Abstract
Context:
Pharmacological agents are used to reduce postoperative blood loss.
Aims:
To assess the effects of tranexamic acid on prevention of bleeding and requirement of blood transfusion after major hip and femoral surgeries.
Settings and Design:
A prospective, randomized, double blinded study was conducted in the tertiary care teaching hospital.
Methods:
Ninety ASA grade I-II patients undergoing hip fracture surgery were included in this prospective study. Forty-five patients received tranexamic acid (TA) given in a bolus dose of 500 mg 15 min before surgical incision followed by continuous infusion. The remaining, 45 patients were allocated as a control group. Postoperative bleeding (volume of blood in the drain), percentage fall of hemoglobin, transfusions and complications were recorded.
Results:
Mean volume of blood in the drain was 39.33±10.09 ml (mean±SD) as compared to 91.11±17.61 ml in placebo group showing a P<0.001. Mean percentage fall in Hb at day 0 was 2.99±3.45 in the study group as compared to 7.70±6.05 in the placebo group (P<0.001), and fall at day 2 in the study group was 0.35±0.74, compared to 2.72±2.70 in the placebo group (P<0.001). The number of patients required blood transfusions were lower in the study group than in the placebo group (P=0.01).
Conclusions:
We conclude that tranexamic acid significantly reduces postoperative blood loss and transfusion requirements during major hip and femoral surgeries.
Keywords: Blood loss, blood transfusion, hip and femoral surgery, tranexamic acid
INTRODUCTION
Major orthopedic surgeries are commonly associated with marked blood loss, and a subsequent need for blood transfusion is often encountered. The causes of bleeding are multifactorial, increased fibrinolytic activity being one of them.[1,2] Although bleeding from these surgical sites is usually controllable, there may be significant blood loss. Several approaches[3] have been used to reduce intraoperative blood loss, including: Hypotensive anesthesia which has its own detrimental consequences.[4,5] The use of allogeneic blood products increases the rate of transmission of infectious diseases, modulates the immune response, and increases the risk of postoperative infection. The alternate approaches are administration of antifibrinolytic agents such as tranexamic acid (TA) perioperatively to stabilize the multiple micro-clots that form within the surgical wound.[6,7]
TA is a synthetic derivative of the amino acid lysine (4-aminoethyl cyclohexane carboxylic acid),[8] that exerts its antifibrinolytic effect through the reversible blockade of lysine binding sites on plasminogen molecules, thereby reducing the conversion of plasminogen to plasmin. Hence, it blocks the dissolution of hemostatic fibrin, which stabilizes fibrin structure and thus may decrease the blood loss secondary to increase fibrinolysis.[9,10] TA has been used in neuro, orthopedic, cardiac, spine and maxillofacial surgeries and has reduced the amount of blood loss and subsequent need for blood transfusion.[11–16] The aim of this study is to evaluate the efficacy of intraoperative IV TA on postoperative blood loss following surgeries for hip and femoral fractures.
METHODS
Ninety patients (ASA grade I/II patients between 18 and 80 years, weighing 40-100 kg) undergoing surgery for femoral fracture like open reduction internal fixation (ORIF), hemiarthroplasty, total hip replacement (THR) was included. Patients with chronic disease like Rheumatoid arthritis, ischemic heart disease, malignancy, history of any previous thromboembolic episodes, hemoglobin <8 g/dl were excluded from the study. The study was approved by the institutional Ethics Committee and written informed consent was obtained from each patient.
They were allocated to two groups. Randomization was done by opaque sealed envelope technique.
Group T: Patients received inj. TA 10 mg/kg body weight.
Group P: Patients received physiological saline 1 ml/kg body weight.
Preoperatively, the hemoglobin concentration, bleeding time, clotting times were measured on the day before operation. All patients in the study group received a bolus intravenous injection of 500 mg TA through 50 ml syringe (as weight of all studied patients was almost equal) during 10 min about 15 min before incision, followed by a continuous infusion of 1 mg/kg/h dissolved in 1 lit of saline until the completion of surgery. Patients in the placebo group received normal saline a bolus intravenous injection of 50 ml about 15 min before the surgery followed by a continuous infusion of 1 liter of saline until the surgery completed. Drug and normal saline were loaded in 50 ml syringe by an independent anesthetist. The anesthetist administrating the drug was unaware of 50 ml being given. Surgery was performed under combined spinal epidural anesthesia with 0.5% bupivacaine heavy (hyperbaric) in 15 mg dose. During induction, an epidural catheter was also inserted to provide postoperative analgesia.
Hemodynamics (heart rate; systolic, diastolic and mean arterial blood pressure (MABP)) was noted starting from preoperational to shifting at regular intervals of 30 min. Ringer's solution was used as the replacement fluid for the estimated intraoperative blood volume lost in a 3:1 ratio. There was no significant difference in amount of fluid in both the groups. Postoperative hemoglobin concentration (on day 0 and day 2) and volume of blood in the drain (when the patient entered into recovery room on the day of operation at 20:00 h) were measured. The number of units of packed red cells transfused during the hospital stay was recorded, and any thromboembolic and other complications were documented. A criterion for blood transfusion was a reduction in hemoglobin exceeding 25% of preoperative level.
For tests of differences between quantitative data, two-sided t-tests were used. In the text, data are presented as mean±standard deviation (SD) and P<0.05 are considered significant.
RESULTS
There were no significant differences between the patients with respect to age, sex, duration and type of surgery and preoperative mean hemoglobin concentration [Table 1 and Figure 1]. Neither heart rate nor MABP has statistically significant difference or results (P>0.05).
Table 1.
Patient characteristics, laboratory and surgical data
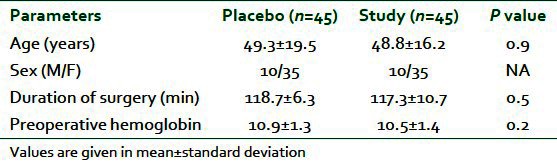
Figure 1.
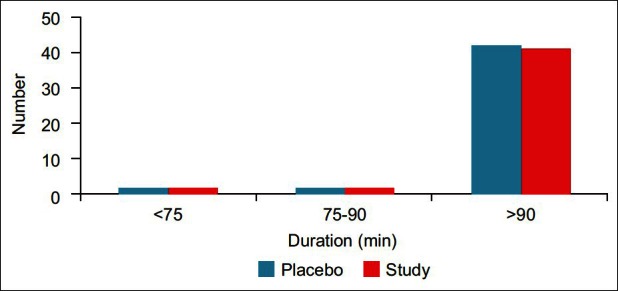
Duration of surgery
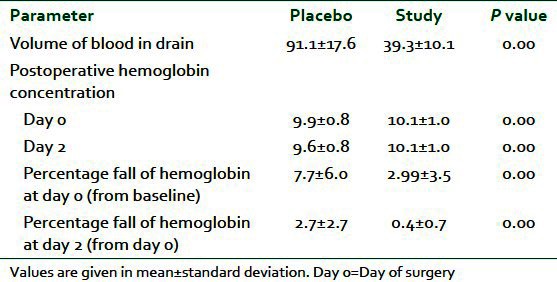
The drains were removed in the evening of the first postoperative day. Mean volume of blood in the drain was 39.33± as compared to 91.11± in placebo group showing a highly significant reduction in postoperative blood loss (P=0.01) [Figure 2]. Mean fall in hemoglobin at day 0 was 2.99±3.457 in the study group as compared to 7.70±6.05 in the placebo that has P value 0.01 making it significant finding [Figure 3]. Similar to this fall in hemoglobin at day 2 in the study group was 0.3578±0.744 and in the placebo was 2.7122±2.70 with P value 0.000 again showing statistically significant [Table 2].
Figure 2.
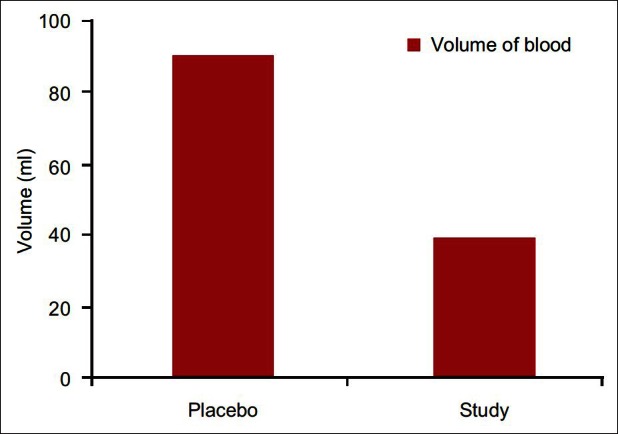
Postoperative volume of blood in drain
Figure 3.
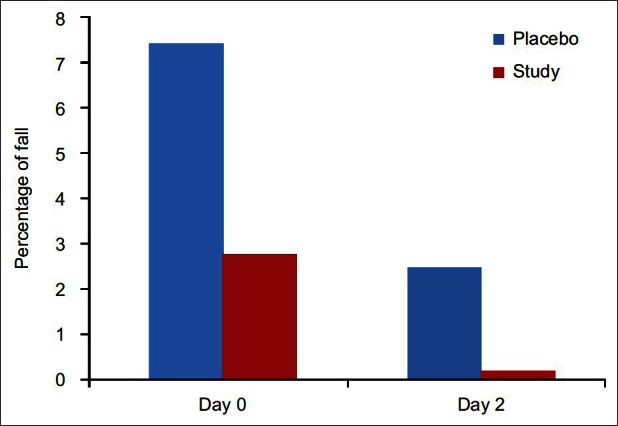
Percentage of fall of mean hemoglobin concentration
Table 2.
Postoperative volume of blood in drain and % fall in hemoglobin
In our study out of 45 patients, 18 patients who fell in the placebo group required blood transfusion and while seven patients out of 45 in the study group need a transfusion (P=0.01). No significant complications like thromboembolic episodes were encountered in both the groups.
DISCUSSION
Several studies have investigated the effect of TA on intraoperative and postoperative blood loss in patients undergoing orthopedic surgeries like total knee replacement (TKR), THR, spinal surgeries and hip fractures but the efficacy of such treatment has not yet been clearly established. In this study, we found that preoperative administration of TA significantly reduces the postoperative blood loss in hip and femoral surgeries, which was indicated by volume of blood in drain and fall in hemoglobin postoperatively. A similar study was conducted by Sadeghi and Mehraein[17] to see the effect of TA on hip fracture surgery. They found perioperative blood loss significantly lower in the TA group (P<0.03). The total blood loss was 960±483 ml in TA group and 1484±724 ml in the control group (P<0.001). Postoperative drainage was lower in TA group (296±85 ml vs. 375±110 ml, P<0.195). There were no differences in coagulation parameters. The rates of transfused patients in TA and control groups were 37 % and 57%.
Other studies like of Benoni et al.,[18] Jansen et al.,[19] Yamasaki et al.,[20] Ekback et al.,[21] and Sano et al.,[22] was conducted on THR or TKR. The volume of blood loss in these procedures may be lesser or greater than during hip fracture surgery and can be attributed to duration as well procedure, but still results were not dissimilar with our study. Benoni et al.[18] administered TA intravenously before tourniquet release and then 3 h later in patients undergoing TKA, and reported that the intra and postoperative blood loss were reduced to one-third as a result. However, in contrast to elective hip or knee surgery; in hip fracture, the fibrinolytic system is activated by trauma and increased during surgery. So we used a single bolus dose of TA 15 min prior to skin incision followed by continuous infusion to ensure that its clot stabilize the effect would cover both the intra as well as the postoperative period.
In agreement with our findings, which were based on a surgical procedure that was of similar duration (120 min) Ekbäck et al. showed that the perioperative blood loss was significantly lower in the TA-treated group than in the control group. Postoperative drainage bleeding was correspondingly less (P=0.001) (520±280 vs. 920±410 ml).[21]
Jansen et al.[19] investigated the effect of TA on blood loss in 42 patients undergoing total knee arthroplasty. Total blood loss measured at 72 h was 678 ml in TA group while 1419 in the control group. Blood loss on discharge from PACU was 58% reduced in TA group. They also found that at first postoperative day, Hb concentration, expressed as a fraction of preoperative value was significantly higher in TA group as compared to control group (83% vs. 73%, respectively; P<0.01) that itself reflect the blood loss.
Yamasaki et al.[20] in their study on cement less THR found a reduction in total blood loss in the TA group (1349±478 ml) than in the control group (1646±469 ml) (P<0.01). Their postoperative laboratory findings showed that the hemoglobin and hematocrit values on the first, seventh, and fourteenth postoperative days were significantly higher in the TA group than in the control group. In addition, the hemoglobin and hematocrit values in the TA group recovered to the first preoperative day level by the fourteenth postoperative day.
This study also demonstrates that transfusion requirements also reduced in the study group. A meta-analysis of nine randomized control trial reveals that use of TA in TKR significantly reduces the proportion of patients requiring blood transfusion.[23] In another study by Camarasa Godoy et al.[24] and Alvarez et al.[25] found similar results. Lozano and colleagues[26] found that only 17.6% patients on TA received blood transfusion in comparison to 54% in the control group. This is said that TA increase hypercoagulability and can lead to chance of thromboembolic episodes, but we did not face any abnormal finding. Benoni et al. also suggested that TA does not affect risk of DVT because it inhibits fibrinolysis in the wound not in circulation.
In conclusion, the present paired study demonstrated that the administration of TA given preoperatively reduces the blood loss in the first 24 h by a highly significant degree in patients undergoing surgeries for hip and femoral fractures as well it causes a significant reduction in postoperative anemia and need for transfusion among these patients. This would in turn, help avoid complications related with transfusion of blood and blood products. However, further investigation is necessary to determine the effectiveness.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356:2301–11. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 2.Sculco TP. Global blood management in orthopaedic surgery. Clin Orthop Relat Res. 1998;357:43–9. doi: 10.1097/00003086-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Keating EM. Preoperative evaluation and methods to reduce blood use in orthopedic surgery. Anesthesiol Clin North America. 2005;23:305–13. doi: 10.1016/j.atc.2005.02.006. vi-vii. [DOI] [PubMed] [Google Scholar]
- 4.Enlund MG, Ahlstedt BL, Andersson LG, Krekmanov LI. Induced hypotension may influence blood loss in orthognathic surgery, but it is not crucial. Scand J Plast Reconstr Surg Hand Surg. 1997;31:311–7. doi: 10.3109/02844319709008977. [DOI] [PubMed] [Google Scholar]
- 5.Pasch T, Huk W. Cerebral complications following induced hypotension. Eur J Anaesthesiol. 1986;3:299–312. [PubMed] [Google Scholar]
- 6.Verstraete M. Clinical application of inhibitors of fibrinolysis. Drugs. 1985;29:236–61. doi: 10.2165/00003495-198529030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Molenaar IQ, Warnaar N, Groen H, Tenvergert EM, Slooff MJ, Porte RJ. Efficacy and safety of antifibrinolytic drugs in liver transplantation: A systematic review and meta-analysis. Am J Transplant. 2007;7:185–94. doi: 10.1111/j.1600-6143.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman LH. Causes and consequences of critical bleeding and mechanisms of blood coagulation. Pharmacotherapy. 2007;27:45S–56S. doi: 10.1592/phco.27.9part2.45S. [DOI] [PubMed] [Google Scholar]
- 9.Dunn CJ, Goa KL. Tranexamic acid: A review of its use in surgery and other indications. Drugs. 1999;57:1005–32. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol) 1980;14:41–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Goobie SM, Meier PM, Pereira LM, McGowan FX, Prescilla RP, Scharp LA, et al. Efficacy of tranexamic acid in pediatric craniosynostosis surgery: A double-blind, placebo-controlled trial. Anesthesiology. 2011;114:862–71. doi: 10.1097/ALN.0b013e318210fd8f. [DOI] [PubMed] [Google Scholar]
- 12.Kakar PN, Gupta N, Govil P, Shah V. Efficacy and Safety of Tranexamic Acid in Control of Bleeding Following TKR: A Randomized Clinical Trial. Indian J Anaesth. 2009;53:667–71. [PMC free article] [PubMed] [Google Scholar]
- 13.Thiagarajamurthy S, Levine A, Dunning J. Does prophylactic tranexamic acid safely reduce bleeding without increasing thrombotic complications in patients undergoing cardiac surgery? Interact Cardiovasc Thorac Surg. 2004;3:489–94. doi: 10.1016/j.icvts.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Fawzy H, Elmistekawy E, Bonneau D, Latter D, Errett L. Can local application of Tranexamic acid reduce post-coronary bypass surgery blood loss? A randomized controlled trial. J Cardiothorac Surg. 2009;4:25. doi: 10.1186/1749-8090-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: A prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976) 2008;33:2577–80. doi: 10.1097/BRS.0b013e318188b9c5. [DOI] [PubMed] [Google Scholar]
- 16.Choi WS, Irwin MG, Samman N. The effect of tranexamic acid on blood loss during orthognathic surgery: A randomized controlled trial. J Oral Maxillofac Surg. 2009;67:125–33. doi: 10.1016/j.joms.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Sadeghi M, Mehr-Aein A. Does a single bolus dose of tranexamic acid reduce blood loss and transfusion requirements during hip fracture surgery? A prospective randomized double blind study in 67 patients. Acta Medica Iranica. 2006;45:431–6. http://www.sid.ir/en/VEWSSID/J_pdf/86520070601.pdf . [Google Scholar]
- 18.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: A prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78:434–40. [PubMed] [Google Scholar]
- 19.Jansen AJ, Andreica S, Claeys M, D'Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83:596–601. doi: 10.1093/bja/83.4.596. [DOI] [PubMed] [Google Scholar]
- 20.Yamasaki S, Masuhara K, Fuji T. Tranexamic acid reduces postoperative blood loss in cementless total hip arthroplasty. J Bone Joint Surg Am. 2005;87:766–70. doi: 10.2106/JBJS.D.02046. [DOI] [PubMed] [Google Scholar]
- 21.Ekbäck G, Axelsson K, Ryttberg L, Edlund B, Kjellberg J, Weckström J, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91:1124–30. doi: 10.1097/00000539-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Sano M, Hakusui H, Kojima C, Akimoto T. Absorption and excretion of tranexamic acid following intravenous, intramuscular and oral administrations in healthy volunteers. Jpn J Clin Pharmacol Therapeutics. 1976;7:375–82. [Google Scholar]
- 23.Cid J, Lozano M. Tranexamic acid reduces allogeneic red cell transfusions in patients undergoing total knee arthroplasty: Results of a meta-analysis of randomized controlled trials. Transfusion. 2005;45:1302–7. doi: 10.1111/j.1537-2995.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- 24.Camarasa Godoy MA, Serra-Prat M, Palomera Fanegas E. Effectiveness of tranexamic acid in routine performance of total knee replacement surgery. Rev Esp Anestesiol Reanim. 2008;55:75–80. doi: 10.1016/s0034-9356(08)70513-9. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez JC, Santiveri FX, Ramos I, Vela E, Puig L, Escolano F. Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion. 2008;48:519–25. doi: 10.1111/j.1537-2995.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 26.Lozano M, Basora M, Peidro L, Merino I, Segur JM, Pereira A, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95:39–44. doi: 10.1111/j.1423-0410.2008.01045.x. [DOI] [PubMed] [Google Scholar]


