Abstract
Background:
Hassall's corpuscles (HC) are commonly used as diagnostic features for identifying human thymus and are still present in thymuses undergoing fatty degeneration in young adults. However, few studies have been performed on human fetuses.
Aim:
A cross-sectional study was done, to study the morphology of HC in human fetuses.
Materials and Methods:
Twenty-eight thymuses were collected from fetuses of gestational age ranging from 11 to 40 weeks. Thymuses were processed by paraffin embedding methods and hematoxylin and eosin staining.
Results:
The size of HC varied from very small (100 microns) to very large corpuscles (> 900 microns). A high level of polymorphism was also observed, from round to unusual or odd shapes corpuscles. The degenerated reticulo-epithelial cells represented the starting point in HC formation. The growth of HC was rapid, especially near 28 weeks, and the level of HC polymorphism was significantly greater after 28 weeks of gestation. In advanced stages of gestation, the increase in size of some corpuscles reduced the spaces between them, and some patterns strongly supported the hypothesis that some HC had fused in a single and larger corpuscle.
Conclusion:
The rapid rise in number and size of HC around 28 weeks of gestation would fit with their role in the negative selection process of thymocytes.
Keywords: Fetuses, gestation, Hassall's corpuscles, polymorphism, reticulo-epithelial cells
INTRODUCTION
Hassall's corpuscles (HC) are characteristic components of the medulla of mammalian thymus. Their dimensions range from 20 to 150 microns in bovines, and from 10 to 1,000 microns in other species.[1–4] Since the first description of concentric corpuscles in the medullary zone of thymus lobules, various structures were considered as HC,[5] which are often described as polymorphic in bovine thymus.[2] Numerous studies previously addressed the issue of origin and function of HC. Most authors agreed that these corpuscles are derived from epithelial cells of thymic medulla,[6–8] though other hypothesis has also been elaborated.[9]
Most of these works have been performed on animals like guinea pigs, mice, hamsters, cats, chicken, monkeys, cattle, nutria thymus, or human adult thymus, rather than in human fetal thymus.[2,9–18] We thus sought to study human fetal thymus to capture morphological changes in HC, according to age of gestation, especially when fetuses reach the period of viability (from 24 to 28 weeks of gestation).[19]
MATERIALS AND METHODS
Twenty eight fetuses fixed in formalin were obtained from the museum of department of Anatomy. Ethical clearance for the study was taken from institutional ethics committee. Ages of fetuses were determined by measuring foot lengths of fetuses and matching them with Streeter's data. Fetuses were divided in two groups, I and II having 14 fetuses each. Gestational age of Group I fetuses were less than 28 weeks, and that of Group II were more than 28 weeks. In both groups, 50% were males, and 50% females.
Thymuses were dissected out by right paramedian incision, and removed for histological preparation. Tissues were processed by paraffin embedding method. Sections of 10mm thickness were stained with hematoxylin and eosin (H and E) stains. Histometric analysis of thymuses was done under Motic-light microscope, and Motic-software was used for all measurements. Initial visualization was done by light microscope, and the images were then digitalized with the help of software. Each HC was examined for its size, shape, and level of degeneration. Classification of HC was performed according to previous suggestions by Raica et al[18] and Liberti et al.[20] Liberti et al classified the HC into solid and cystic, depending on the presence or absence of empty space inside HC. Raica et al classified the HC into 4 types: juvenile, premature, mature, senescent or advanced. The Chi-square test was used to assess the level of significance for polymorphism of HC.
RESULTS
A total of 8,724 HC were examined. Of these, 2,783 (31.9%) HC were from fetuses aged below 28 weeks and 5,941 (68.1%) were from fetuses aged above 28 weeks. These HC had variable sizes, from very small to very large. The smallest size class was represented by corpuscles in early stages of organization, composed of one or two hypertrophic reticulo-epithelial cells (juvenile stage). Next category was represented by small groups of hypertrophic cells showing early processes of keratinization, but without a flattened aspect, or a tendency to concentric disposition (pre-mature stage). In mature stage, the reticulo-epithelial cells appeared flattened and disposed concentrically around keratin and a mix of degenerated lymphocytes and macrophages, with or without empty space. In advance stage (mainly observed in older fetuses), some HC showed varying degrees of deposition of materials at their center or periphery, whereas others HC with a distorted shape seemed to try and fuse with other nearby HC [Figure 1]. Matured and advanced HC were more significantly present in fetuses above 28 weeks of gestation, and HC were significantly more polymorphic in Group II fetuses [Table 1].
Figure 1.
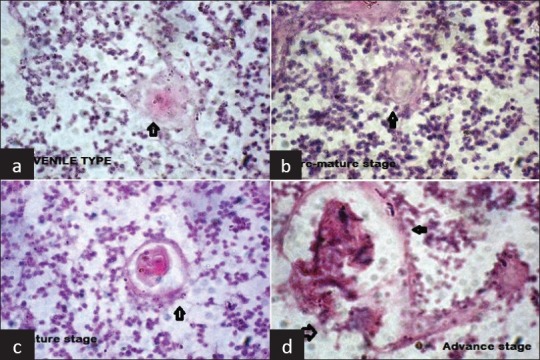
Hassall's Corpuscles in different stages of development (a): Juvenile (b): Pre-mature (c): Mature (d): Advance (H and E staining, ×40)
Table 1.
Different stages of Hassall's corpuscles in two groups

The keratinization process of reticulo-epithelial cells was triggered at different moments from one corpuscle to another, with no obvious correlation between the size of the HC and the development of this process. For instance, some small HC were highly keratinized, whereas the keratinization process was in a very early stage in larger ones. However, keratin appeared in various stages of degradation in almost all medium-sized corpuscles.
Most HC showed a well-organized peripheral zone, consisting of concentric reticulo-epithelial cells, with the central area occupied with material derived from both keratinization and degeneration of reticulo-epithelial cells (and possibly other cellular types, like lymphocytes), in different proportion from one formation to another. Large corpuscles had same general organization and structure as the medium size ones, only differences were in the dimensions and degree of degeneration of the components in central area [Figure 2].
Figure 2.

Polymorphism of Hassallæs Corpuscles: Variation in size (marked with black arrow) (a): 93 microns (b): 199.5 microns (c): 428 microns (d): 618 microns (H and E staining, ×40)
The shape of HC varied significantly, from spherical (size varying from small to very large), to much deformed, with a gradient of intermediary aspects like tricycle appearance, biconcave lens appearance, club shapes, elongated, comet and comma shaped (more in fetuses above 28 weeks of gestation) [Figure 3]. These patterns were created by the way reticulo-epithelial cells anchored to peripheral area of HC. Some HC presented with area of partial or total lysis, with spaces devoid of content, leading to large central or peripheral vacuoles (cystic pattern), while others were solid [Figure 4]. Such cystic patterns were quite infrequent in fetuses below 28 weeks of gestation where most of HC were solid; but were very frequent in fetuses above 28 weeks of gestation, especially near term fetuses [Table 2].
Figure 3.
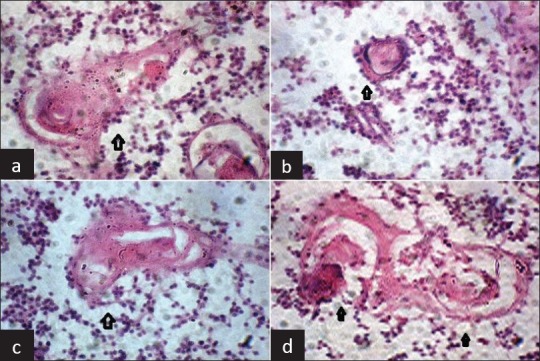
Ploymorphism of Hassall's Corpuscles: Variation in shapes (a) Comet shaped (b) Club shaped (c) Elongated shape (d) Tricyclic shaped (HandE staining, ×40)
Figure 4.
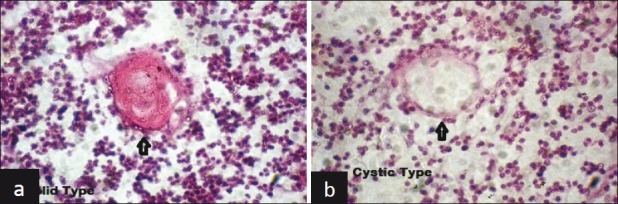
Solid and cystic types of Hassall's Corpuscles (HandE staining, ×40)
Table 2.
Solid and cystic Hassall's corpuscles in two groups

DISCUSSION
In lower animals like guinea pigs, mice and rats, HC have a small size and a spherical shape, with few polymorphisms in size and shape.[9] An experiment was performed by Blau to study the dynamic behavior of HC in guinea-pig by low dose radiation, showing that just after radiation, the HC grew in number and size, as if they tried to remove the dead thymocytes.[21] Similar findings were also observed in thymus of animals previously treated by high dose steroids. In human fetuses, HC are probably involved in the negative selection of thymocytes, and might also help to remove apoptotic self-reacting thymocytes. This might occur mainly towards 28 weeks of gestation. Indeed, we could observe a rise of HC at this period, together with a rapid growth of the size of HC. After 28 weeks of gestation, the rise in number and size of HC slows down, although HC become even more polymorphic. It can be speculated that these changes of HC are also linked to the process of negative selection.
Hammer studied sequential changes in human HC on 250 normal thymus and 750 pathological thymus glands and examined about 100,000 HC.[22] He described two types of HC - so called progressive and regressive; with invasion of HC by lymphocytes and cystic degeneration leading to resorption of corpuscles and their inside content. We did not find any feature suggestive of such regressive type of HC in fetal thymuses. This discrepancy might be explained by the active and ongoing role of HC in thymic negative selection in fetal thymus, which should prevent fetal HC from such regression.
The presence of numerous and large HC, with highly polymorphic shape in human fetuses, makes it one of the best model to study formation and evolutionary pattern of HC. All observed aspects support the idea that the reticulo-epithelial cells represent the starting point in the formation of HC. The variable anchoring of those cells to the outer layers of HC might be responsible for the striking variations of shape and size of HC noticed in the present work. Future works should be performed to assess the phenotype and function of lymphocytes surrounding HC, and look for correlations between those phenotypes and the various HC patterns observed in the present work.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Izard I. Ultrastructure of Hassall's corpuscles during experimental involution of the thymus provoked by foliculine. Z Zellforschung. 1965;66:276–92. [PubMed] [Google Scholar]
- 2.Rotaru O. Cluj-Napoca, Romania: University of Agricultural Sciences and Veterinary Medicine; 1977. Normal and pathological aspect of bovine thymus [dissertation] [Google Scholar]
- 3.Bodey B. Sofia, Bulgaria: Inst Morphol Bulg Acad Sci; 1977. Histo-morphology and histochemistry of the human thymus during its prenatal ontogenesis [Dissertation] [Google Scholar]
- 4.Bodey B, Hadjioloff AI. Thymus development, Structure and function. Nature. 1977;26:11–9. [Google Scholar]
- 5.Hassall AH. Vol. 1. London: Samuel Highley; 1849. The microscopic anatomy of the human body, in health and disease; pp. 3–12. 477-79. [Google Scholar]
- 6.Laster AJ, Itoh T, Palker TJ, Haynes BF. The human thymic micro-environment: Thymic epithelium contains specific keratins associated with early and late stages of epidermal keratinocyte maturation. Differentiation. 1986;31:67–77. doi: 10.1111/j.1432-0436.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 7.Nicolos JF, Reano A, Kaiserlian D, Thivolet J. Epithelial cells heterogeneity in mammalian thymus: Monoclonal antibody to high molecular weight keratins exclusively binds to Hassall's corpuscles. Histochem J. 1989;21:357–64. doi: 10.1007/BF01798499. [DOI] [PubMed] [Google Scholar]
- 8.Boyd RL, Tucek CL, Godfrey DI, Izon DJ, Wilson T, Davidson NJ, et al. The thymic environment. Immunol Today. 1993;14:445–59. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- 9.Kohnen P, Weiss L. An electron microscopic study of thymic corpuscles in the Guinea pig and the mouse. Anat Rec. 1964;148:29–57. doi: 10.1002/ar.1091480104. [DOI] [PubMed] [Google Scholar]
- 10.Mandel T. The development and structure of Hassall's corpuscles in guinea pig. Z Zellforschung. 1968;89:180–92. doi: 10.1007/BF00347291. [DOI] [PubMed] [Google Scholar]
- 11.Cesarini JP, Benkoel L, Bonneau H. Compared ultrastructure of the thymus at young and adult hamster. C R Soc Biol. 1968;162:1975–9. [PubMed] [Google Scholar]
- 12.Cabanie P, Mirouze P. Observations on the ultrastructure of the thymus in cat at different ages. Rev Med Vet. 1971;122:639–52. [Google Scholar]
- 13.Frazier JA. Ultrastructure of the chick thymus. Z Zellforschung. 1973;136:191–205. doi: 10.1007/BF00307440. [DOI] [PubMed] [Google Scholar]
- 14.Chapman WL, Allen JR. The fine structure of the thymus of the fetal and neonatal monkey. Z Zellforschung. 1971;114:220–33. doi: 10.1007/BF00334002. [DOI] [PubMed] [Google Scholar]
- 15.Miclaus V, Rotaru O, Puscasiu D, Mihaiu M. Histological study concerning the thymus age involution in cattle. Bul USAMV-ZMV. 1997;51:75–8. [Google Scholar]
- 16.Kendall MD, Stebbings RJ. The endocrine thymus. Endocrine J. 1994;2:333–9. [Google Scholar]
- 17.Gaudecker B. Functional histology of the human thymus. Anat Embryo. 1991;183:1–15. doi: 10.1007/BF00185830. [DOI] [PubMed] [Google Scholar]
- 18.Raica M, Encica S, Motoc A, Cimpean AM, Scridon T, Barsan M. Structural heterogeneity and immunohistochemical profile of Hassall's corpuscles in normal human thymus. Ann Anat. 2006;188:345–52. doi: 10.1016/j.aanat.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Finney PA. St Louis: Herder Book Co; 1922. Moral problems in hospital practice - A practical handbook; p. 24. [Google Scholar]
- 20.Liberti EA, Fagundas TP, Perito MA, Matson E, Konlg JB. On the size of Hassall's corpuscles in human fetuses. Bull Asso Anat. 1994;242:15–8. [PubMed] [Google Scholar]
- 21.Blau JN. The dynamic behavior of Hassall's corpuscles and the transport of particulate matter in the thymus of guinea-pig. Immunology. 1967;13:281–92. [PMC free article] [PubMed] [Google Scholar]
- 22.Hammer JA. Uber progressive und regressive formen von Hassallschen korpern. Z Anat Entwickl Gesch. 1924;70:466–88. [Google Scholar]


