Abstract
Background & objectives:
With improvement in the early diagnosis of breast cancer, breast conserving therapy (BCT) is being increasingly used. Precise preoperative evaluation of the incision margin is, therefore, very important. Utilizing three dimentional (3D) images in a preoperative evaluation for breast conserving surgery has considerable significance, but the currently 3D CT scan reconstruction commonly used has problems in accurately displaying breast cancer. Thin slice 3D reconstruction is also widely used now to delineate organs and tissues of breast cancers. This study was aimed to compare 3D CT with thin slice 3D reconstruction in breast cancer patients to find a better technique for accurate evaluation of breast cancer.
Methods:
A total of 16-slice spiral CT scans and 3D reconstructions were performed on 15 breast cancer patients. All patients had been treated with modified radical mastectomy; 2D and 3D images of breast and tumours were obtained. The specimens were fixed and sliced at 2 mm thickness to obtain serial thin slice images, and reconstructed using 3D DOCTOR software to gain 3D images.
Results:
Compared with 2D CT images, thin slice images showed more clearly the morphological characteristics of tumour, breast tissues and the margins of different tissues in each slice. After 3D reconstruction, the tumour shapes obtained by the two reconstruction methods were basically the same, but the thin slice 3D reconstruction showed the tumour margins more clearly.
Interpretation & conclusions:
Compared with 3D CT reconstruction, thin slice 3D reconstruction of breast tumour gave clearer images, which could provide guidance for the observation and application of CT 3D reconstructed images and contribute to the accurate evaluation of tumours using CT imaging technology.
Keywords: 3D reconstruction, breast cancer, computed tomography, thin slices anatomy
Advances in imaging has contributed greatly to the early diagnosis of breast cancer. Diversification of breast cancer therapy has led to improvement in breast conserving surgery, which is now in common practice1–4. However, the incision margin is one of the key problems in conservative therapy; if no clear incision margin can be delineated, this will directly affect the success of the operation5,6. Therefore, accurate preoperative evaluation of breast tumours is extremely important. In recent years, three dimentional (3D) images, especially 3D CT or MRI reconstructions, have played an important role in preoperative evaluation7,8, but highly precise imaging is required for accurate preoperative evaluation. Currently thin slice and 3D reconstructions are widely used9,10, for 3D reconstruction to show more clearly organs and tissues. Likewise, 3D reconstruction of the breast can clearly reveal the morphological features of tumours within the breast11. In this study, we, therefore, compared 3D CT with thin slice 3D reconstruction in breast cancer patients to find a more precise imaging technique.
Material & Methods
Clinical data: Breast cancer specimens were obtained from 15 patients aged 30-65 yr (mean 46.13±9.49 yr) treated in Southwest Hospital, Third Military Medical University, Chongqing, China between July 2004 and September 2006. They underwent preoperative core biopsy and pathological diagnosis. Thirteen of them had infiltrating ductal carcinoma and two had infiltrating lobular carcinoma. The study protocol was approved by the Ethics Committee of the University and all patients signed an informed consent form.
Multi-slice spiral CT scan: Before surgery, 16-slice spiral CT scanning (Somatom Sensation 16, Siemens, Erlangen, Germany) was carried out. Patients were placed supine for CT axial scanning of the breast, and the data were recorded in CT.DICOM format in the computer. The scanning conditions were a voltage of 120 kV and an electric current of 100 mA; scanning parameters included FOV 330, matrix 512×512, W 350, L 40, slice thickness (ST) 1 mm, pitch 1, and reconstruction slice thickness of 2 mm. An Envision CT high-pressure injector was used to enhance CT examination, with a non-ion contrast agent (90 ml, Iohexol Injection, Omnipaque Amersham Health, Cork, Ireland) being injected via the ulnar vein at 2.6 ml/sec. The delay time for enhancement scanning was 40 sec.
3D reconstruction using SYNGO software (Siemens, Germany): Volume data were transferred to SYNGO workstation for 2D and 3D reconstructions, including multiplanar reconstruction and volume reappearance to obtain coronal, sagittal and any other plane images in addition to 3D images. The shape and location of tumours in the breast and their relation to the adjacent tissues were examined. Tumour size was measured, with false colour visualization of the tumour. The multiplanar image reconstruction included mainly sagittal and coronal reconstructions of the lesions.
Thin slice and 3D image reconstructions of breast tissue specimen in vitro: All the patients underwent modified radical mastectomy. The fatty tissue at the margin of the specimens was trimmed, the specimens were fixed on a level board and soaked in 10 per cent formalin for >1 wk before being stored in a -25° C refrigerator for frozen shaping. The specimens were placed in a specific mold, added with a gelatin solution for fixation12,13, and stored in a frozen tank (-20 to -25°C) for 1 wk.
Slicing of specimens at low temperature: Frozen gelatin-embedded specimens including the mold were removed from the frozen tank and bathed in cool water at room temperature. The mold was removed as soon as the ice had begun to melt. The ice-embedded specimens were placed on a slicing table in the low temperature laboratory14,15, the upper and lower parts were fixed with specific clamps, and both sides were fixed with positioning clamps to avoid displacement. The minor axis of the specimen was made parallel with the horizontal plane and X-axis of slicing using a plumb and set square. In a comparative airtight -20 to -25° C thermostatic laboratory, the specimens were sliced using a TK-T6350 digital horizontal mill (Shanghai Machinery Company, China). The mill consisted of a bed, a control table and a voltage-regulated power supply. It was located in the thermostatic laboratory and digitally controlled in the machine's main axis with an FANUCO-MD operating system on an outdoor control console. With the mill bed in operation, specimens were sliced and photographed with a digital camera. The pitch value was revised before every single slicing until the whole specimen had been completely cut.
Data collection: Digital camera (EOS-IDS Canon digital camera, Japan 11 mega pixels/image, with a resolving power of 4064×2704 pixels) was used to collect anatomical information of the sliced specimen images. The image-acquiring computer outside the thermostatic laboratory was connected to the digital camera via a data line. The digital information of the images was controlled to record, store and manipulate the form, along with the photographic parameters of the images. The RAW data format of the images by digital camera was converted into TIFF format, before being converted into the widely-applied BMP format.
Data processing: A computer (basic frequency: 3.0 GHz, memory: 512 MB, hard disc: 160 GB, resolution: 1,024×768 pixels) was used to obtain breast cancer image data, which were reconstructed in 3D. Since the 3D DOCTOR software (Able Software, America) allows a maximum of 450 pictures, slices for digitizing the internal structure of the breast were used at 0.2 mm thickness. The BMP format was converted into JPG format using ACDSEE6.0 (ACD Systems, Canada), and PHOTOSHOP7.0 (Adobe Systems, America) was used to set up the action. JPG images of each breast were obtained by batch cutting in the same range and size, which were then imported into 3D DOCTOR1.0. These were edited to produce an image directory file (.1st) and volume format file (.PRJ), which were then enlarged. The breast margin, skin, fat, mammary glands, tumour and the surrounding vessels of each image and the interface were drawn layer by layer using a dot-to-dot straight line drawing method15.
Results
Characteristics of multi-slide CT scan and multiplanar reconstruction of human breast cancer: Any 2D plane images of scan images of slices of the breast after multiplanar reconstruction could be observed. The characteristics of tumours, glands and fat were clearly displayed. The fat appeared as gray (low) density, the glands as medium density, and the tumour as medium to high density. There were 10 cases of high-density and five cases of isodensity with the gland. Sentus sign, sub-lobe sign or irregular shape were seen, and the shapes and margins of all the tumours were more clearly displayed, with strong contrast after enhanced scanning (Fig. 1).
Fig. 1.
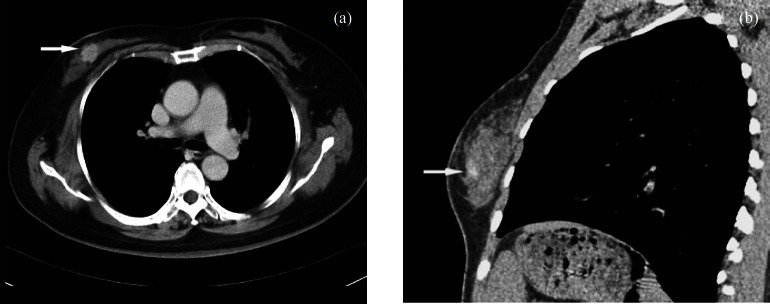
CT Cross section scan image and multiplanar reconstruction of breast cancer: (a) CT Cross section scan image of human breast cancer, the tumour can be clearly seen with enhanced scanning (arrow); (b) Sagittal reconstruction of CT scan images of human breast cancer (arrow).
Thin-slice anatomical features of breast cancer: More anatomic structures were observed on the serial thin sections of in vitro breast cancer specimens. The layers, the mammary papilla, skin, subcutaneous tissue, glands, and the structural features of tumours were continually and more clearly displayed compared with CT scan. On normal structure sections, the skin, subcutaneous tissue, and gland distribution and colour were observed to obtain the basic characteristics of the specimens. On tumour sections, the intra-tumoural structures were seen; the border between the tumour and normal glandular tissue could be clearly distinguished, and the internal colour of tumours was different from that of normal glands. The tumour parenchyma was heterogeneous, some regions being dense and some sparse. The colour of tumour tissue was also different from that of normal glands, being uniform white. CT scan images were in grey scale, and all these features could not be seen on CT images. Moreover, the blood flow distribution of the tumour within and surrounding it could be observed, with abundant blood flow around the tumour (Fig. 2). This was not seen in CT images.
Fig. 2.
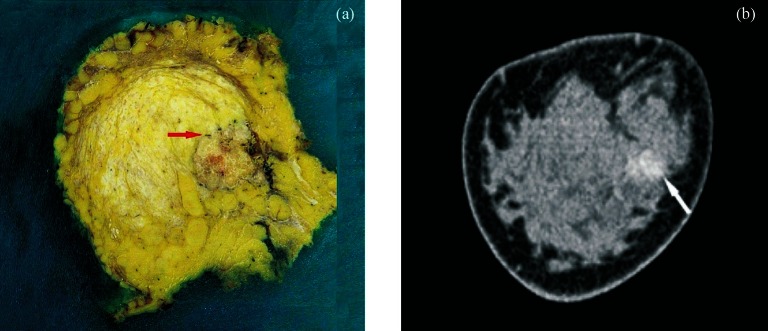
Thin-slice anatomical features and coronal reconstruction of breast cancer: (a) Thin slice anatomical section of breast and breast tumour, with the surrounding blood vessels can be seen (arrow); (b) Coronal reconstruction of CT image of the breast and tumour (arrow).
Comparison of the characteristics of thin slice 3D reconstruction and CT 3D reconstruction: SYNGO volume rendering (VR) by CT and thin slice 3D reconstruction by 3D DOCTOR were used to show the spatial structures of breast tumour for a comparison of their characteristics.
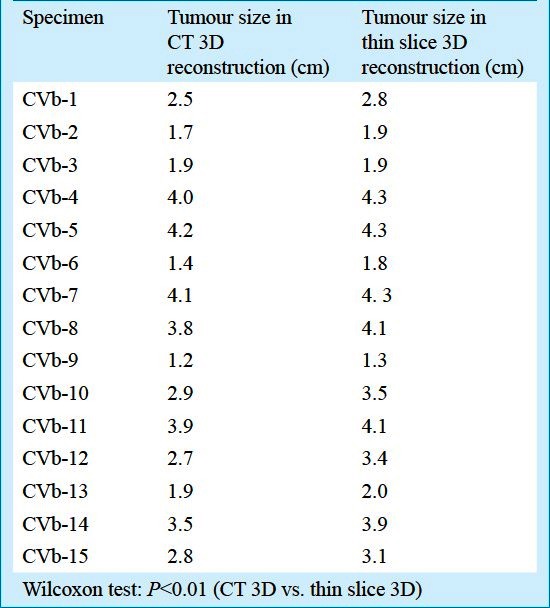
Comparison of tumour long diameter: The RECIST standard16 was used for tumour size comparison. The longest diameter of tumour in CT reconstruction was generally smaller than in thin slice 3D reconstructions, varing by 0-7 mm, which was statistically different (P<0.01; Table).
Table.
Comparison of tumour size between thin slice 3D reconstruction and CT 3D reconstruction
Comparison of tumour shapes: The general shape of the tumour after 3D thin slice multiplanar reconstruction was basically consistent with CT, but the tumour margins were much clearer than by CT. Some regular tumours examined by CT scan were irregular by multiplanar 3D reconstruction with 3D DOCTOR. Irregular tumours, which were roundish by CT, were seen in five cases in this study.
Comparison of tumour infiltration: Both SYNGO volume rendering (VR) 3D reconstruction of CT images and the thin slice 3D reconstruction of specimens in vitro by 3D DOCTOR displayed tumour infiltrating the subcutaneous tissues and the main duct, but more accurately by 3D image reconstruction of the specimen in vitro. CT images revealed tumour infiltration into the subcutaneous tissues in four cases and the major duct below the papillae in one case. SYNGO volume rendering (VR) 3D reconstruction of the specimen in vivo showed “pseudopodia” stretching into subcutaneous tissues in five cases; the same was found in four cases by CT images, and one case was also observed with tumour invasion into the sub-nipple area (Fig. 3).
Fig. 3.
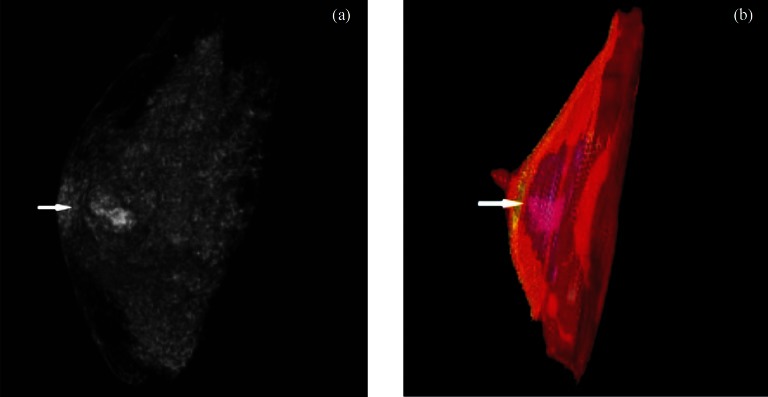
CT image 3D reconstruction and thin slice 3D reconstruction of breast: (a) CT image 3D reconstruction of breast tumour (arrow), (b) Thin slice 3D reconstruction of breast and breast tumour (arrow); a and b can all be observed with tumour invasion into sub-nipple area.
Discussion
Rapid developments in the field of radiology and availability of sophisticated equipment with software are playing a very important role in clinical examination, diagnosis and treatment. Improvement in minimally invasive surgery is based on the correct guidance of imaging. Combination of digital virtual techniques with imaging is the precondition of realizing virtual surgery. In recent years, 3D image reconstruction has been widely used, and has greatly raised the level of surgical therapy17,18. The use of CT in breast cancer is initially to examine distant metastasis. With increasing use of multi-slice spiral CT in clinics, CT scan began to be used in the diagnosis of breast cancer. Several investigators have reported the importance of 3D spiral CT in preoperative evaluation of breast cancer and its significance in assessing the extent of surgery needed in breast conserving therapy, and proposed applying the value of navigation surgery7,19.
Using multi-slice CT scan images, reconstructions in transversal, sagittal and coronal planes (or at any other angle) can be done, and the tumour characteristics can be observed in the 2D plane. Further, 3D reconstruction images can be obtained using volume reconstruction, so that the tumour shape, size and margin status can be observed from every direction. CT scans do not show the morphological characteristics and blood flow of isodense tumours completely, and the tumour margin is poorly displayed. Contrast enhanced CT scan shows more accurately the shape and margin of tumours, and also clearly some isodense tumours. In our study, contrast enhanced CT scan rapidly enhanced tumour margins, which further explains an abundant blood flow around breast carcinoma.
Clinically there are often certain deviations between actual lesion structure, tumour size and radiological reports; and radiological reports obtained using a variety of imaging techniques give different results for the same lesion and tumour size. If 3D reconstruction is used only in qualitative or localization diagnosis, little influence on the assessment of tumour size and margin will be found; but for breast conserving surgery, higher accuracy regading tumour size, its margin and the incision margin is required.
Since slice anatomy and 3D reconstruction of real organs approximates well the anatomic features of human organs and tissues, which is a precise representation of the human structures, it is of great instructional/directional significance in clinical work, especially for surgical operations, and has great reference value in clinical imaging diagnosis when combined with contrast imaging. Although 3D slice reconstruction method cannot be used in the diagnosis of clinical diseases, as a study method it plays an instructional/directional role for diagnosis and treatment of clinical diseases. Slice anatomy can clearly show 2D characteristics of tumours and intra-tumour structures; 3D reconstruction can show the blood flow distribution of tumours, thus raising the accuracy of assessing the structural characteristics of tumours. With 3D slice reconstruction as a real representation of tumour shape and size, one can find out and revise the possible bias from imaging diagnosis when it is combined with the contrast study of imaging, thus providing a more precise diagnosis that is closer to the reality. This offers a basis for the development of image-assisted navigation surgery for breast cancer.
In this study growing features of tumour were clearly observed in all directions and of the tumour margin from the 3D thin slice reconstruction of breast. In five cases, the coronal and sagittal planes of CT scans before operation showed the tumour roundish, and the margins smooth. But 3D thin slice reconstruction revealed irregular spicular-like appearance on the tumour margin. When the preoperative CT data were reconstructed in multiple directions, typical spicular sign of breast cancer was found in the five cases. In multi-slice CT examination of breast cancer where lesions are hard to determine, multi-dimensional reconstruction made it possible to view the shape and features of tumours from different directions.
Three dimentional CT reconstruction of breast tumours can display the tumour characteristics in the breast and define the tumour margin and surroundings. This information helps surgeons to perform more precise excision procedures. But the morphological characters and size of tumours by CT images are different from those in reality, so for CT-guided breast conserving therapy the image should be properly extended in order that a tumour can be completely resected.
In summary, thin slice 3D reconstruction of breast cancer generates clearer images compared with 3D CT reconstruction, and is helpful in accurately evaluating breast cancer.
References
- 1.Siponen ET, Vaalavirta L, Joensuu H, Vironen J, Heikkilä P, Leidenius MH. Ipsilateral breast recurrence after breast conserving surgery in patients with small (≤2 cm) breast cancer treated with modern adjuvant therapies. Eur J Surg Oncol. 2011;37:25–31. doi: 10.1016/j.ejso.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Offersen BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: A systematic review. Radiother Oncol. 2009;90:1–13. doi: 10.1016/j.radonc.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Winzer KJ, Sauerbrei W, Braun M, Liersch T, Dunst J, Guski H, et al. German Breast Cancer Study Group (GBSG) Radiation therapy and tamoxifen after breast-conserving surgery: Updated results of a 2 × 2 randomised clinical trial in patients with low risk of recurrence. Eur J Cancer. 2010;46:95–101. doi: 10.1016/j.ejca.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Kirby RM, Basit A, Manimaran N. Patient choice significantly affects mastectomy rates in the treatment of breast cancer. Int Semin Surg Oncol. 2008;5:20–2. doi: 10.1186/1477-7800-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrow M. Margins in breast-conserving therapy: have we lost sight of the big picture? Expert Rev Anticancer Ther. 2008;8:1193–6. doi: 10.1586/14737140.8.8.1193. [DOI] [PubMed] [Google Scholar]
- 6.Londero V, Zuiani C, Panozzo M, Linda A, Girometti R, Bazzocchi M. Surgical specimen ultrasound: Is it able to predict the status of resection margins after breast-conserving surgery? Breast. 2010;19:532–7. doi: 10.1016/j.breast.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T, Tamaki Y, Hamada S, Yamamoto S, Sato Y, Tamura S, et al. Usefulness of three-dimensional multidetector-row CT images for preoperative evaluation of tumor extension in primary breast cancer patients. Breast Cancer Res Treat. 2005;89:119–25. doi: 10.1007/s10549-004-1477-7. [DOI] [PubMed] [Google Scholar]
- 8.Carter T, Tanner C, Beechey-Newman N, Barratt D, Hawkes D. MR navigated breast surgery: method and initial clinical experience. Med Image Comput Comput Assist Interv. 2008;11:356–63. doi: 10.1007/978-3-540-85990-1_43. [DOI] [PubMed] [Google Scholar]
- 9.Li QY, Zhang SX, Heng PA, Liu ZJ, Lin ZF, Tan LW, et al. Segmentation and three-dimension reconstruction of Chinese digitized human cerebrum. Comput Med Imaging Graph. 2006;30:89–94. doi: 10.1016/j.compmedimag.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Guo YL, Heng PA, Zhang SX, Liu ZJ, Tan LW, Li QY, et al. Thin sectional anatomy, three-dimensional reconstruction and visualization of the heart from the Chinese Visible Human. Surg Radiol Anat. 2005;27:113–8. doi: 10.1007/s00276-004-0282-7. [DOI] [PubMed] [Google Scholar]
- 11.Moyer HR, Carlson GW, Styblo TM, Losken A. Three-dimensional digital evaluation of breast symmetry after breast conservation therapy. J Am Coll Surg. 2008;207:227–32. doi: 10.1016/j.jamcollsurg.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Li K, Tan LW, Zhang SX, Liu ZJ. A study on data acquisition of liver of visible human. J Reg Anat Operat Surg. 2008;17:3–5. [Google Scholar]
- 13.Samani A, Bishop J, Luginbuhl C, Plewes DB. Measuring the elastic modulus of ex vivo small tissue samples. Phys Med Biol. 2003;48:2183–98. doi: 10.1088/0031-9155/48/14/310. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer V, Ackerman MJ, Scherzinger AL, Whitlock D. The visible human male: a technical report. J Am Med Inform Assoc. 1996;3:118–30. doi: 10.1136/jamia.1996.96236280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang SX, Heng PA, Liu ZJ, Tan LW, Qiu MG, Li QY, et al. Creation of the Chinese visible human data set. Anat Rec B New Anat. 2003;275:190–5. doi: 10.1002/ar.b.10035. [DOI] [PubMed] [Google Scholar]
- 16.Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol. 2006;24:3245–51. doi: 10.1200/JCO.2006.06.5599. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes R, DiPasquale J. Computer-aided surgery using 3D rendering of maxillofacial pathology and trauma. Int J Med Robot. 2007;3:203–6. doi: 10.1002/rcs.137. [DOI] [PubMed] [Google Scholar]
- 18.De Greef S, Willems G. Three-dimensional cranio-facial reconstruction in forensic identification: latest progress and new tendencies in the 21st century. J Forensic Sci. 2005;50:12–7. [PubMed] [Google Scholar]
- 19.Uematsu T, Sano M, Homma K, Shiina M, Kobayashi S. Three-dimensional helical CT of the breast: accuracy for measuring extent of breast cancer candidates for breast conserving surgery. Breast Cancer Res Treat. 2001;65:249–57. doi: 10.1023/a:1010641223012. [DOI] [PubMed] [Google Scholar]


