Abstract
Background:
Paravertebral block (PVB) has been an established technique for providing analgesia to the chest and abdomen. We conducted the current study to compare single-dose PVB versus single-dose epidural blockade (EP) for pain relief after renal surgery.
Methods:
Eighty patients scheduled for renal surgery were randomly assigned into two groups according to the analgesic technique, PVB group or EP group. General anesthesia was induced for all patients. Postoperative pain was assessed over 24 h using 10-cm visual analog scale (VAS). Postoperative total pethidine consumption was recorded. Any postoperative events, such as nausea, vomiting, shivering, or respiratory complications, were recorded. Hemodynamics and blood gasometry were also recorded.
Results:
EP group showed significant decrease of both heart rate and mean blood pressure at most of the operative periods when compared with PVB group. There was no difference in total rescue analgesic consumption. Postoperative VAS showed no significant difference between the studied groups. Postoperative events were comparable in both the groups.
Conclusion:
Single injection PVB resulted in similar analgesia but greater hemodynamic stability than epidural analgesia in patients undergoing renal surgery, therefore this technique may be recommended for patients with coexisting circulatory disease.
Keywords: Anesthesia, epidural, pain and renal surgery, paravertebral
INTRODUCTION
Renal surgeries (RS) are usually associated with significant postoperative pain. Postoperative analgesia following RS is essential to allow effective coughing, early mobilization, and to reduce the incidence of postoperative respiratory complications.[1] A considerable portion of those patients may suffer from comorbidities, such as impaired renal function, hypertension, and ischemic heart diseases. So morbidity rates can be reduced by analgesia after RS in such a high-risk group.
A variety of techniques for postoperative analgesia following RS were applied.[2,3] Administration of systemic analgesia after RS may be precluded by impaired renal function and respiratory complications from the use of opioids.[4] Other techniques include intramuscular and/or intravenous injection of paracetamol or nonsteroidal anti-inflammatory drugs. However, none of these methods was proven to be highly effective. On the other hand, postoperative analgesia by utilizing epidural blockade (EP) demonstrated high rates of safety and efficacy in upper abdominal surgery, including RS.[5]
Paravertebral blocks (PVBs) are effective techniques in controlling pain after lower thoracic and upper abdominal surgery. The use of paravertebral catheter to provide unilateral or bilateral analgesics has been described in adults and children.[6,7] A single shot technique using bupivacaine, levobupivacaine, or ropivacaine can provide analgesia for up to 18 h.[8] However, PVB has not been extensively studied as analgesic tool in renal surgery. In addition, to the best of our knowledge, effect of single-injection PVB in comparison with EP after renal surgery in adult patients has not been undertaken previously. Therefore, in the current study we have conducted a prospective randomized trial aiming at the assessment of hemodynamics and analgesic response after single-injection PVB versus EP.
METHODS
After approval by the hospital ethical committee, written informed consent was obtained from patients scheduled to undergo elective renal surgery. Study population: 80 patients (American Society of Anesthesiologists (ASA) physical status I or II), were included in the study. All were scheduled for elective renal surgery, including nephrectomy, pyelolithotomy, and pyeloplasty. Patients with any contraindication for regional anesthesia, such as infection of the puncture site, anatomic deformities, or coagulation disorders, were excluded. The patients were randomized into two groups by use of sealed envelopes (40 patients each) to receive epidural block (EP group) n=40, or paravertebral block (PVB group) n=40.
All the patients were thoroughly assessed preoperatively by history, physical examination, and laboratory evaluations (complete blood picture, liver function, and renal function tests). The day before surgery, the study protocol, EP, and PVB procedures were explained to each patient. All patients were made familiar with the use of 10-cm visual analog scale score (VAS) identifying 0 as no pain and 10 as the worst imaginable pain. A 10 mg diazepam was administrated orally at the night of surgery for all patients.
On arrival of the patients to the theater suite, and after routine monitoring, peripheral intravenous cannula (18 G) was inserted. Lactated Ringer's solution was infused at a rate of 8 mL/kg to replenish the overnight fasting hours. All patients were premedicated with fentanyl 1.5 μg/kg and midazolam 0.05 mg/kg. Arterial catheter was inserted in all patients after modified Allen's test in the nondominant hand and arterial blood gases were taken preoperatively (basal reading).
In the EP group, patients were placed sitting upright with the neck and back flexed and the shoulders relaxed forward. Under complete aseptic technique through a midline approach, the block was done at T10 interspace using Tuohy needle with loss of resistance technique (with air) by 1-1.5 mg/kg of bupivacaine 0.5%.
Patients of the PVB group were placed sitting upright with the neck and back flexed and the shoulders relaxed forward. The spinous process of T10 is palpated and marked at its superior aspect. From the midpoint of the spinous process, the 8-10 cm Tuohy needle entry site is marked 2.5 cm laterally. Under aseptic technique, place a skin wheal of lidocaine local anesthetic 2% at T10. The Tuohy needle is attached via extension tubing to a syringe of local anesthetic. Grasp the shaft of the needle in your dominant hand, insert the needle through the skin wheal, and advance it anteriorly perpendicular to the back until it contacts the transverse process. With the needle contacting the transverse process, grasp the needle shaft with fingers 1 cm from the skin surface. The fingers now serve as a “backstop” to prevent the needle passing beyond 1 cm into the paravertebral space and possibly into the pleura of the lung. Then withdraw the needle tip to the subcutaneous tissue and angle it to “walk off” the caudal edge of the transverse process, advancing no more than 1 cm into the space. Often, a loss of resistance or “pop” is appreciated, indicating that the needle tip has penetrated the superior costotransverse ligament. After gentle aspiration of the syringe for blood and air, inject 1.5 mg/kg of bupivacaine 0.5% into the paravertebral space.[9]
General anesthesia was induced to all patients with thiopental sodium until loss of eye lash reflex and rocuronium bromide (0.6 mg/kg) to facilitate tracheal intubation. Anesthesia was maintained with isoflurane (1-2%) and 60% air in oxygen mixture and top up dose of rocuronium. Controlled ventilation was achieved by (Drager model (Primus), S. No: 5370893, Germany, 2006) ventilator with tidal volume of 8-10 mL/kg and I/E ratio 1:2 to maintain endtidal carbon dioxide tension around 35 mmHg.
Electrocardiography, noninvasive blood pressure, pulse oximetry, and endtidal carbon dioxide (ETCO2) was monitored throughout surgery by (Datex–Ohmeda model (S/5) AN. S.No: 3422715, Finland, 1998) monitor. Heart rate (HR), mean arterial blood pressure(MBP), pulse oximetry was monitored as preoperative (basal), 15 min, 30 min, 1 h, 1½, 2, 2½, 3, 3½ from start of surgery, then 15 min, 30 min, 1 h, 4, 8, 12, 16, and 24 h postoperatively. And arterial blood gases were taken at the same time of hemodynamic monitoring. At the end of surgery neuromuscular block was antagonized in all patients with neostigmine 0.04 mg/kg and atropine 0.02 mg/kg.
Postoperative assessment
Post-operative pain was assessed over 24 h using 10-cm VAS where 0 = no pain and 10 = unbearable pain. VAS was recorded at times (early postoperative, 15 min, 30 min, 1 h, 2, 4, 6, 12, 18, and 24 h). When the patients experienced pain (VAS ≥4) pethidine was given intramuscularly 50-100 mg. Total pethidine consumption were recorded.
Any postoperative events like nausea, vomiting, shivering or respiratory distress were recorded (through counting the respiratory rate).
Statistical analysis
The statistical analysis of data done by using excel program for figures and Statistical Package for Social Science (SPSS, Inc, Chicago, IL, USA) program version 16. To test the normality of data distribution Kolmogorov–Smirnov test was done only significant data revealed to be nonparametric. The description of the data done in the form of mean (±SD) for quantitative data and frequency and proportion for qualitative data.
The analysis of the data was done to test statistical significant difference between groups.
Chi-square test was used for qualitative data. Any difference or change showing probability (P) less than 0.05 was considered statistically significant at confidence interval 95%.
Sample size was calculated using online statistical calculator (G power® version 3). Minimal sample size of 80 patients was needed to get power level: 0.8, alpha level: 0.05 anticipated effect size (f2): 0.15. VAS score as the primary variant.
RESULTS
Fifty males and 30 females were prospectively enrolled in the current study with a mean age±SD of 43.57±11.22 years (range, 29-58), 45 of patients were ranked as ASA I, whereas 35 were ASA II. Simple or radical nephrectomies were carried out in 70 patients, pyelolithotomy in 7, and pyeloplasty in 3.
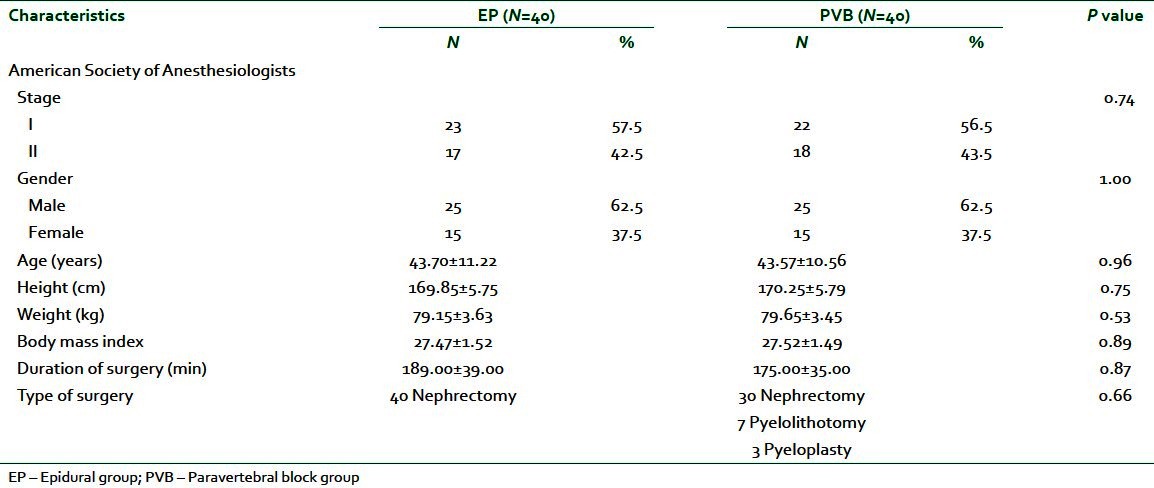
In this study, there were no significant differences between EP group and PVB group with respect to demographic data, duration, and types of surgery [Table 1].
Table 1.
Patients' characteristics and duration of surgery (min)
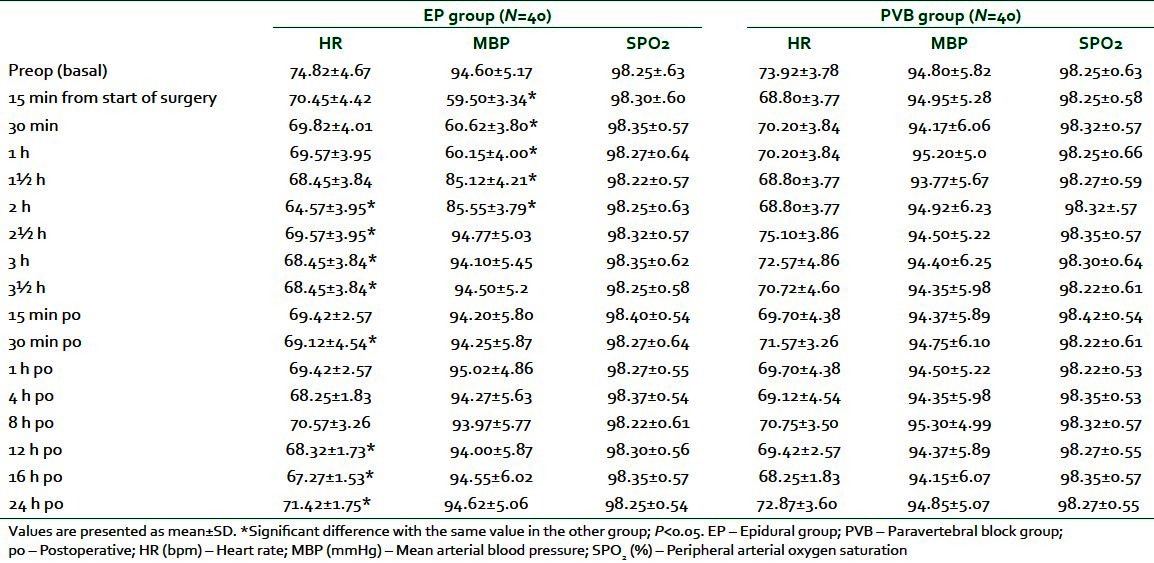
No patients had abnormal finding, such as hypotension, arrhythmia, or respiratory failure, throughout monitoring. There was a significant decrease in HR in EP group compared with PVB group at 2, 2½, 3, and 3½ h and at 30 min, 12 h,16 h, and 24 h postoperatively (P ranged from <0.01 to <0.001). Similarly, MBP showed a significant decrease in EP group compared with PVB group 15 min, 30 min, 1 h, 1½, and 2 h from the start of surgery (P<0.001).
Peripheral arterial oxygen saturation (SPO2) displayed no significant difference between the studied groups throughout the study period [Table 2].
Table 2.
Perioperative hemodynamic data in EP and PVB groups
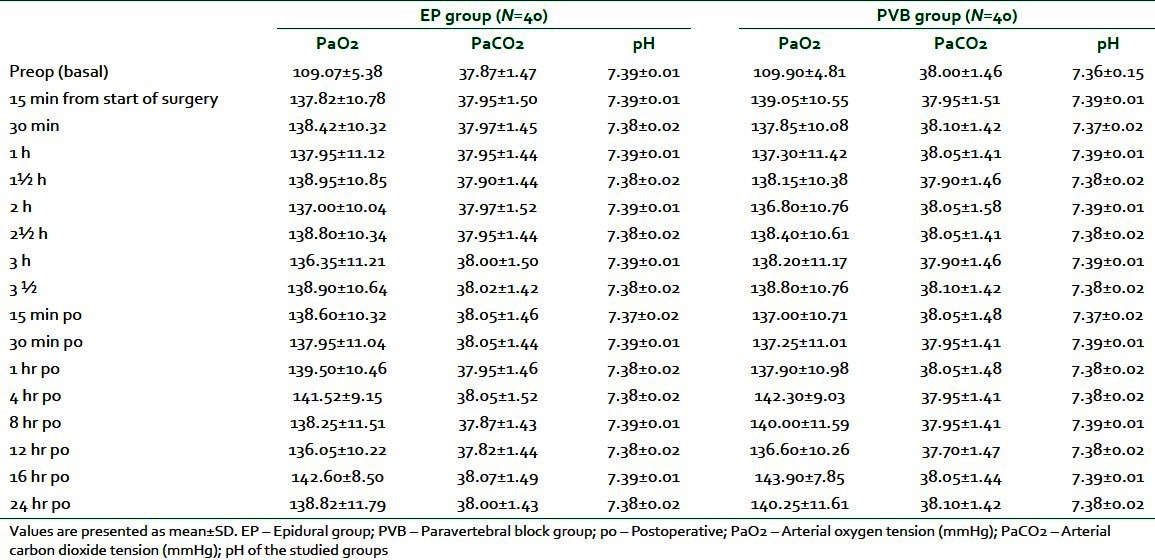
On the other hand, no significant differences were found concerning arterial oxygen tension (PaO2), arterial carbon dioxide tension (PaCO2), and pH, comparing the two studied groups at different time points [Table 3].
Table 3.
Perioperative blood gasometric data in EP and PVB groups
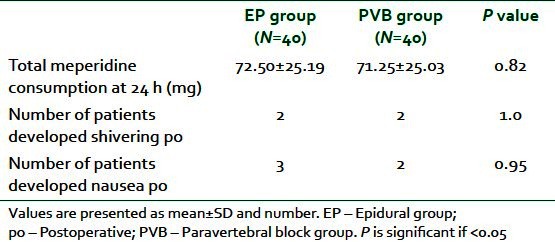
The total analgesic consumption (meperidine) at 24 h postoperatively showed no significant difference between the two groups). Postoperative shivering developed in 2 patients in each group. Whereas 3 patients in EP group suffered from nausea postoperatively compared with 2 patients in the PVB group [Table 4].
Table 4.
Total consumption of postoperative analgesia and postoperative complications
Mean postoperative VAS scores demonstrated no significant difference between both groups throughout the duration of monitoring. After 24 h, all patients had the worst pain with VAS score 4 mm in the EP group, versus 5 mm in the PVB group [Figure 1]. The postoperative respiratory rate showed no significant difference between the studied groups [Figure 2].
Figure 1.
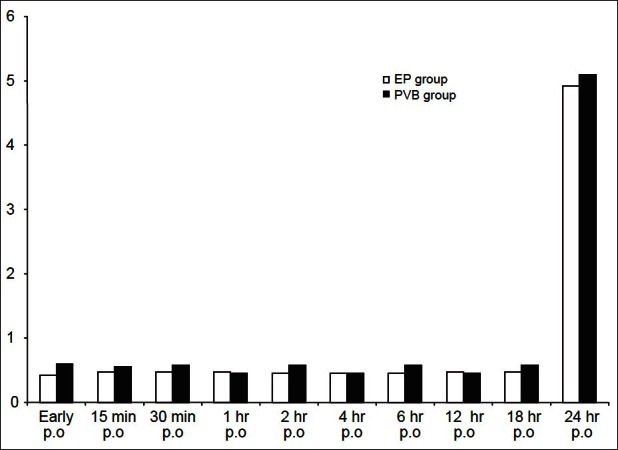
Visual analogue scale score (VAS) of the studied groups. White column (EP) n=40 and black column (PVB) n=40, PO=postoperative
Figure 2.
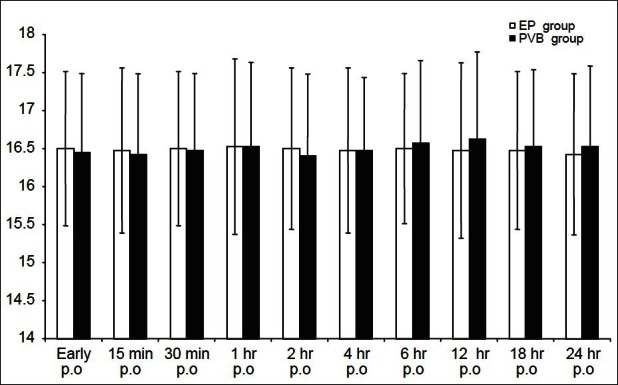
Post operative respiratory rate (RR) of the studied groups. White column (EP) n=40 and black column (PVB) n=40 PO=postoperative
Regarding the intraoperative complications in the PVB group, we have 2 patients excluded from the study. One patient developed block failure that diagnosed before induction of general anesthesia by pinprick test that revealed 3 unblocked adjacent dermatomes and confirmed at the end of the surgery by VAS scoring more than 5 mm as well as the need for opioid administration in the recovery room.
Another patient with inadvertent intravascular placement of the needle was defined as positive aspiration of blood was also excluded. This might be attributed to the highly vascularized paravertebral space and inadvertent vascular puncture will often occur and this highlights the need for a meticulous technique involving frequent aspiration in order to detect intravascular placement of the needle.
In the current study, we did not confront with any other intraoperative complications that frequently occurred with PVB, such as accidental pleural puncture and subsequent development of pneumothorax or dural cuff puncture with subsequent intrathecal spread of the local anesthetic.
DISCUSSION
In the present study, EP and PVB groups were compared with regard to hemodynamics, blood gasometric profile, their analgesic effect, the need for postoperative analgesia, and postoperative complication. Patients in both the groups demonstrated comparable demographic characteristics. In the current study, patients in the EP group demonstrated a significant decrease in hemodynamics (HR and MBP) compared with patients in the PVB group. Both groups displayed stable blood gases parameters (PaO2, PaCO2 and pH). In addition, the total analgesic consumption at 24 h was equal in both the groups. Two patients developed postoperative shivering in the 2 studied groups, whereas 3 patients in the EP group developed postoperative nausea and only 2 patients developed nausea in the PVB group postoperatively.
In spite of many advances in the issue of pain management, postoperative pain remains a serious cause of severe suffering that is often undermanaged despite our best efforts.
Unrelieved postoperative pain may result in clinical and psychological changes that increase morbidity and mortality as well as costs and that decreases quality of life.[10]
Negative clinical outcomes resulting from ineffective postoperative pain management include deep vein thrombosis, pulmonary embolism, coronary ischemia, myocardial infarction, pneumonia, poor wound healing, and insomnia.[11] Associated with these complications are economic and medical implications, such as extended lengths of stay, readmissions, and patient dissatisfaction with medical care.[12]
The current study showed that HR was reduced in EP group than in the PVB group nearly at all times of the operative period, but this reduction was significant (P<0.001) at 2 h, 2½ h, 3 h, 3½ h from the start of surgery and at 30 min postoperatively most probably due to the intensive sympathetic blockade induced by the EP, which was more observed than with PVB. PVB is characterized by unilateral regional blockade of several dermatomes without sympathicolysis and with effective blockade of pain stimuli.[9,13] These unique characteristics are attributed to ipsilateral blockade of the spinal nerves and sympathetic chain, without blocking of the contralateral sympathetic chain.[14] Also we speculated the reason for this significant difference in HR might be explained by qualitatively greater block of the somatic nerves together with block of the sympathetic chain and the rami communicans when local anesthetic is placed alongside the vertebral column rather than anatomically distant from it in the paravertebral space.[15]
The mean arterial blood pressure in our study demonstrated highly significant decrease in EP group than PVB group (P<0.001), which was in accordance with previous study that used local anesthetic through thoracic epidural analgesia during major abdominal surgery proved that significant hypotension has been occurred in all groups after application of epidural solution.[16]
One of the most complicated issues needed to be addressed are how to distinguish between adequate anesthesia and effective analgesia and how to quantify intraoperative analgesia. We used the definition of intraoperative analgesia in the review by Guignard,[17] where adequate analgesia is defined in terms of maintaining a stable hemodynamic status, both during and after a noxious stimulus. So thoracic PVB, through its hemodynamic stability considered an effective and safe analgesic.
The global view of blood gasometrical changes sensed through measurements of arterial oxygen tension (PaO2), arterial carbon dioxide tension (PaCO2), and pH is reflecting a similar effect of EP block compared with the PVB as there were no significant changes recorded between the studied groups during or after the procedure at any time interval.
In the current study, the two different techniques used were equally effective with respect to pain control (VAS), with no difference in postoperative meperidine consumption at 24 h. Interestingly, after 24 h the score was still lower in both the groups. However, at this time a pharmacologic effect of bupivacaine cannot be expected. This finding may be explained by a pre-emptive effect of the PVB: Reducing the nociceptive input to the central nervous system in the first hour after surgery may have attenuated central sensitization, thereby leading to less postoperative pain.[18]
Our results confirm the findings of previous studies showed that single-injection thoracic PVB reduced the severity of postoperative pain after breast surgery.[19,20]
Also the result of our study pass in accordance with another study that used preoperative continuous epidural or paravertebral bupivacaine on postthoracotomy pain and proved significant lower VAS scores at rest and on coughing.[21]
Vogt and colleagues studied the effect of single-injection PVB for postoperative pain treatment after thoracoscopic surgery.[22] They found low pain scores at rest and on coughing.
One of the interesting studies on the same patients group that used intraoperative intravenous ketamine in combination with epidural analgesia for postoperative pain relieve after renal surgery was found that the VAS scores were significantly lower in the ketamine group during rest and strain.[8]
In another study, continuous PVB has been reported in pediatric patients undergoing renal surgery. Thirty-five pediatric patients undergoing renal surgery, receiving either PVB (n=15) or EP (n=20), were reviewed retrospectively. It was proved that postoperative need for supplemental morphine administration was significantly lower (P=0.046) and the number of patients with no need for supplemental morphine administration postoperatively was significantly higher (P=0.019) in patients treated with PVB versus EP. This study indicates that PVB may possess a potential for postoperative analgesia equal to or may be even superior to conventional lumbar EP in pediatric patients undergoing renal surgery.[5]
In our study, the overall incidence of nausea and shivering in the 24 h postoperative was comparable with the previous studies. This is probably related to the little opioid administered rather than the preoperative fentanyl dose.[23,24]
The absence of postoperative nausea contrasts with recent observations of Klein et al. and Pusch et al. A possible explanation for this finding might be the difference in patient selection with both studies, because we limited our study to the renal surgical procedures.[25,26]
Renal surgery includes a special position during the procedure as well as a characteristic surgical incision; both have a detrimental effect on pulmonary mechanics. However, the early postoperative respiratory deterioration is thought to be caused mainly by the respiratory effects of severe postoperative pain.[27] We recorded the postoperative respiratory rate as a sign for effective pain control. In our study there was no significant difference between the studied groups with regard to postoperative respiratory rate. This result passes in agreement with a previous study.[28]
The current study has certain limitations, including the inability to use ultrasound guided blockade. It is conceivable that ultrasound guidance will further enhance the performance of PVB. Single-injection techniques are limited by the duration of the local anesthetic used. Also one of the potential study design drawbacks is the shorter duration of postoperative followup for analgesia; we recommend further study including long-time follow-up for at least 48 h postoperatively.
CONCLUSION
In summary, our study showed that single-injection PVB resulted successfully in pain relief during the first 24 h after a renal surgery. Further studies are needed to determine the optimal pain control for this group of patients and if the use of continuous or repetitive analgesic control through a catheter is more effective than the single-shot technique used in our study.
Although PVB resulted in satisfactory postoperative pain relief, the advantages over EP were marginal in this patient group (renal surgery). Considering that the technique has a certain complication rate, we conclude that at present the risk/benefit ratio of PVB does not favor routine use in renal surgery; however, this technique may be recommended for patients with coexisting circulatory disease.
ACKNOWLEDGMENTS
We thank Dr. Ahmed Abo Alyazed, Assistant Professor of Community Medicine, Mansoura University, Egypt, for his statistical assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Rudge CJ. Renal transplantation. Curr Anesthesia Crit Care. 1992;3:156–61A. [Google Scholar]
- 2.Kararmaz A, Kaya S, Karaman H, Turhanoglu S, Ozyilmaz MA. Intraoperative intravenous ketamine in combination with epidural analgesia: Postoperative analgesia after renal surgery. Anesth Analg. 2003;97:1092–6. doi: 10.1213/01.ANE.0000080205.24285.36. [DOI] [PubMed] [Google Scholar]
- 3.Shoeibi G, Babakhani B, Mohammadi SS. The efficacy of ilioinguinal-iliohypogastric and intercostal nerve co-blockade for postoperative pain relief in kidney recipients. Anesth Analg. 2009;108:330–3. doi: 10.1213/ane.0b013e31818c1b13. [DOI] [PubMed] [Google Scholar]
- 4.Ghods AJ. Organ transplantation in Iran. Saudi J Kidney Dis Transpl. 2007;18:648–55. [PubMed] [Google Scholar]
- 5.Lönnqvist PA, Olsson GL. Paravertebral vs epidural block in children. Effects on postoperative morphine requirement after renal surgery. Acta Anaesthesiol Scand. 1994;38:346–9. doi: 10.1111/j.1399-6576.1994.tb03905.x. [DOI] [PubMed] [Google Scholar]
- 6.Buckenmaier CC, III, Klein SM, Nielsen KC, Steele SM. Continuous paravertebral catheter and outpatient infusion for breast surgery. Anesth Analg. 2003;97:715–17. doi: 10.1213/01.ANE.0000075836.53838.EB. Erratum in Anesth Analg 2004;98:101. [DOI] [PubMed] [Google Scholar]
- 7.Karmakar MK, Booker PD, Franks R, Pozzi M. Continuous extrapleural paravertebral infusion of bupivacaine for post-thoracotomy analgesia in young infants. Br J Anaesth. 1996;76:811–15. doi: 10.1093/bja/76.6.811. [DOI] [PubMed] [Google Scholar]
- 8.Bondár A, Szűcs S, Iohom G. Thoracic paravertebral blockade. Med Ultrason. 2010;12:223–7. [PubMed] [Google Scholar]
- 9.Eason MJ, Wyatt R. Paravertebral thoracic block: A reappraisal. Anaesthesia. 1979;34:638–42. doi: 10.1111/j.1365-2044.1979.tb06363.x. [DOI] [PubMed] [Google Scholar]
- 10.Carr DB, Goudas LC. Acute pain. Lancet. 1999;353:2051–8. doi: 10.1016/S0140-6736(99)03313-9. [DOI] [PubMed] [Google Scholar]
- 11.Breivik H. Postoperative pain management: Why is it difficult to show that it improves outcome? Eur J Anaesthesiol. 1998;15:748–51. doi: 10.1097/00003643-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Twersky R, Fishman D, Homel P. What happens after discharge? Return hospital visits after ambulatory surgery. Anesth Analg. 1997;84:319–24. doi: 10.1097/00000539-199702000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Richardson J, Lonnqvist PA. Thoracic paravertebral block. Br J Anaesth. 1998;81:230–8. doi: 10.1093/bja/81.2.230. [DOI] [PubMed] [Google Scholar]
- 14.Cheema SP, Ilsley D, Richardson J, Sabanathan S. A thermographic study of paravertebral analgesia. Anaesthesia. 1995;50:118–21. doi: 10.1111/j.1365-2044.1995.tb15092.x. [DOI] [PubMed] [Google Scholar]
- 15.Richardson J, Jones J, Atkinson R. The effect of thoracic paravertebral blockade on intercostals somatosensory evoked potentials. Anesth Analg. 1998;87:373–6. doi: 10.1097/00000539-199808000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Panousis P, Heller AR, Koch T, Litz RJ. Epidural ropivacaine concentrations for intraoperative analgesia during major upper abdominal surgery: A prospective, randomized, double-blinded, placebo-controlled study. Anesth Analg. 2009;108:1971–6. doi: 10.1213/ane.0b013e3181a2a301. [DOI] [PubMed] [Google Scholar]
- 17.Guignard B. Monitoring analgesia. Best Pract Res Clin Anaesthesiol. 2006;20:161–80. doi: 10.1016/j.bpa.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 19.Kairaluoma PM, Bachmann MS, Korpinen AK, Rosenberg PH, Pere PJ. Single injection paravertebral block before general anesthesia enhances analgesia after breast cancer surgery with and without associated lymph node biopsy. Anesth Analg. 2004;99:1837–43. doi: 10.1213/01.ANE.0000136775.15566.87. [DOI] [PubMed] [Google Scholar]
- 20.Naja MZ, Ziade MF, Lonnqvist PA. Nerve-stimulator guided paravertebral blockade vs. general anaesthesia for breast surgery: A prospective randomized trial. Eur J Anaesthesiol. 2003;20:897–903. doi: 10.1017/s0265021503001443. [DOI] [PubMed] [Google Scholar]
- 21.Richardson J, Sabanathan S, Jones J, Shah RD, Cheema S, et al. A prospective, randomized comparison of preoperative and continuous balanced epidural or paravertebral bupivacaine on post-thoracotomy pain, pulmonary function and stress responses. Br J Anaesth. 1999;83:387–92. doi: 10.1093/bja/83.3.387. [DOI] [PubMed] [Google Scholar]
- 22.Vogt A, Stieger DS, Theurillat C, Curatolo M. Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery. Br J Anaesth. 2005;95:816–21. doi: 10.1093/bja/aei250. [DOI] [PubMed] [Google Scholar]
- 23.Snijdelaar DG, Hasenbos MA, van Egmond J, Wolff AP, Liem TH. High thoracic epidural sufentanil with bupivacaine: Continuous infusion of high volume versus low volume. Anesth Analg. 1994;78:490–4. doi: 10.1213/00000539-199403000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Mourisse J, Hasenbos MA, Gielen MJ, Moll JE, Cromheecke GJ. Epidural bupivacaine, sufentanil or the combination for post thoracotomy pain. Acta Anaesthesiol Scand. 1992;36:70–4. doi: 10.1111/j.1399-6576.1992.tb03425.x. [DOI] [PubMed] [Google Scholar]
- 25.Klein SM, Bergh A, Steele SM, Georgiade GS, Greengrass RA. Thoracic paravertebral block for breast surgery. Anesth Analg. 2000;90:1402–5. doi: 10.1097/00000539-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Pusch F, Freitag H, Weinstabl C, Obwegeser R, Huber E, Wildling E. Single-injection paravertebral block compared to general anaesthesia in breast surgery. Acta Anaesthesiol Scand. 1999;43:770–4. doi: 10.1034/j.1399-6576.1999.430714.x. [DOI] [PubMed] [Google Scholar]
- 27.Sabanathan S, Eng J, Mearns AJ. Alterations in respiratory mechanics following thoracotomy. J R Coll Surg Edinb. 1990;35:144–50. [PubMed] [Google Scholar]
- 28.Ballantyne JC, Carr DB, deFerranti S, Suarez T, Lau J, Chalmers TC, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: Cumulative meta-analyses of randomized, controlled trials. Anesth Analg. 1998;86:598–612. doi: 10.1097/00000539-199803000-00032. [DOI] [PubMed] [Google Scholar]


