Abstract
Background & objectives:
Pre-clinical studies in swine have demonstrated that a supratherapeutic concentration of sildenafil citrate decreased defibrillation efficacy and facilitated cardiac arrhythmia. We therefore, decided to investigate the effects of Kaempferia parviflora (KP) extract on these parameters in the swine heart. The underlying assumption was that in the heart, KP might be producing effects similar to sildanafil citrate as KP has long been used in southeast Asian traditional medicine to correct erectile dysfunction.
Methods:
The study was conducted as the defibrillation study, and ventricular fibrillation (VF) induction study. In both studies, the defibrillation threshold (DFT), the upper limit of vulnerability (ULV) and VF threshold were determined before and after KP extract administration.
Results:
In both studies KP extract at high concentrations (100 and 50 mg/kg) significantly increased the DFT and ULV, without altering the VF threshold. At these concentrations, systolic and diastolic blood pressures were also attenuated.
Interpretation & conclusions:
High concentrations of KP extract attenuated defibrillation efficacy and increased cardiac vulnerability to arrhythmia in a normal swine heart. When used in appropriate concentrations, its blood pressure lowering effect may be useful in hypertensive states. Further studies need to be done to elucidate its mechanism of action.
Keywords: Cardiac arrhythmia, heart, Kaempferia parviflora, slidenafil, ventricular defibrillation
Kaempferia parviflora Wall. Ex. Baker (KP), one of the plants in the Zingiberaceae family and locally known in Thai language as Krachai-dam, has been used in Thai traditional medicine as a tonic for promoting health, relieving body pains and gastrointestinal disorders and rectifying male impotence1–4. KP contains many kinds of flavonoids, including 11 flavones, three flavanones and two chalcones1,5–7. Previous studies have reported that KP has anti-plasmodial1, anti-fungal1, anti-mycobacterial1, anti-gastric ulcer2, anti-allergic3 and anti-acetylcholine-esterase (AChE) activities8. Some of these effects are attributed to the presence of certain specific flavonoids in KP extract.
Traditionally, KP has been used as herbal medicine to alleviate male impotence4. Even though its mechanism of action is not known, recent in vitro study indicated that it may be mediated through cGMP by promoting inhibition of phosphodiesterase type 5 (PDE -5) activity9 which breaks down cGMP by cleaving it to 5’-GMP10. cGMP is essential for penile erection as it causes smooth muscle relaxation in the corpora cavernosa10. Formation of cGMP is increased by nitric oxide (NO) released from the cavernous nerves and endothelial cells in response to sexual stimuli10. KP extract also promotes NO release by enhancing mRNA and protein expression in human umbilical vein endothelial cells11. Additionally, it causes relaxation of rat aortic rings through a NO signaling pathway12–14. All these actions lead to an increase in cGMP level and may rectify erectile dysfunction.
Sildenafil citrate is used worldwide for correction of impotence10. Its mode of action is promoting accumulation of cGMP in the corpora cavernosa by inhibiting its breakdown by PDE-510. However, recent pre-clinical studies have demonstrated that supratherapeutic concentration of sildenafil citrate administered with or without an NO donor significantly decreased defibrillation efficacy and might facilitate arrhythmias15–18. It remains to be seen whether KP extract also produces similar effects in the heart. Hence the present investigation was carried out to study the effects of KP extract on these parameters in the swine heart its anatomy, size, physiology and the perfusion distribution of blood flow are similar to the human heart19.
Material & Methods
Preparation of KP extract: KP rhizomes were obtained in the form of coarsely ground powder from Thanyaporn Co., Ltd., Samutprakarn Province, Thailand. The coarse powder of this plant was weighed for 100, 50, 25, or 12.5 mg/kg body weight, and extracted with 70 ml saline for 15 min at 90 °C. The solution was filtered with Whatman filtered paper No.1 to collect the extract. The final volume of the extract was approximately 50 ml. It was expected that such an extraction would retain the bioactive components of KP rhizomes and would produce biological effects without cytotoxicity1,20.
Animal preparation and electrode placement: The study was approved by the Institutional Animal Care and Use Committees of the Faculty of Medicine, Chiang Mai University, Thailand. Pigs (~30 kg) of either sex were anaesthetized, monitored, and maintained under physiological conditions as described earlier16,17. In each pig, anaesthesia was induced with a combination of atropine (0.04 mg/kg), xylazine (2 mg/kg) and zolitil (5 mg/kg), injected intramuscularly and maintained by 0.5-2.0 per cent isoflurane delivered in 100 per cent oxygen. After cuffed-endotracheal intubation, mechanical ventilation (volume controlled, tidal volume=12 ml/kg, respiratory rate=10-15 cycles/min) was started with the pig in a restrained dorsally recumbent position. The surface electrocardiogram (lead II), PaO2, end-tidal CO2, femoral arterial blood pressure, core temperature, respiratory rate, blood gases and electrolytes were monitored continuously throughout the entire study. The pigs were operated on to expose and isolate the left and right external jugular veins. A catheter with a 34-mm platinum-coated titanium coil electrode (Guidant Corporation, Natick, MA, USA) was inserted into the right ventricular apex. A 68-mm electrode catheter was placed at the junction between the right atrium and superior vena cava. The positions of the catheters were verified with fluoroscopy and secured at the venotomy sites to stabilize their positions. Succinylcholine (1 mg/kg loading, 0.25 mg/kg maintenance every 45 min) was administered intravenously to minimize skeletal muscle contraction during shock testing or rescue shock.
Two sets of experiments were performed; defibrillation study and VF induction study. In each study, the pigs were divided into five groups (n=8 each). The KP extract of 100, 50, 25, 12.5 mg/kg and saline, each of 50 ml, were intravenously infused in groups 1, 2, 3, 4 and 5, respectively. The KP extract or saline was infused at the rate of two ml/min for 25 min in both series. All parameters in the defibrillation and VF induction studies were determined before (control) and at 30 min after KP extract or saline administration. This time point was chosen since our data indicated that the effect of KP extract on haemodynamics and cardiac electrophysiology peaked at 30 min after infusion and declined to the baseline within three hours.
Defibrillation protocol: Ventricular fibrillation (VF) was induced by a 50-Hz alternating current delivered via an electrode at the tip of the right ventricular catheter. After 10 sec of VF induction, the defibrillation was attempted with biphasic truncated exponential shocks (Ventak, Guidant Corporation, Natick, MA, USA) with electrodes at the right ventricular apex as cathode, and at the superior vena cava as anode for the first phase. A minimum of four minutes was allowed to elapse between VF episodes. If the shock failed to defibrillate, a rescue shock (20-30 J) was delivered to restore sinus rhythm within ten seconds. The defibrillation threshold (DFT) was determined using a three-reversal up/down protocol with the initial strength at 400 V16. The lowest shock strength that is successful to defibrillate after the third reversal was defined as DFT16. In the present study, the DFT was shown as the delivered voltage and the total energy delivered by the defibrillator to the fibrillating heart. The other two parameters that involved in the determination of DFT were pulse width and impedance. Pulse width represented the duration of each shock given in each episode for defibrillation. The impedance represented the resistance on the heart for each defibrillation shock. Normally, the unaltered pulse width and impedance are used to confirm that the change in the DFT is not due to the influence of those two parameters.
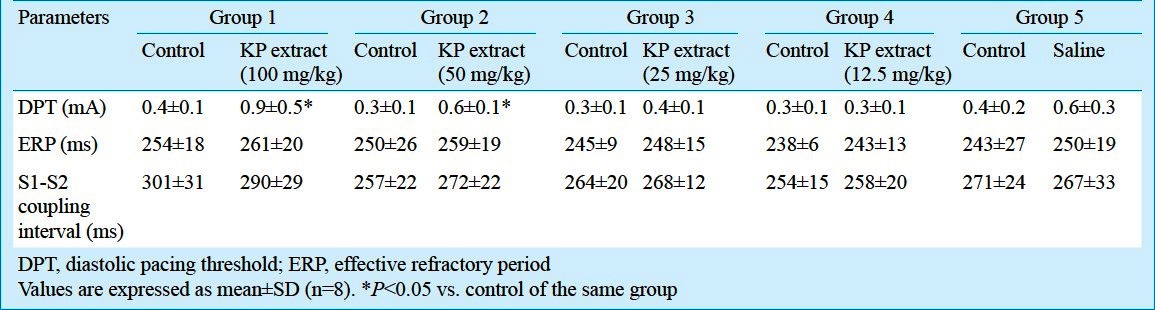
Diastolic pacing threshold (DPT) and effective refractory period (ERP) measurement: The diastolic pacing threshold (DPT) was defined as the lowest current strength required causing the ventricular response (capture). The DPT testing was determined by delivering 10 stimuli of a five msec square pulse (S1) via the pacing electrode at the tip of right ventricular (RV) catheter at 500 msec intervals. The current strength began from 0.1 mA and increased with increments of 0.1 mA until all S1 stimuli could capture the ventricle. After the DPT determination, the effective refractory period (ERP) was determined. A train of 10 S1 stimuli was delivered, and an S2 stimulus was introduced in late diastole (350 msec) after the tenth S1. The basic S1-S2 coupling interval was decreased in 10 msec steps until S2 failed to elicit a capture. The ERP was defined as the longest interval between S1 and extra stimulus (S2), where S2 is the stimulus that fails to elicit a ventricular response18.
Ventricular fibrillation threshold (VFT) and upper limit of vulnerability (ULV) determination protocol: The ventricular fibrillation threshold (VFT) testing was performed by using the step-down protocol. Initially, a train of 10 S1 stimuli (unipolar, 5 msec monophasic pulses) were paced from a pacing electrode at the tip of the RV apex. An S2 shock (biphasic truncated exponential single-capacitor waveform) was delivered at the mid electrical diastole (mid T wave) with initial intensity of 100 V to induce VF. If VF occurred, the strength of S2 shock would be decreased by 10 V for each VF induction episode until VF could no longer be induced. If the first S2 shock was not able to induce VF, the shock strength would be increased in 10 V steps until VF could be induced. The lowest shock strength required for inducing VF was defined as the VFT18. Similar to the DFT, the VFT was expressed as the delivered voltage and the total energy delivered by the defibrillator. Additionally, the pulse width and impedance were also measured in the VFT study, similar to that of the DFT study.
The upper limit of vulnerability (ULV) determination was performed by the use of a three-reversal up/down protocol18. The initial S2 shock strength was chosen at 400 V and delivered at the mid T wave of the last tenth S1 pacing stimuli. The lowest shock strength above VFT that did not induce VF at the mid T wave was defined as the ULV18. Thus, the ULV was the lowest electrical shock strength higher than the VFT that could no longer induce VF when delivered during the mid T wave of a normal sinus rhythm. Similar to the DFT, the ULV was expressed as the delivered voltage and the total energy delivered by the defibrillator. Additionally, the pulse width and impedance were also measured in the ULV study, similar to that of the DFT study.
In the present study, the range of shock strengths between the VFT and the ULV was also determined, and expressed as the window width of VF induction. It refers to the range of the shock strength that could induce VF when delivered during the mid T wave of a normal sinus rhythm. Any condition that expands the window width of VF induction indicated the increased cardiac vulnerability to VF in this study. The window width of VF induction was calculated by subtracting the VFT from the ULV.
Statistical analysis: Values were expressed as mean±SD. Comparisons of electrophysiological and haemodynamic parameters within groups were analyzed using the Wilcoxon Signed Ranks test21.
Results
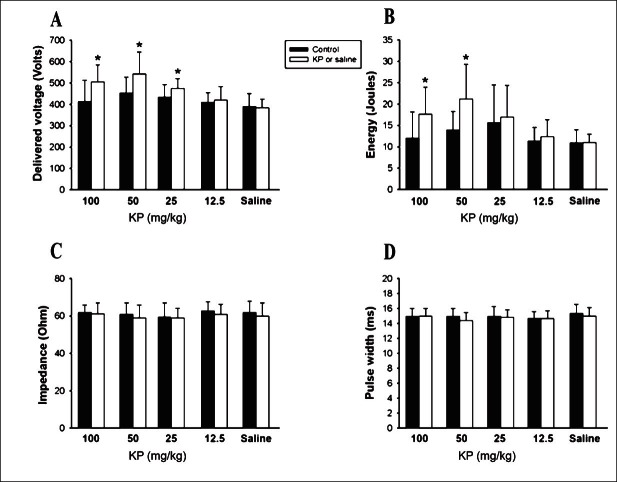
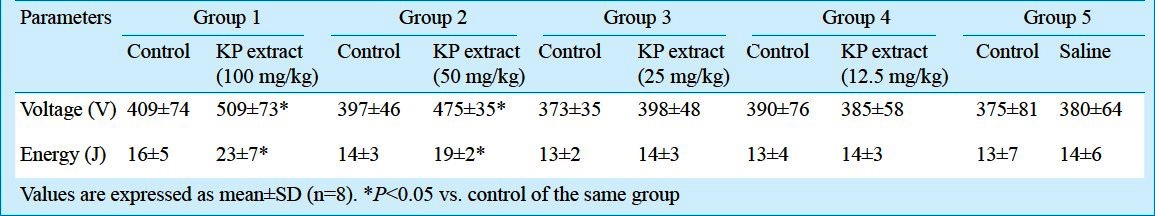
Effect of KP extract on the defibrillation efficacy: In the defibrillation study, the delivered voltage and the total energy for the DFT in groups 1 and 2 (100 and 50 mg/kg KP extracts) were found to be significantly higher than that in their respective controls (Fig. 1A and B). The delivered voltage and total energy in group 1 increased by 22 and 46 per cent, respectively. In group 2, these increased by 20 and 50 per cent, respectively. The delivered voltage alone increased significantly (8%, P<0.5) in group 3 (25 mg/kg KP extract). No change was noted in both these parameters in group 4 (12.5 mg/kg KP extract). The delivered voltage and total energy for the DFT before and after saline administration were not significantly different from each other in group 5. The impedance and pulse width did not change significantly from the control in all groups (Fig. 1C and D).
Fig. 1.
Effects of K. parviflora extract and saline (n=8 in each group) on the delivered voltage (A) and total energy (B) of the defibrillation threshold (DFT). The impedance (C) and pulse width (D) of DFT in all groups are demonstrated. (*P < 0.05 vs. control).
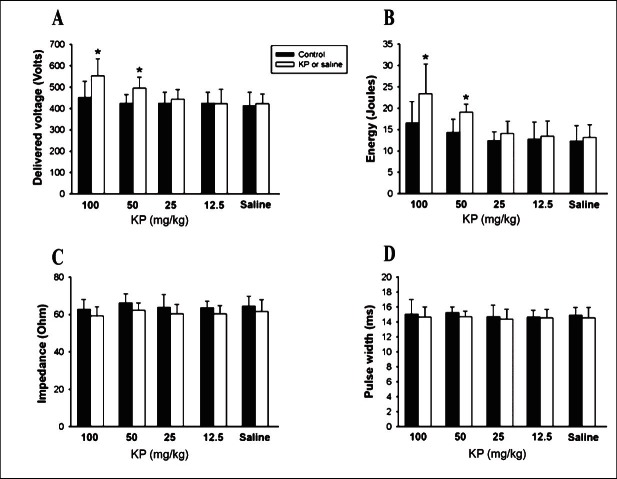
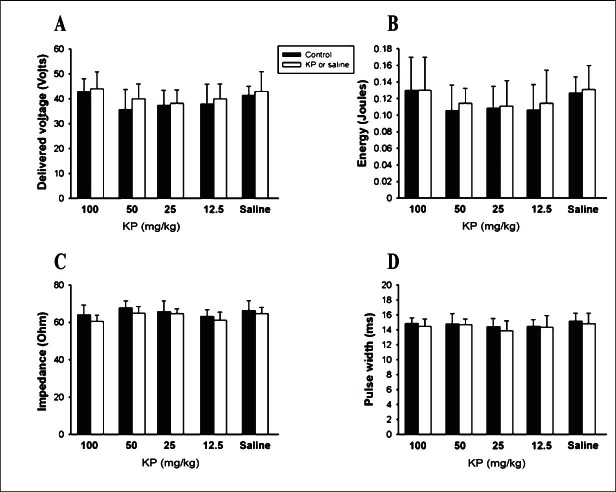
Effect of KP extract on the VF inducibility: In the VF induction study, the delivered voltage and total energy for the ULV in groups 1 and 2 were significantly higher than the control (Fig. 2A and B). In group 1, the delivered voltage and the total energy of the ULV were significantly (P<0.05) increased by 22 and 41 per cent, respectively, while in group 2 by 14 and 30 per cent, respectively. In groups 3 and 4, the delivered voltage and total energy were not changed from the control. The delivered voltage and total energy for the ULV before and after saline administration were not different in group 5. The impedance and pulse width were not significantly changed from the control in any group (Fig. 2C and D). For VFT determination, the delivered voltage, total energy, impedance and pulse width before and after all concentrations of KP extracts or saline administration were not different in any group (Fig. 3). Further, after 100 and 50 mg/kg KP extract administration, the window width of VF induction (calculated by subtracting the VFT from the ULV) was significantly increased compared to the control (Table I). This effect was not observed in 25, 12.5 mg/kg KP extract or saline administration.
Fig. 2.
Effects of K. parviflora extract and saline (n=8 in each group) on the delivered voltage (A) and total energy (B) of the upper limit of vulnerability (ULV). The impedance (C) and pulse width (D) of ULV in all groups are demonstrated. (*P < 0.05 vs. control).
Fig. 3.
Effects of K. parviflora extract and saline (n=8 in each group) on the delivered voltage (A) and total energy (B) of the ventricular fibrillation threshold (VFT). The impedance (C) and pulse width (D) of VFT in all groups are demonstrated. (Not significant between groups).
Table I.
The mean window width of ventricular fibrillation (VF) induction in all groups
Effect of KP extract on the basic cardiac electrophysiology: The basic cardiac electrophysiological parameters (DPT, ERP and S1-S2 coupling interval) in all experimental groups are shown in Table II. In groups 1 and 2, the DPT was significantly increased, compared to the control. It did not change from the control in groups 3, 4 and 5. The ERP and S1-S2 coupling interval after the administrations of each of the different concentrations of the KP extract or saline did not differ significantly from their corresponding controls.
Table II.
Cardiac electrophysiological parameters measured before and after KP extract or saline administration
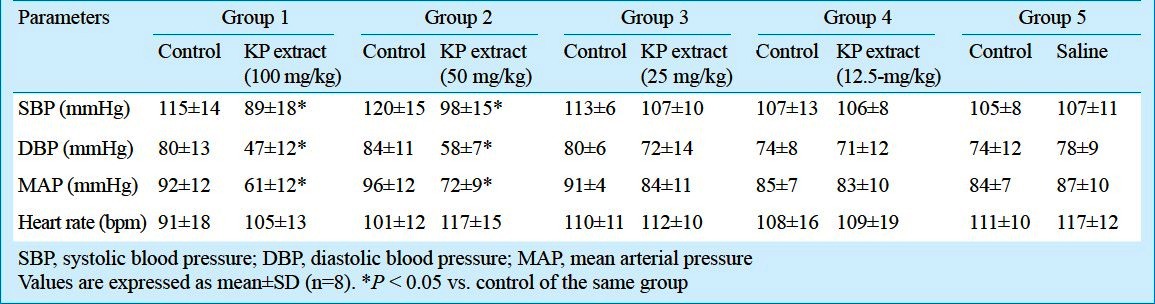
Effect of KP extract on the hemodynamic parameters: Heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) in all experimental groups are shown in Table III. When compared with the controls, the SBP, DBP and MAP were decreased significantly (P<0.05) following the administration of higher concentrations of KP extract (100 and 50 mg/kg) in groups 1 and 2. The heart rate did not change significantly from the control with any of the concentrations of KP tested and by the saline administered.
Table III.
Hemodynamic parameters measured before and after KP extract or saline administration
Discussion
In the present study the intravenous administration of KP extract at high concentrations (100 and 50 mg/kg) increased the DFT, ULV and DPT, but decreased SBP, DBP and MAP significantly. Even at these concentrations, KP did not alter ERP and HR. A small but significant increase was elicited in the delivered voltage of DFT with a KP extract of lower concentration (25 mg/kg).
Although KP extract has been used in traditional medicine for many decades, its effects on cardiac electrophysiology remain mostly unknown. Our results indicated that higher concentrations of KP (50 and 100 mg/kg) could decrease the defibrillation efficacy and increase cardiac vulnerability to fatal ventricular arrhythmia. These findings are consistent with the supratherapeutic effect of sildenafil citrate reported previously16–18. It is proposed that KP extract could have increased cGMP levels in the heart, an effect similar to sildenafil citrate leading to cardiac vulnerability to arrhythmia.
The effects of NO and cGMP have been investigated in the heart to regulate many types of ions via the modulation of voltage-gated ion channels22–26, ligand-gated ion channels27 as well as ion-handling proteins such as ryanodine receptor28, sarco-endoplasmic reticulum calcium ATPase (SERCA)29 and phospholamban29. The intracellular Ca2+ regulation which plays an important role in the genesis and maintenance of cardiac arrhythmia, was found to be altered by NO-cGMP signaling pathway22,23,28,29. Moreover, the effects of NO and cGMP on other cardiac ion channels involved in the formation of cardiac action potential including Na+channel24 and various type of K+ channels25–27 have also been reported. Since NO-cGMP signaling plays a critical role in various ionic regulations of cardiomyocyte, it is proposed that KP extract would have also produced its effects through these mechanisms. The disturbance in the ionic regulation in the heart by effect of KP extract could lead to the increased cardiac vulnerability to ventricular arrhythmia by an increase in the DFT and ULV as shown in the present study. Future studies on the effects of KP extract on cardiac ion channels are required to elucidate this hypothesis.
Our results also demonstrated that both 100 and 50 mg/kg KP extract administrations decreased significantly the SBP by approximately 26 and 22 mmHg, respectively. This finding is consistent with the previous studies that KP has the vasorelaxant effect12,13. Despite this hypotensive effect, both high concentrations of KP extract did not change significantly the HR. This could be due to the depressed baroreceptor reflex of isoflurane used for anaesthetization30.
The present study had certain limitations. Since this study was performed in normal swine hearts, it remains to be seen whether the findings can be extrapolated to diseased hearts of pigs and hearts from other species. In the present study, neither the cGMP level nor PDE-5 activity was determined. Moreover, the metabolism of this herbal plant could vary substantially between species.
In conclusion, our study demonstrated that high concentrations of KP extract decreased the blood pressure and modulated the cardiac electrophysiology, thus causing an alteration of the defibrillation process by decreasing the defibrillation efficacy and increasing the cardiac vulnerability to VF. It is proposed that the observed responses could be mediated through NO-cGMP signaling pathway. However, cellular and molecular studies are required to confirm this proposition.
Acknowledgment
The authors thank Ms. Rodjana Tawan and Ms. Petnoi Petsophonsakul for technical assistance. This study was supported by the CHE-PhD-SW Scholarship, Office of the Higher Education Commission, Ministry of Education, Thailand (P.W. and N.C.), and grants from the National Research Council of Thailand (N.C.), the Faculty of Medicine Endowment Fund, Chiang Mai University (N.C.), and the Thailand Research Fund BRG 5480003 (S.C.), RTA 5580006 (N.C.) and RTA 5280006 (N.C.).
References
- 1.Yenjai C, Prasanphen K, Daodee S, Wongpanich V, Kittakoop P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia. 2004;75:89–92. doi: 10.1016/j.fitote.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Rujjanawate C, Kanjanapothi D, Amornlerdpison D, Pojanagaroon S. Anti-gastric ulcer effect of Kaempferia parviflora. J Ethnopharmacol. 2005;102:120–2. doi: 10.1016/j.jep.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Tewtrakul S, Subhadhirasakul S, Kummee S. Anti-allergic activity of compounds from Kaempferia parviflora. J Ethnopharmacol. 2008;116:191–3. doi: 10.1016/j.jep.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 4.Trisomboon H. Kaempferia parviflora, a Thai herbal plant, neither promote reproductive function nor increase libido via male hormone. Thai J Physiol Sci. 2008-2009;21:83–6. [Google Scholar]
- 5.Jaipetch T, Reutrakul V, Tuntiwachwuttikul P, Santisuk T. Flavonoids in the black rhizomes of Boesenbergia pandurata. Phytochemistry. 1983;22:625–6. [Google Scholar]
- 6.Herunsalee A, Pancharoen O, Tuntiwachwuttikul P. Further studies of flavonoids of the black rhizomes Boesenbergia pandurata. J Sci Soc Thail. 1987;13:119–22. [Google Scholar]
- 7.Sutthanut K, Sripanidkulchai B, Yenjai C, Jay M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J Chromatogr A. 2007;1143:227–33. doi: 10.1016/j.chroma.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Sawasdee P, Sabphon C, Sitthiwongwanit D, Kokpol U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother Res. 2009;23:1792–4. doi: 10.1002/ptr.2858. [DOI] [PubMed] [Google Scholar]
- 9.Temkitthawon P, Hinds TR, Beavo JA, Viyoch J, Suwanborirux K, Pongamornkul W, et al. Kaempferia parviflora, a plant used in traditional medicine to enhance sexual performance contains large amounts of low affinity PDE5 inhibitors. J Ethnopharmacol. 2011;137:1437–41. doi: 10.1016/j.jep.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen RC, Kostis JB. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am J Cardiol. 2003;92:9M–18M. doi: 10.1016/s0002-9149(03)00824-5. [DOI] [PubMed] [Google Scholar]
- 11.Wattanapitayakul SK, Suwatronnakorn M, Chularojmontri L, Herunsalee A, Niumsakul S, Charuchongkolwongse S, et al. Kaempferia parviflora ethanolic extract promoted nitric oxide production in human umbilical vein endothelial cells. J Ethnopharmacol. 2007;110:559–62. doi: 10.1016/j.jep.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 12.Chaturapanich G, Chaiyakul S, Verawatnapakul V, Pholpramool C. Effects of Kaempferia parviflora extracts on reproductive parameters and spermatic blood flow in male rats. Reproduction. 2008;136:515–22. doi: 10.1530/REP-08-0069. [DOI] [PubMed] [Google Scholar]
- 13.Wattanapitayakul SK, Chularojmontri L, Herunsalee A, Charuchongkolwongse S, Chansuvanich N. Vasorelaxation and antispasmodic effects of Kaempferia parviflora ethanolic extract in isolated rat organ studies. Fitoterapia. 2008;79:214–6. doi: 10.1016/j.fitote.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Tep-areenan P, Ingkaninan K, Randall MD. Mechanisms of Kaempferia parviflora extract (KPE)-induced vasorelaxation in the rat aorta. Asian Biomed. 2010;4:103–11. [Google Scholar]
- 15.Swissa M, Ohara T, Lee MH, Kaul S, Shah PK, Hayashi H, et al. Sildenafil-nitric oxide donor combination promotes ventricular tachyarrhythmias in the swine right ventricle. Am J Physiol Heart Circ Physiol. 2002;282:H1787–92. doi: 10.1152/ajpheart.00607.2001. [DOI] [PubMed] [Google Scholar]
- 16.Shinlapawittayatorn K, Sungnoon R, Chattipakorn S, Chattipakorn N. Effects of sildenafil citrate on defibrillation efficacy. J Cardiovasc Electrophysiol. 2006;17:292–5. doi: 10.1111/j.1540-8167.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 17.Shinlapawittayatorn K, Chattipakorn S, Sungnoon R, Chattipakorn N. Effects of combined sildenafil-nitric oxide donor on defibrillation efficacy. J Med Assoc Thai. 2007;90:2143–9. [PubMed] [Google Scholar]
- 18.Kanlop N, Shinlapawittayatorn K, Sungnoon R, Chattipakorn S, Lailerd N, Chattipakorn N. Sildenafil citrate on the inducibility of ventricular fibrillation and upper limit of vulnerability in swine. Med Sci Monit. 2008;14:BR205–9. [PubMed] [Google Scholar]
- 19.Hughes HC. Swine in cardiovascular research. Lab Anim Sci. 1986;36:348–50. [PubMed] [Google Scholar]
- 20.Sudwan P, Saenphet K, Saenphet S, Suwansirikul S. Effect of Kaempferia parviflora Wall. ex. Baker on sexual activity of male rats and its toxicity. Southeast Asian J Trop Med Public Health. 2006;37(Suppl. 3):210–5. [PubMed] [Google Scholar]
- 21.Palee S, Weerateerangkul P, Surinkeaw S, Chattipakorn S, Chattipakorn N. Effect of rosiglitazone on cardiac electrophysiology, infarct size and mitochondrial function in ischaemia and reperfusion of swine and rat heart. Exp Physiol. 2011;96:778–89. doi: 10.1113/expphysiol.2011.057885. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Kim E, Lee SH, Yoo S, Ho WK, Earm YE. cGMP facilitates calcium current via cGMP-dependent protein kinase in isolated rabbit ventricular myocytes. Pflugers Arch. 1998;435:388–93. doi: 10.1007/s004240050528. [DOI] [PubMed] [Google Scholar]
- 23.Gallo MP, Ghigo D, Bosia A, Alloatti G, Costamagna C, Penna C, et al. Modulation of guinea-pig cardiac L-type calcium current by nitric oxide synthase inhibitors. J Physiol. 1998;506(Pt 3):639–51. doi: 10.1111/j.1469-7793.1998.639bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmmed GU, Xu Y, Hong Dong P, Zhang Z, Eiserich J, Chiamvimonvat N. Nitric oxide modulates cardiac Na(+) channel via protein kinase A and protein kinase G. Circ Res. 2001;89:1005–13. doi: 10.1161/hh2301.100801. [DOI] [PubMed] [Google Scholar]
- 25.Bai CX, Namekata I, Kurokawa J, Tanaka H, Shigenobu K, Furukawa T. Role of nitric oxide in Ca2+ sensitivity of the slowly activating delayed rectifier K+ current in cardiac myocytes. Circ Res. 2005;96:64–72. doi: 10.1161/01.RES.0000151846.19788.E0. [DOI] [PubMed] [Google Scholar]
- 26.Gómez R, Núñez L, Vaquero M, Amorós I, Barana A, de Prada T, et al. Nitric oxide inhibits Kv4.3 and human cardiac transient outward potassium current (Ito1) Cardiovasc Res. 2008;80:375–84. doi: 10.1093/cvr/cvn205. [DOI] [PubMed] [Google Scholar]
- 27.Han J, Kim N, Joo H, Kim E, Earm YE. ATP-sensitive K(+) channel activation by nitric oxide and protein kinase G in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H1545–54. doi: 10.1152/ajpheart.01052.2001. [DOI] [PubMed] [Google Scholar]
- 28.Zahradníková A, Minarovic I, Venema RC, Mészáros LG. Inactivation of the cardiac ryanodine receptor calcium release channel by nitric oxide. Cell Calcium. 1997;22:447–54. doi: 10.1016/s0143-4160(97)90072-5. [DOI] [PubMed] [Google Scholar]
- 29.Khan SA, Skaf MW, Harrison RW, Lee K, Minhas KM, Kumar A, et al. Nitric oxide regulation of myocardial contractility and calcium cycling: independent impact of neuronal and endothelial nitric oxide synthases. Circ Res. 2003;92:1322–9. doi: 10.1161/01.RES.0000078171.52542.9E. [DOI] [PubMed] [Google Scholar]
- 30.Seagard JL, Elegbe EO, Hopp FA, Bosnjak ZJ, von Colditz JH, Kalbfleisch JH, et al. Effects of isoflurane on the baroreceptor reflex. Anesthesiology. 1983;59:511–20. doi: 10.1097/00000542-198312000-00005. [DOI] [PubMed] [Google Scholar]