Abstract
Background & objectives:
The osteoporotic risk for women increases soon after menopause. Bone turnover markers are known to be associated with bone loss and fracture risk. This study was aimed to assess bone turnover using bone markers and their correlation with bone mineral density (BMD) in pre- and post-menopausal women.
Methods:
A total of 255 healthy women (160 pre- and 95 post-menopausal) were enrolled. Serum bone alkaline phosphatase (sBAP) and serum N-terminal telopeptide of type I collagen (NTX) were measured to evaluate the bone formation and resorption, respectively. Bone mineral density was determined at lumbar spine (L2-L4) anteroposteriorly, femoral neck and Ward's triangle using Prodigy dual-energy X-ray absorptiometry (DXA) system. The comparison of years since menopause with respect to BMD and bone markers was also evaluated.
Results:
NTX and sBAP showed significant negative correlation with BMD of femur neck and Ward's triangle in postmenopausal women. BMD of all three sides were significant variables for NTX and BMD of femur neck and Ward's triangle for sBAP in postmenopausal women. BMD lumbar spine was a significant variable for sBAP in premenopausal women. The mean values of NTX increased significantly with increase in the duration of years since menopause. The BMD of all three sides decreased significantly with increase in the duration of years since menopause.
Interpretation & conclusions:
Serum NTX and sBAP were inversely correlated to BMD of femur neck and Ward's triangle in post-menopausal women. Simultaneous measurements of NTX and BMD in the north Indian women, suggest that bone resorption in women with low BMD remains high after menopause.
Keywords: Bone mineral density (BMD), bone turnover, postmenopause, premenopause, serum bone alkaline phosphatase (sBAP), serum N, terminal telopeptide of type I collagen (NTX)
The level of bone mass can be assessed by measuring bone mineral density (BMD) using dual X-ray absorptiometry (DXA). This measurement does not capture all risk factors for fracture. Bone fragility depends on the morphology, the architecture and remodelling of bone as well as properties of the bone matrix that cannot be readily assessed. It has been suggested that bone strength may be indirectly reflected by BMD, ultrasonic measurement of bone and by measuring bone turnover markers. The use of biochemical markers of bone turnover as indicators of overall bone metabolism has been a potentially valuable clinical method for screening, diagnosis, and monitoring of osteoporosis1. Decreased bone mass and architectural deterioration of bone tissue are shown to be related to abnormalities of bone turnover. Biochemical markers reflect small changes in bone turnover of the entire skeleton in a shorter time frame compared with absorptiometry method2, and also capture bone properties independent of BMD measurements3. The role of biochemical markers in monitoring the effectiveness of anti-fracture drugs has been reported4. It has been suggested that combined biochemical markers and BMD screening might be a useful predictor of future fractures than BMD alone5.
BMD greatly decreases and biochemical markers of bone turnover markedly increase in postmenopausal women. This is due to accelerated bone remodelling as a consequence of estrogen withdrawal6. As a result, the osteoporotic risk for women increases soon after menopause. The rate of postmenopausal bone loss is not similar in all women. In women with low bone mass, bone turnover markers are independent predictors of fracture risk7. The clinical value in the diagnosis of osteoporosis as well as in defining osteoporosis risk before bone fractures in postmenopausal women is not completely defined8.
Serum bone specific alkaline phosphatase (sBAP) is one of the most specific markers of bone formation and type I collagen cross-linked N-telopeptides (NTX) is among the most specific markers of resorption7,9. Both markers have been reported to be associated with bone loss and fracture risk10. The aim of this study was to assess bone turnover using bone markers and their correlation with bone mineral density (BMD) in pre- and post-menopausal women.
Material & Methods
This cross-sectional study was conducted in the Department of Obstetrics and Gynaecology, Maulana Azad Medical College, and the associated Lok Nayak Hospital, New Delhi, India.
The study protocol was approved by the Institutional Ethics Committee.
Sample size: The sample size was calculated by considering the prevalence of osteoporosis to be 27 per cent11 (varying 21.5 to 32.5%) with alpha 5 per cent. It was found that a minimum of 247 women need to be enrolled in the study. Therefore, a total of 255 women were recruited for the study over a period of two years (April 2006 to March 2008).
Study subjects: The subjects enrolled in this study were healthy normal women who were relatives of patients admitted in the obstetrical and gynaecological wards of the hospital. A total of 255 healthy women (160 premenopausal and 95 postmenopausal women of 20-69 yr of age) were included. This study population was taken from the previous cross-sectional study12. Various demographic characteristics including socio-economic status and serum parameters in relationship to BMD were evaluated. In addition, the daily dietary intake of energy, protein, fat, and calcium and the amount of physical activity were assessed. All these women, who consecutively volunteered themselves and gave prior written informed consent, were evaluated. A detailed medical history and menstrual history including age at menarche, the duration, cyclicity, and flow of menstrual period were obtained. Postmenopausal women were defined as those who had their last menstrual period at least a year ago, in accordance with the clinical definition of the World Health Organization (WHO)13.
Women with history of current or past chronic medical diseases such as hyperparathyroidism, collagen diseases, Cushing's disease, chronic renal, gastrointestinal, or lung disease, or ovarian tumour were excluded. The women were also screened to exclude diseases of the kidney, liver, parathyroid, thyroid, diabetes mellitus, hyperprolectinemia, oophorectomy, rheumatoid arthritis, ankylosing spondylitis, malabsorption syndromes, malignant tumours, haematologic diseases, previous pathological fractures and traumatic fractures within one year. Women were also excluded if they had prior treatment or were receiving medications known to interfere with calcium metabolism such as anticonvulsants, corticosteroids, thiazides, thyroxine, heparin, oral contraceptive pills, calcium, or vitamin D. All subjects were ambulatory, and none was pregnant or lactating or had a history of fracture. None of the postmenopausal women had been treated with hormone replacement therapy containing an estrogen or progesterone or treated for osteoporosis.
Venous blood sample (10 ml) was collected between 0800 and 0900 h after overnight fasting. Serum samples were obtained by centrifugation and stored at -20°C for analysis of biochemical parameters and bone markers.
Biochemical measurement: Levels of serum calcium, phosphorus and albumin were estimated by semi automatic analyzer (Minitecno; I.S.E. S.r.l., Italy) by using commercially available kits manufactured by Sistemi Intelligenti Elettronici (Rome, Italy). The reference range of serum calcium was 8.0-11.0 mg/dl. The intra- and inter-assay coefficients of variation were 1.2 and 4.0 per cent, respectively. The reference range of serum phosphorus was 2.7- 4.5 mg/dl. The intra- and inter-assay coefficients of variation were 1.6 and 2.8 per cent, respectively. The reference range of serum albumin was 3.0-5.0 g/l, with intra- and inter-assay coefficients of variations of 1.7 and 2.6 per cent, respectively.
Markers of bone turnover: Serum bone-specific alkaline phosphatase was measured by enzyme immunoassay using the Metra BAP EIA kit (Quidel, San Diego, CA, USA) as a marker of bone formation. The procedure was performed according to the manufacturer's instructions. sBAP is expressed in U/l, where 1 Unit represents 1 mmol of p-nitrophenyl phosphate (pNPP) hydrolyzed per minute at 25°C in 2-amino-2-methyl-1-propanol buffer. The reference range for BAP was 11.6-29.6 U/l in women aged 25-45 yr and 14.2-42.7 U/l in women more than 45 yr of age. The intra- and inter-assay coefficients of variation were 5.2 and 5.0 per cent, respectively. Serum NTX was quantified by enzyme linked immunosorbent assay (ELISA) using OSTEOMARK NTX Serum (Wampole Laboratories, Princeton, NJ, USA) as a marker of bone resorption, according to the manufacturer's instruction. NTX was expressed in nanomoles bone collagen equivalents per litre (nM BCE). The reference range for NTX was 7.7-19.3 nM BCE. The intra- and inter-assay coefficients of variation were 4.6 and 6.9 per cent, respectively.
Bone mineral density measurement: Bone mineral density was determined at the lumbar spine (L2-L4) anteroposteriorly and at the femoral neck and Ward's triangle by using the Prodigy dual-energy X-ray absorptiometry (DXA) system (software version 8.50) manufactured by GE Medical Systems LUNAR. The calibration of the absorptiometer was checked daily. The results of the measurements were expressed in g/cm2. Subjects with lumbar osteophytes or deformities were excluded. In this DXA system, NHANES/USA population aged 20-40 yr was used for anterioposterior (AP) spine, femur and Ward's triangle reference BMD and 68 per cent of repeat scans fell within 1SD (± 0.010 g/cm2 for AP spine L1-L4, and ± 0.012 g/cm2 for total femur and Ward's triangle). A single technician measured the BMD of all the participants. The in vivo precision was determined after two repeated BMD measurements at different skeletal sites in 30 women using ISCD advance precision calculating tool [International Society for Clinical Densitometry (ISCD); www.iscd.org]. The Root Mean Square SD (RMS SD) for the spine, femur and Ward's triangle regions were 0.011, 0.009 and 0.007 g/cm2, respectively. The percentage coefficient of variation (% CV) for the spine, femur and Ward's triangle regions were 1.37, 1.01 and 0.98 per cent, respectively.
Statistical analysis: Data were statistically analyzed using Statistical Package for Social Sciences (SPSS) software for windows, version 15.0 (SPSS, Illinois, USA). Student's t test and Mann-Whitney test were applied where required to compare between pre- and postmenopausal cases of continuous data. The correlation and regression analysis between NTX and sBAP with other variables was done by converting the values of NTX and sBAP into logarithmic formed. Relationship between continuous data was found by applying Pearson correlation method and the average relationship by applying linear regression analysis for sBAP and NTX with respect to BMD of lumbar spine, femur neck and Ward's triangle. The years since menopause (YSM) of postmenopausal women were divided into three groups (<5, 5-15 and >15). The comparison of years since menopause with respect to BMD and bone markers was done by kruskal-Wallis test and one way (ANOVA), followed by Post-hoc comparison by Bonferroni method. P<0.05 was considered as statistically significant. To investigate the distribution of NTX and sBAP using logarithmic values, the population was stratified into four categories of BMD (lumbar spine, femur neck and Ward's triangle) corresponding to the quartiles of BMD lumbar spine, femur neck and Ward's triangle (Q1=25%, Q2=50% and Q3=75%). Significant differences of NTX and sBAP across the different categories of the quartiles of the BMD lumbar spine, femur neck and Ward's triangle were observed through one way ANOVA test.
Results
The age at menarche for pre-menopausal women (n=160) was 14.9 ± 1.2 yr and 15.2 ± 1.2 yr for postmenopausal women (n=95). The age at menopause was 46.1 ± 4.5 yr for postmenopausal women.
Age related changes and correlation with serum sBAP and NTX: The study population was divided into five decades according to age. The mean levels (±SD) of serum sBAP (U/l) and NTX (nM BCE) were 53.84 ± 33.46 and 24.31 ± 14.14 in the age range of 20-29 yr, 57.96 ± 36.21 and 23.05 ± 13.45 in 30-39 yr, 39.38 ± 33.27 and 12.63 ± 12.61 in 40-49 yr, 31.06 ± 28.53 and 18.12 ± 15.23 in 50-59 yr, and 35.12 ± 39.15 and 27.51 ± 15.37 in 60-69 yr. The mean level of sBAP was highest in the age group of 30-39 yr and that NTX in the age group of 60-69 yr (Fig.). Age had negative and significant correlation with sBAP, marker of bone formation (r=-0.230; P=0.0001) and negative but non-significant correlation with NTX, marker of bone resorption (r=-0.045; P=0.474). BMI showed positive correlation with sBAP (r = 0.127; P= 0.043) and negative correlation with NTX (r = -0.184; P=0.0001).
Fig.
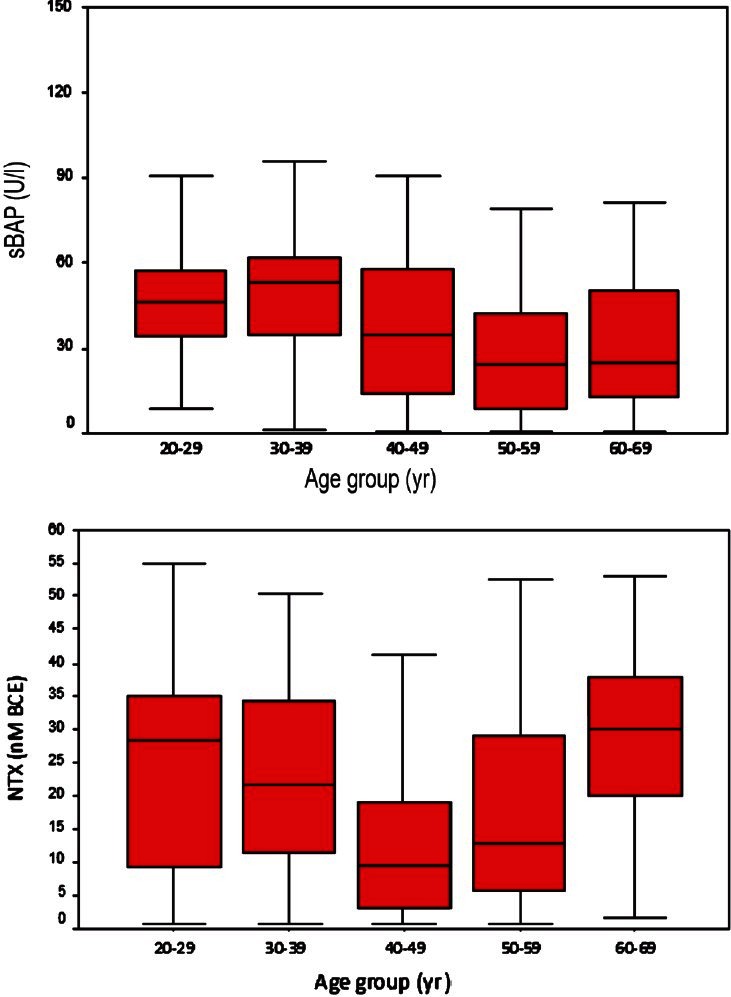
Levels of sBAP and NTX with age group.
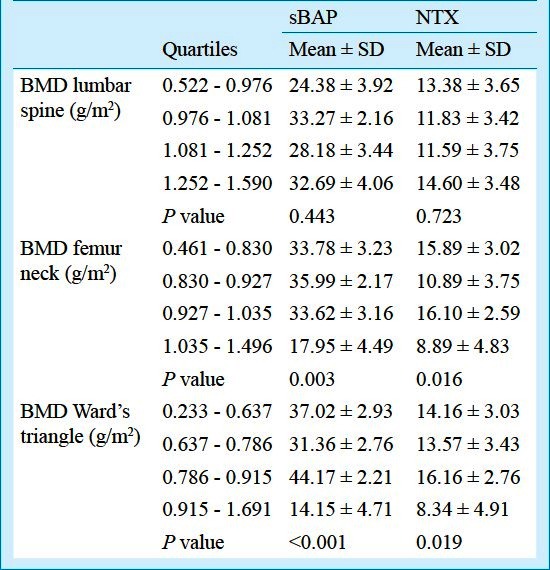
NTX and sBAP according to the quartiles of BMD of lumbar spine, femur neck and Ward's triangle are shown in Table I. The mean and SD of NTX and sBAP in all quartiles varied significantly among categories of BMD of femur neck (P=0.003 and P=0.016, respectively). The mean and SD of NTX and sBAP in all quartiles varied significantly among categories of BMD of Ward's triangle (P<0.001 and P= 0.019) (Table I).
Table I.
N-telopeptide of type 1 collagen (NTX) and serum bone-specific alkaline phosphatase (sBAP) at different sites according to quartiles of BMD lumbar spine, femur neck and Ward's triangle
Relationship between serum levels of sBAP and NTX and BMD: In the study population, sBAP had negative correlation with BMD of femur neck and Ward's triangle (r = -0.141; P =0.024 and r = -0.207; P =0.001, respectively) and positive correlation with BMD of lumbar spine (r = 0.148; P = 0.018). NTX showed negative correlation with BMD of lumbar spine, femur neck and Ward's triangle but was not significant (r = -0.018; P = 0.771, r = -0.095; P = 0.129, r = -0.080; P = 0.205, respectively).
Pre- and postmenopausal groups: In premenopausal women, significant increase in the level of sBAP was observed when compared with postmenopausal women (P<0.001) (Table II). The marker of bone resorption (NTX) showed significant negative correlation with age (r = -0.198; P = 0.012) and positive correlation with serum albumin (r=0.163; P=0.039). sBAP had negative correlation with BMD of Ward's triangle (r = -0.232; P= 0.003) (Table III). On linear regression analysis, the variations in bone markers (sBAP and NTX level) in relation to the BMD (lumbar spine, femur neck, Ward's triangle) are shown in Table IV. Beta values denote change in bone markers in relation to the per unit change in BMD. BMD Ward's triangle showed negative linear relationship with sBAP and non linear relationship with NTX. BMD lumbar spine and femur neck had non linear relationship with sBAP and NTX (Table IV).
Table II.
Characteristics of the pre- and post menopausal women
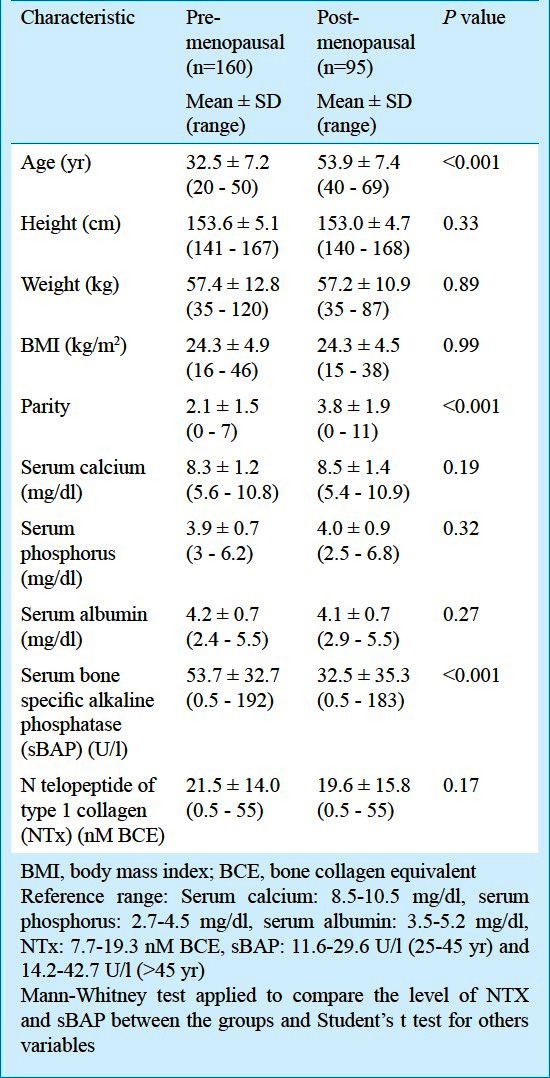
Table III.
Correlation of bone markers with characteristics in premenopausal and postmenopausal women
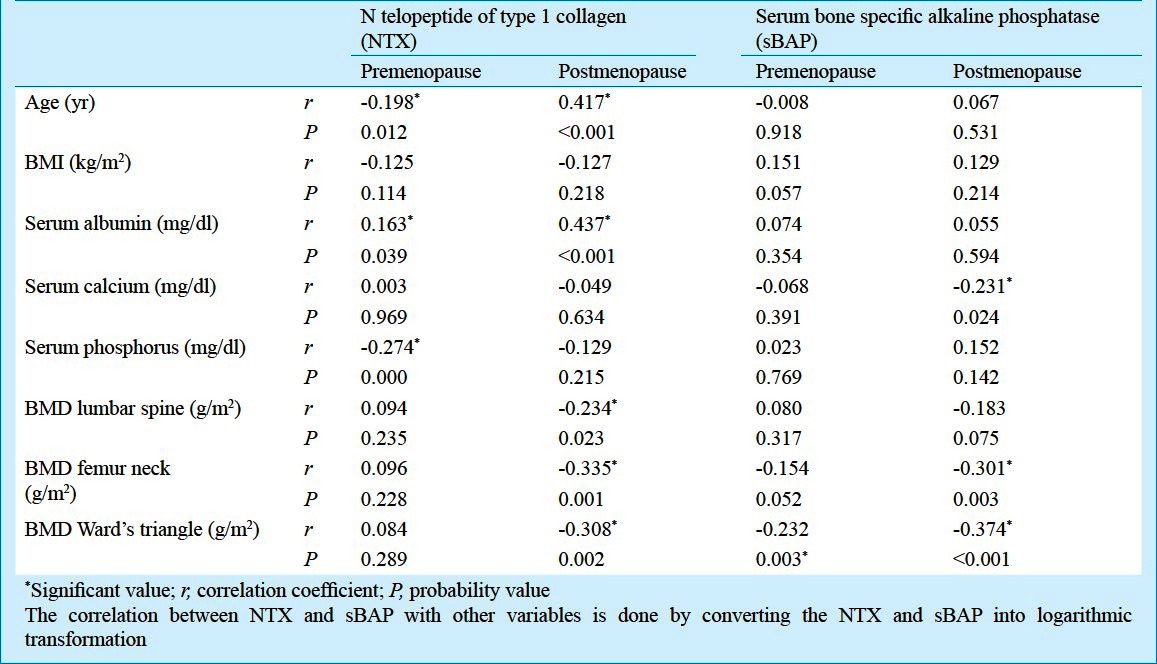
Table IV.
Relationship of bone markers and BMD: Results of linear regression analysis
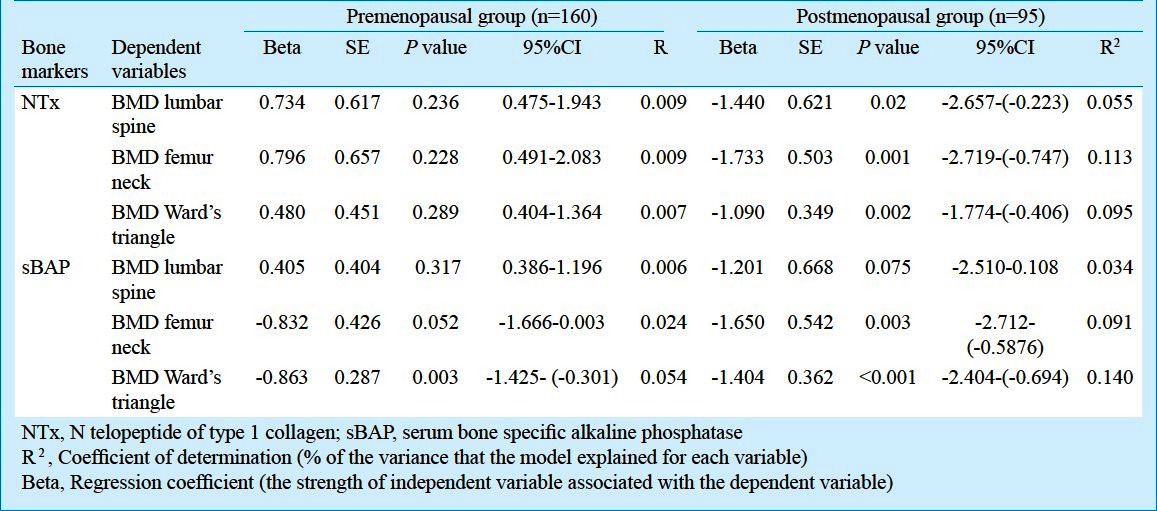
In postmenopausal women, the marker of bone resorption (NTX) showed positive correlation with age (r=0.417; P<0.001). NTX also showed significant positive correlation with serum albumin (r=0.437; P<0.001) and negative correlation with serum calcium (r=-0.049; P=0.634) and phosphorus (r=-0.274; P<0.001). Both the bone markers i.e. sBAP and NTX showed negative correlation with BMD of lumbar spine, femur neck and Ward's triangle. NTX had negative significant correlation with BMD of lumbar spine, femur neck and Ward's triangle (r = -0.234; P = 0.023, r = -0.335; P = 0.001 and r = -0.308; P= 0.002, respectively) and sBAP had negative significant correlation with BMD of femur neck and Ward's triangle (r = -0.301; P= 0.003 and r = -0.374; P =<0.001, respectively) (Table III). On linear regression analysis, BMD femur neck and Ward's triangle has negative linear relationship with sBAP and NTX. BMD lumbar spine has no linear relationship with sBAP and negative linear relationship with NTX (Table IV).
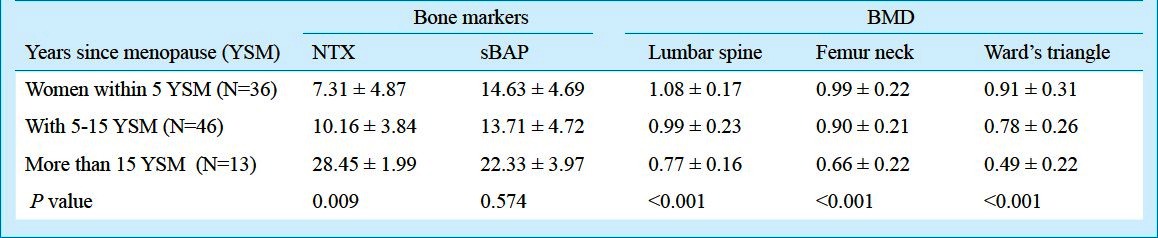
When the postmenopausal women were divided into three subgroups according to the years since menopause (YSM), the variation was observed in both bone markers and BMD. The NTX showed a significant different values with different YSM (P=0.009). A significant increase in NTX levels was observed in women with more than 15 YSM compared to women within 5 YSM and women with 5 to 15 YSM (P=0.007 and 0.047, respectively), whereas, sBAP did not show a significant difference with YSM (P=0.574).
The level of sBAP shows no significant difference in women with >5 YSM compared to women within 5 YSM and women with 5 to 15 YSM (P=1.000 and P=0.894, respectively). DMD of lumbar spine, femur neck and Ward's triangle showed significant different values with YSM (P<0.001). BMD of lumbar spine was significantly decreased in women with more than 15 YSM compared to women within 5 YSM and women with 5 to 15 YSM (P= 0.001 and P = 0.002, respectively). BMD of femur neck was significantly decreased in women with more than 15 YSM compared to women within 5 to 15 YSM (P= 0.000 and P= 0.002, respectively). BMD of Ward's triangle was significantly decreased in women with more than 15 YSM compared to women within 5 YSM and women with 5 and 5 to 15 YSM (P = 0.000 and P = 0.005, respectively) (Table V).
Table V.
Mean values of bone markers and bone mineral density according to years since menopause
On linear regression analysis, serum albumin showed positive linear relationship with NTX, in women within 5, 5 to 15 and >15 YSM (β=3.216; P=0.022; β=2.467; P=0.031 and β=4.187; P=0.002, respectively). Serum phosphorus had negative linear relationship with more than 15 YSM (β = -2.741; P=0.024). BMD of femur neck showed negative linear relationship with sBAP in women within 5 and 5-15 YSM (β= -2.373; P=0.029 and β= -2.881; P=0.002, respectively). Total protein and BMI had positive linear relationship with sBAP in women within 5 YSM (β=1.36; P =0.03 and β =2.35; P=0.03, respectively). BMD of Ward's triangle had negative linear relationship with sBAP in women with <5 and 5 to 15 YSM (β= -2.001; P=0.006 and β= -1.978; P=0.001, respectively).
Discussion
The study population was not different from the general population of Indian women in terms of nutritional status and dietary habits12. The mean age at menarche of the study population was similar to that reported in others studies14.
The clinical usefulness of bone turnover biochemical markers in the contemporary management of postmenopausal osteoporosis remains a controversial issue. It has been suggested that several of these markers can be used to target women at increased risk for osteoporosis and for the development of future fractures15,16.
Several prospective studies have shown that an increased bone resorption evaluated by specific biochemical markers was associated with increased risk of the hip, spine and non-vertebral fractures independently of BMD16–18. The use of bone markers in individual may be appropriate in some situations, especially in women who are not detected at risk by BMD measurements. Thus, bone markers may be used in the assessment of fracture risk in selected cases in which BMD and clinical risk factors are not enough to take a treatment decision19.
In the present study, the levels of sBAP reached at maximum in the age range of 30-39 yr and minimum at the age range 50-59 yr. The level of NTX reached maximum in the age range of 60-69 yr and minimum at the age range 40-49 yr. A cross sectional study reported increase in bone turnover (sBAP and sCTX) with increase in age showing minimal level in the 30-39 yr and maximum in 40-59 yr groups20. In another study, the levels of bone resorption markers, C-terminal crosslinking telopeptide of type-I collagen (CTX-I) and deoxypyridinoline (DPD) increased significantly across the age showing a negative correlation with BMD21. NTX showed significant negative correlation with age in premenopausal women and significant negative correlation in postmenopausal women. No significant correlation was observed with age and sBAP.
In premenopausal women, sBAP was found to be positively correlated with BMD of the lumbar spine as also shown in a recent study on young women22. NTX and sBAP levels in the present study were negatively correlated with BMD in both femur neck and Ward's triangle, as measured by DXA scan in postmenopausal women. These findings are in accordance with other studies showing an inverse correlation between sBAP and BMD15,23 and suggest that the assays of markers could be of clinical utility to better define bone status and future risk for osteoporosis-related fractures. The biochemical markers of bone formation and resorption were reported to be significantly correlated with change in bone mineral density in a group of postmenopausal women24. It has also been observed that NTX was the only marker to correlate significantly with BMD changes at the femoral neck, but not at the spine25. Lofman et al observed an inverse correlation between bone markers serum levels (osteocalcin and sBAP) and BMD (hip and lumbar spine) in a cross-sectional and longitudinal study conducted in postmenopausal women26. Probably NTX and sBAP measurements in menopausal women are useful clinically for long-term prediction of future bone loss.
The NTX showed negative correlation with BMI in both pre- and post-menopausal women and no correlation was observed with sBAP and BMI. This is in contrast with the study that concluded that BMI was inversely associated with levels of bone formation marker, serum carboxyl terminal pro-peptide of type-I pro-collagen (PICP)27. In the present study, the mean value of NTX increased significantly with increase in the duration of YSM. The mean value of BMD of lumbar spine, femur neck and Ward's triangle decreased significantly with increase in the duration of YSM. There is an exponential relationship between rate of bone loss and duration of YSM, indicating higher rate of bone loss with increase in the duration of menopause.
The study observed a positive correlation of NTX with serum albumin in both pre- and postmenopausal women showing that serum albumin may have a role in bone resorption. It has been shown that in healthy postmenopausal women the serum albumin level does not play a significant role in the pathogenesis of bone density reduction, which is mainly due to the number of years since menopause and advancing age. The hypoalbuminemia may be related to the reduction of bone mass only in the subjects affected by diseases associated with a significant albumin reduction28. Indians from low income groups subsist on diets that have inadequate calcium coupled with too few calories, proteins and micronutrients. Moreover, absorption of calcium could be hampered by vitamin D deficiency. There are reports of high prevalence of suboptimal dietary calcium intake and 25(OH) D insufficiencies in Indian populations29. Study observed a significant relation between serum 25(OH) D concentrations and hip MBD but not with lumbar spine or forearm MBD in an urban Delhi cohort30.
Osteomalacia occurs due to lack of vitamin D in the body or inability to absorb it, leading to impaired mineralization of the bones. Several social and environmental exposures including deficiencies of calcium and vitamin D are responsible for impaired mineralization of bone, reflected clinically as osteomalacia and osteoporosis. The population belonging to low-income groups subsist on diets with inadequate calcium coupled with low calories, proteins and micronutrients. In addition, socio-economic status, per capita income and education are also the demographic factors associated with poor bone mineralization. Nutrition is closely link with education and socio-economic status15.
The limitations of this study were its sample size and the subjects who volunteered themselves, were recruited among the visitors of hospital which might have generated a selection bias. A larger population based study may provide future directions regarding the present observations and to define the role of these bone markers and BMD in the long term prediction of bone loss.
In conclusion, this study showed that bone resorption significantly affected BMD of lumbar spine, femur neck and Ward's triangle with increase in the duration of menopause. Simultaneous measurements of NTX and BMD in the north Indian pre- and postmenopausal women, suggest that bone resorption in women with low BMD remains high many years after menopause. NTX measurement can be useful in providing information about the changes in bone density and subsequent bone loss in healthy postmenopausal north Indian women.
References
- 1.Nishizawa Y, Nakamura T, Ohata H, Kushida K, Gorai I, Shiraki M, et al. Committee on the guidelines for the use of biochemical markers of bone turnover in osteoporosis: Japan Osteoporosis Society. Guidelines on the use of biochemical-markers of bone turnover in osteoporosis. J Bone Miner Metab. 2001;19:338–44. doi: 10.1007/s007740170002. [DOI] [PubMed] [Google Scholar]
- 2.Demers LM. Clinical usefulness of markers of bone degradation and formation. Scand J Clin Invest. 1997;57(Suppl 227):12–20. [PubMed] [Google Scholar]
- 3.Vergnaud P, Lunt M, Scheidt-Nave C, Poor G, Gennari C, Hoszowski K, et al. Is the predictive power of previous fractures for new spine and non-spine fractures associated with biochemical evidence of altered bone remodeling? The EPOS study. European Prospective Osteoporosis Study. Clin Chim Acta. 2002;322:121–32. doi: 10.1016/s0009-8981(02)00164-x. [DOI] [PubMed] [Google Scholar]
- 4.Cremers S, Garnero P. Biochemical markers of bone turnover in the clinical development of drugs for osteoporosis and metastatic bone disease. Potential uses and pitfalls. Drugs. 2006;66:2031–58. doi: 10.2165/00003495-200666160-00001. [DOI] [PubMed] [Google Scholar]
- 5.Melton LJ, III, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Min Res. 1997;12:1083–91. doi: 10.1359/jbmr.1997.12.7.1083. [DOI] [PubMed] [Google Scholar]
- 6.Nohara T, Kamei T, Ohta A. Accelerated decrease in bone mineral density in women aged 52-57 years. Tohoku J Exp Med. 2006;210:341–7. doi: 10.1620/tjem.210.341. [DOI] [PubMed] [Google Scholar]
- 7.Seibel MJ, Lang M, Geilenkeuser WJ. Interlaboratory variation of biochemical markers of bone turnover. Clin Chem. 2001;47:1443–50. [PubMed] [Google Scholar]
- 8.Kamel H. Postmenopausal osteoporosis: Etiology, current diagnostic strategies, and non-prescription interventions. J Manag Care Pharm. 2006;12(6 Suppl A):4–6. doi: 10.18553/jmcp.2006.12.S6-A.S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller PD, Baran DT, Bilezikian JP, Greenspan SL, Lindsay R, Riggs BL, et al. Practical clinical application of biochemical markers of bone turnover: Consensus of an expert panel. J Clin Densitom. 1999;2:323–42. doi: 10.1385/jcd:2:3:323. [DOI] [PubMed] [Google Scholar]
- 10.Ross PD. Predicting bone loss and fracture risk with biochemical markers. A review. J Clin Densitom. 1999;2:285–94. doi: 10.1385/jcd:2:3:285. [DOI] [PubMed] [Google Scholar]
- 11.Shatrugna V, Kulkarni B, Kumar PA, Rani KU, Balakrishna N. Bone status of Indian women from a low-income group and its relationship to the nutritional status. Osteoporos Int. 2005;16:1827–35. doi: 10.1007/s00198-005-1933-1. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Mittal S, Orito S, Ishitani K, Ohta H. Impact of dietary intake, education, and physical activity on bone mineral density among North Indian women. J Bone Miner Metab. 2010;28:192–201. doi: 10.1007/s00774-009-0118-y. [DOI] [PubMed] [Google Scholar]
- 13.WHO Tech Rep Ser 670. Geneva: World Health Organization; 1981. World Health Organization. Research on the menopause, report of a WHO Scientific Group. [PubMed] [Google Scholar]
- 14.Sharma Krishan. Age at menarche in Northwest Indian females and a review of Indian data. Ann Hum Biol. 1990;17:159–62. doi: 10.1080/03014469000000912. [DOI] [PubMed] [Google Scholar]
- 15.Ross P, Knowlton W. Rapid bone loss is associated with increased levels of biochemical markers. J Bone Miner Res. 1998;13:297–302. doi: 10.1359/jbmr.1998.13.2.297. [DOI] [PubMed] [Google Scholar]
- 16.Ravn P, Rix M, Andreassen H, Clemmensen B, Bidstrup M, Gunnes M. High bone turnover is associated with low bone mass and spinal fracture in postmenopausal women. Calcif Tissue Int. 1997;60:255–60. doi: 10.1007/s002239900225. [DOI] [PubMed] [Google Scholar]
- 17.Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS prospective study. J Bone Miner Res. 1996;11:1531–8. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]
- 18.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: The OFELY study. J Bone Miner Res. 2000;15:1526–36. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 19.Garnero P, Delmas PD. Contribution of bone mineral density and bone turnover markers to the estimation of risk of osteoporotic fracture in postmenopausal women. J Musculoskelet Neuronal Interact. 2004;4:50–63. [PubMed] [Google Scholar]
- 20.Shan PF, Wu XP, Zhang H, Luo XH, Cao XZ, Xie H, et al. Age-related changes of serum bone alkaline phosphatase and cross-linked C-telopeptides of type I collagen and the relationship with bone mineral density in Chinese women. Clin Chim Acta. 2006;366:233–8. doi: 10.1016/j.cca.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Desai MP, Khatkhatay MI, Bhanu PKV, Savardekar LS, Shah RS, Ansari Z. Hormonal profiles and biochemical indices of bone turnover in Indian women. Osteoporos Int. 2007;18:923–9. doi: 10.1007/s00198-006-0318-4. [DOI] [PubMed] [Google Scholar]
- 22.Ikeuchi K, Umesaki N. Factors affecting bone mineral density of young women and predictive factors of low bone mineral density. Clin Exp Obstet Gynecol. 2009;36:87–90. [PubMed] [Google Scholar]
- 23.Bruyere O, Collette J, Delmas P, Rouillon A, Roux C, Seidel L, et al. Interest of biochemical markers of bone turnover for long-term prediction of new vertebral fracture in postmenopausal osteoporotic women. Maturitas. 2003;44:259–65. doi: 10.1016/s0378-5122(03)00042-2. [DOI] [PubMed] [Google Scholar]
- 24.Rogers A, Hannon RA, Eastell R. Biochemical markers as predictors of rates of bone loss after menopause. J Bone Miner Res. 2000;15:1398–404. doi: 10.1359/jbmr.2000.15.7.1398. [DOI] [PubMed] [Google Scholar]
- 25.Donescu OS, Battié MC, Videman T, Risteli J, Eyre D. The predictive role of bone turnover markers for BMD in middle-aged men. Aging Male. 2006;9:97–102. doi: 10.1080/13685530600708631. [DOI] [PubMed] [Google Scholar]
- 26.Lofman O, Magnusson P, Toss G, Larsson L. Common biochemical markers of bone turnover predict future bone loss, A 5-year follow-up study. Clin Chim Acta. 2005;356:75–6. doi: 10.1016/j.cccn.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Papakitsou EF, Margioris AN, Dretakis KE, Trovas G, Zoras U, Lyritis G, et al. Body mass index (BMI) and parameters of bone formation and resorption in postmenopausal women. Maturitas. 2004;47:185–93. doi: 10.1016/S0378-5122(03)00282-2. [DOI] [PubMed] [Google Scholar]
- 28.D’Erasmo E, Pisani D, Ragno A, Raejntroph N, Letizia C, Acca M. Relationship between serum albumin and bone mineral density in postmenopausal women and in patients with hypoalbuminemia. Horm Metab Res. 1999;31:385–8. doi: 10.1055/s-2007-978760. [DOI] [PubMed] [Google Scholar]
- 29.Harinarayan CV, Ramalakshmi T, Venkataprasad U. High prevalence of low dietary calcium and low vitamin D status in healthy south Indians. Asia Pac J Clin Nutr. 2004;13:359–64. [PubMed] [Google Scholar]
- 30.Vupputuri MR, Goswami R, Gupta N, Ray D, Tandon N, Kumar N. Prevalence and functional significance of 25-hydroxyvitamin D deficiency and vitamin D receptor gene polymorphisms in Asian Indians. Am J Clin Nutr. 2006;83:1411–9. doi: 10.1093/ajcn/83.6.1411. [DOI] [PubMed] [Google Scholar]


