;60:52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 4.Rocha AB, Lopes RM, Schwartsmann G. Natural products in anticancer therapy. Curr Opin Pharmacol. 2001;11:364–9. doi: 10.1016/s1471-4892(01)00063-7. [DOI] [PubMed] [Google Scholar]
- 5.Newman DJ, Cragg GM. Advanced preclinical and clinical trials of natural products and related compounds from marine sources. Curr Med Chem. 2004;11:1689–709. doi: 10.2174/0929867043364982. [DOI] [PubMed] [Google Scholar]
- 6.Kavitha A, Vijayalakshmi M. Cultural parameters affecting the production of bioactive metabolites by Nocardia levis MK-VL_113. J Appl Sci Res. 2009;5:2138–47. doi: 10.1111/j.1472-765X.2009.02697.x. [DOI] [PubMed] [Google Scholar]
- 7.Kavitha A, Vijayalakshmi M. Influence of cultural conditions on the production of bioactive metabolites by Streptomyces tendae TK-VL_333. Res J Biotechnol. 2009;4:56–64. [Google Scholar]
- 8.Kavitha A, Prabhakar P, Vijayalakshmi M, Venkateswarlu Y. Production of bioactive metabolites by Nocardia levis MK-VL_113. Lett Appl Microbiol. 2009;49:484–90. doi: 10.1111/j.1472-765X.2009.02697.x. [DOI] [PubMed] [Google Scholar]
- 9.Kavitha A, Prabhakar P, Narasimhulu M, Vijayalakshmi M, Venkateswarlu Y, Venkateswara Rao K, et al. Isolation, characterization and biological evaluation of bioactive metabolites from Nocardia levis MK-VL_113. Microbiol Res. 2010;165:199–210. doi: 10.1016/j.micres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Kavitha A, Prabhakar P, Vijayalakshmi M, Venkateswarlu Y. Purification and biological evaluation of the metabolites produced by Streptomyces tendae TK-VL_333. Res Microbiol. 2010;161:335–45. doi: 10.1016/j.resmic.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid calorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Cassinelli G, Arlandini E, Ballabio M, Bordoni T, Geroni C, Giuliani F, et al. New biosynthetic anthracyclines related to barminomycins incorporating barbiturates in their moiety. J Antibiot. 1990;43:19–28. doi: 10.7164/antibiotics.43.19. [DOI] [PubMed] [Google Scholar]
- 13.Abe N, Nakakita Y, Nakamura T, Enoki N, Uchida H, Munekata M. New antitumor antibiotics, saptomycins. I. Taxonomy of the producing organism, fermentation, HPLC analysis and biological activities. J Antibiot. 1993;46:1530–5. doi: 10.7164/antibiotics.46.1530. [DOI] [PubMed] [Google Scholar]
- 14.McGuire JN, Wilson SR, Rinehart KL. Cremeomycin, a novel cytotoxic antibiotic from Streptomyces cremeus. Structure elucidation and biological activity. J Antibiot. 1995;48:516–9. doi: 10.7164/antibiotics.48.516. [DOI] [PubMed] [Google Scholar]
- 15.Fujii N, Katsuyama T, Kobayashi E, Hara M, Nakano H. Clecarmycins, new antitumor antibiotics produced by Streptomyces: Fermentation, isolation and biological properties. J Antibiot. 1995;48:768–72. doi: 10.7164/antibiotics.48.768. [DOI] [PubMed] [Google Scholar]
- 16.Bitzer J, Gesheva V, Zeeck A. Actinomycins with altered threonine units in the beta-peptidolactone. J Nat Prod. 2006;69:1153–7. doi: 10.1021/np060063g. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Fattah MS, Kharel MK, Hitron JA, Baig I, Rohr J. Moromycins A and B: Isolation and structure elucidation of C-glycosylangucycline type antibiotics from Streptomyces sp. KYOO2. J Nat Prod. 2008;71:1569–73. doi: 10.1021/np800281f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nemoto A, Tanaka Y, Karasaki Y, Komaki H, Yazawa K, Mikami Y. Brasiliquinones A, B and C, new benz[a]anthraquinone antibiotics from Nocardia brasiliensis. I. Producing strain, isolation and biological activities of the antibiotics. J Antibiot. 1997;50:18–21. doi: 10.7164/antibiotics.50.18. [DOI] [PubMed] [Google Scholar]
- 19.Otani T, Sugimoto Y, Aoyagi Y, Igarashi Y, Furumai T, Saito N, et al. New Cdc25B tyrosine phosphate inhibitors, nocardiones A and B, produced by Nocardia sp. TP-A0248: Taxonomy, fermentation, isolation, structural elucidation and biological properties. J Antibiot. 2000;53:337–44. doi: 10.7164/antibiotics.53.337. [DOI] [PubMed] [Google Scholar]
- 20.Sun CH, Wang Y, Wang Z, Zhou JQ, Jin WZ, You HG, et al. Chemomicin A: A new angucyclinone antibiotic produced by Nocardia mediterranei subsp. kanglensis 1747-64. J Antibiot. 2007;60:211–5. doi: 10.1038/ja.2007.25. [DOI] [PubMed] [Google Scholar]


