Abstract
Background:
There are conflicting reports regarding the effect of exercise on cognition. We therefore planned to assess the acute effect of moderate exercise on cognition, studied by event-related brain potential P300, in subjects having sedentary lifestyles.
Materials and Methods:
Sixty adults (40 males and 20 females) in the age-group of 18–30 years having sedentary lifestyles were included in the study. Baseline P300 was first measured and after that the subjects were divided into two equal age- and sex-matched groups. The test group was subjected to moderate exercise (that is, to achieve 60%–80% of maximum heart rate during exercise, where 100%=200–age) on a bicycle ergometer for 5 minutes, following which postexposure P300 was measured. In the control group P300 was re-recorded 15 minutes after baseline recording, without any intervening exercise.
Results:
The latency of P300 was found to be significantly reduced after acute moderate exercise in the test group.
Conclusion:
It appears that acute moderate exercise improves the cognitive brain functions of adults with sedentary lifestyles.
Keywords: Cognition, event-related potential, exercise, sedentary lifestyle
INTRODUCTION
The effect of physical activity on brain and cognition has attracted the interest of many researchers in recent years, with an increasing number of reports indicating that both regular exercise as well as isolated acute bouts of exercise may benefit human cognitive processes.[1] But there are conflicting reports regarding the type of cognitive processing that is most affected by aerobic exercise.[2,3]
Measurement of event-related potentials (ERP) is a noninvasive technique to assess the function of the central nervous system (CNS).[4] ERPs are patterns of neuroelectric activation that occur in response to a stimulus. The amplitude of the P300 is directly related to the allocation of attentional resources during stimulus engagement.[5] The latency of the P300 is used for stimulus classification and for evaluation of speed, with increased latency indicating longer processing time.[6] Earlier studies have observed increased amplitude and shorter latency, relative to a basal state, following single acute bouts of moderately intense exercise.[7] However, other researchers, examining a different aspect of cognition (other than cognitive P300), failed to demonstrate a beneficial effect of acute aerobic exercise.[8] Therefore, the present study was designed to study the effect of acute moderate exercise of short duration on the cognitive (P300) functions of young males and females with sedentary lifestyles.
MATERIALS AND METHODS
Sixty right-handed healthy volunteers (40 males, 20 females) in the age-group of 18–30 years with sedentary lifestyles were recruited from among the undergraduate and postgraduate students and staff members of a medical college. Sedentary lifestyle was determined as physical activity less than 30 minutes per day for last 6 months. None of the participants reported any adverse health conditions such as previous history of stroke, diabetes, depression, hypertension, osteoarthritis, chronic obstructive lung disease, visual or auditory impairment, or smoking habits. Before taking part in the experiment, all participants signed consent forms and were fully informed about the protocol. The ethical guidelines were followed as per Harriss et al.[9]
All subjects were asked to arrive at the lab at the same time of the day, i.e., between 9 AM and 11 AM. Basal recording of ERP was done using the RMS EMG EP Mark-II (RMS, Chandigarh, India). After this, the subjects were divided into two equal age- and sex-matched groups: the control and test groups. The test group was asked to perform exercise for 5 minutes on a bicycle ergometer (Mag Cycle™). The severity of exercise was maintained at a moderate level as indicated by the heart rate (that is, we sought to achieve 60%–80% of maximum heart rate during exercise, where 100%= 200–age). Immediately after the 5 minutes of exercise, when the heart rate monitored by a pulse oximeter had returned to normal, the ERP was recorded again. In the control group no exercise was done and the second recording of ERP was done 15 minutes after the first.
ERP recordings
Silver chloride electrodes were placed at Pz and Cz actively, according to the international 10–20 system,[10] referenced to a linked earlobe electrode and a forehead electrode as ground. Impedances were maintained below 5 kΩ and were measured from each lead at the beginning and the end of each session. P300 potentials were recorded with a bandpass of 0.1–50 Hz. Recordings were made for 300 seconds, excluding the rejection errors, at a sweep speed of 50 msec/division. P300 potentials were obtained from an auditory oddball paradigm. The target tones (2000 Hz) were presented randomly with a probability of 20%, whereas the non-target tones (1000 Hz) were presented randomly with a probability of 80%; the tones were presented binaurally over headphones at a sound intensity of 80 dB and a post-stimulus delay of 1 second. At the end of each session, each participant's count was compared with the actual number of target tones given to assess the accuracy of the task performance. All participants performed the tasks with an error rate <5% in all trials. Principal peaks and their identification were made according to the standard recommendations for long-latency auditory ERPs of the International Federation of Clinical Neurophysiology and the principal component analysis technique.[10] The amplitude of the P300 wave was measured as an absolute value between the peak points of N200 and P300 (N2P3).
Statistical analysis
The paired Student's ‘t’ test and ANOVA with post hoc Tukey's test was applied to compare the values between the groups. SPSS® version 10 and Microsoft® Excel® 2007 were used for statistical analysis. Level of significance was taken at P≤.05.
RESULTS
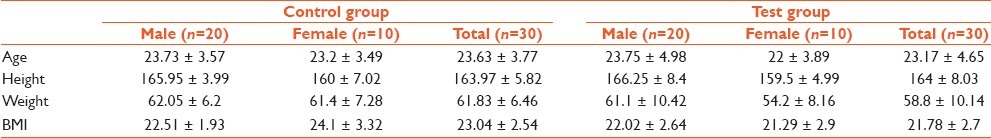
Age, weight, and body mass index (BMI) were comparable between the control and test groups. The height of subjects showed a significant difference between the groups, but on individual comparison with the post hoc Tukey's test there was no significant difference [Table 1].
Table 1.
Comparison of anthropometric data and basal P300 values between control and test groups
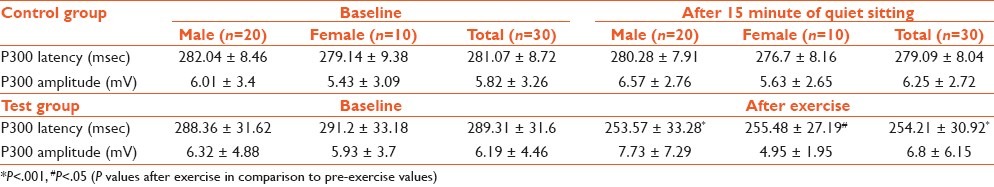
There was no significant difference between the baseline values of the control and test groups. While there was no significant difference between the two recordings of P300 latency and amplitude values in the control group, there was a significant improvement in cognitive P300 latency after exercise in the test group, in males and females separately as well as in the total values (P=.000, P=.019, and P=.000, respectively) [Table 2].
Table 2.
Comparison of P300 latency and amplitude in control and test groups using post hoc Tukey's test
DISCUSSION
In our study there was significant decrease in the P300 latencies, which is consistent with the other studies.[11–13] A short bout of exercise was found to decrease the latency of ERP P300 in the study by Hillman et al.[14] It has been shown that aerobic exercise promotes cerebral blood flow. In animal models it has been proved that exercise improves neurotransmitter function and cerebral vascularization, besides inducing other neurobiological changes.[15] Isaacs et al. observed significantly shorter diffusion-distances from blood vessels in the cerebellum of rats that were on exercise training.[16] Our study showed no significant improvement in P300 amplitude, probably because of the relatively short duration of exercise in our study. Some theories of P300 suggest that the amplitude reflects allocation of attention and context updating of working memory resources.[17] It has also been shown to be proportional to the amount of resources allocated to a particular task or stimulus, implying that acute bouts of cardiovascular exercise may facilitate the allocation of attentional and memory resources and hence benefit executive control function.[18] Magnie et al. interpreted the observed increases in P300 amplitude as suggesting that acute exercise facilitates cognitive processing via a general arousal effect.[11] Similarly, Polich et al. have suggested that P300- exercise effects occur in a global fashion related to increases in general arousal in the body.[19] Although it is known that exercise contributes to increased P300 amplitude,[13] no change in P300 amplitude to significant levels was observed in an endurance-training group in another study.[3] The absence of significant improvement in P300 amplitude in our study may be because of difference in the exercise protocol as compared to the other studies.
There has not been much investigation of the role of brisk exercise in different situations and further research on this topic is necessary.
The present study had some limitations. The low proportion of female volunteers meant that we could not make comparisons between males and females. Awareness of the intention to do exercise may also lead to CNS arousal, and this was an unavoidable limitation of our study.
We conclude that acute moderate exercise of short duration may enhance the cognitive functions of brain of persons having sedentary lifestyles as evidenced by the reduction in latencies of ERP P300 in this study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 2.Jedrziewski MK, Lee VM, Trojanowski JQ. Physical activity and cognitive health. Alzheimers Dement. 2007;3:98–108. doi: 10.1016/j.jalz.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 4.Beck EC, Dustman RE. Changes in evoked responses during maturation and aging in man and macaque. In: Burch N, Altshuler HL, editors. Behavior and Brain Electrical Activity. New York: Plenum Press; 1975. pp. 431–72. [Google Scholar]
- 5.Polich J. Task difficulty, probability and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroencephalogr Clin Neurophysiol. 1987;63:251–9. doi: 10.1016/0168-5597(87)90052-9. [DOI] [PubMed] [Google Scholar]
- 6.Duncan-Johnson CC. P3 latency: A new metric of information processing. Psychophysiology. 1981;18:207–15. doi: 10.1111/j.1469-8986.1981.tb03020.x. [DOI] [PubMed] [Google Scholar]
- 7.Kamijo K, Nishihira Y, Hatta A, Kaneda T, Wasaka T, Kida T. Differential influences of exercise intensity on information processing in the central nervous system. Eur J Appl Physiol. 2004;92:305–11. doi: 10.1007/s00421-004-1097-2. [DOI] [PubMed] [Google Scholar]
- 8.Tomporowski PD, Davis CL, Miller PH, Naglieri JA. Exercise and children's intelligence, cognition, and academic achievement. Educ Psychol Rev. 2008;20:111–31. doi: 10.1007/s10648-007-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harriss DJ, Atkinson G. Ethical Standards in Sport and Exercise Science Research. Int J Sports Med. 2009;30:701–2. doi: 10.1055/s-0029-1237378. [DOI] [PubMed] [Google Scholar]
- 10.Heinze HJ, Münte TF, Kutas M, Butler SR, Naatanen R, Nuwer MR. Cognitive event related potentials.The International Federation of Clinical Neurophysiology. Electroencephalogr and Clin Neurophysiol. 1999;52:91–7. [PubMed] [Google Scholar]
- 11.Magnie MN, Bermon S, Martin F, Madany-Lounis M, Suisse G, Muhammad W. P300, N400, aerobic fitness and maximal aerobic exercise. Psychophysiology. 2000;37:369–77. [PubMed] [Google Scholar]
- 12.McDowell K, Kerick SE, Santa Maria DL, Hatfield BD. Aging, physical activity, and cognitive processing: An examination of P300. Neurobiol Aging. 2003;24:597–606. doi: 10.1016/s0197-4580(02)00131-8. [DOI] [PubMed] [Google Scholar]
- 13.Polich J, Lardon MT. P300 and long-termphysical exercise.Electroencephalogr. Clin Neurophysiol. 1997;103:493–8. doi: 10.1016/s0013-4694(97)96033-8. [DOI] [PubMed] [Google Scholar]
- 14.Hillman CH, Snook EM, Jerome GJ. Acute cardiovascular exercise and executive control function. Int J Psychophysiol. 2003;48:307–14. doi: 10.1016/s0167-8760(03)00080-1. [DOI] [PubMed] [Google Scholar]
- 15.Dustman RE, Emmerson RE, Shearer DE. Electrophysiology and aging: Slowing, inhibition and aerobic fitness. In: Howe ML, Stones MJ, Brainerd CJ, editors. Cognitive and Behavioral Performance Factors in Atypical Aging. New York: Springer-Venley; 1990. pp. 103–49. [Google Scholar]
- 16.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: Angiogenesis in the adult rat cerebellumafter vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12:110–9. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 17.Donchin E, Coles MG. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:357–74. [Google Scholar]
- 18.Wickens C, Kramer AF, Vanasse L, Donchin E. The performance of concurrent tasks: A psychophysiological analysis of the reciprocity of information processing resources. Science. 1983;221:1080–2. doi: 10.1126/science.6879207. [DOI] [PubMed] [Google Scholar]
- 19.Polich J, Kok A. Cognitive and biological determinants of P300: An integrative review. Biol Psychol. 1995;41:103–46. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]


