Abstract
Mutations in ACTN4, the gene encoding the actin-binding protein α-actinin-4, are a cause of familial FSGS. We examined kidney biopsies from patients with ACTN4 mutations to characterize systematically the histopathology of kidney damage in these patients and to determine whether distinctive morphologic changes are associated with mutations in this gene. The changes observed with light microscopy were typical of FSGS and were morphologically heterogeneous, similar to other inherited podocytopathies. The ultrastructural characteristics, however, were distinctive: Most notably, the presence of cytoplasmic electron-dense aggregates in podocytes. Indirect immunofluorescence using antibodies to a conserved domain of α-actinin-4 (present in both wild-type and mutant proteins) revealed a segmental and irregular granular staining pattern in the capillary walls of preserved glomeruli of ACTN4 mutants, whereas preserved glomeruli of patients with other podocyte diseases retained a global linear staining pattern for α-actinin-4. These characteristics resemble features observed in mouse models of this disease and may aid in the identification of patients and families who harbor ACTN4 mutations.
FSGS is a common pattern of renal injury. There is a multiplicity of processes that can lead to this histologic pattern. These include multiple genetic causes and some recognized environmental causes, although a large fraction remain “idiopathic.” In addition, a spectrum of morphologic appearances to the characteristic lesions has prompted the definition and refinement of a histopathologic classification scheme for FSGS that might facilitate correlation with clinical presentation or prognosis.1,2 Recent proposals to unify the histopathologic classification of FSGS with disease etiology have led to increased clarity in describing this lesion,3 yet in the absence of knowledge of disease etiology, it is difficult to know what meaning to give to specific morphologic features.
Mutations in ACTN4, encoding α-actinin-4, lead to a familial form of FSGS with an autosomal dominant pattern of inheritance. Kidney disease develops through an apparent “gain of function” mechanism that seems to involve primarily the podocyte.4,5 The disease-causing ACTN4 mutations that have been identified thus far are part of a growing list of “inherited podocytopathies” that includes mutations in NPHS1, NPHS2, CD2AP, and TRPC6.6 Patients carrying mutations in ACTN4 initially present with proteinuria or nephrotic syndrome in early adulthood and often progress to renal failure. Although the clinical picture in this rare form of FSGS is fairly well understood, the histopathology in the kidneys of patients with confirmed ACTN4 mutations has not been described in detail. Here, we present a detailed description of pathologic findings in biopsies from patients who were found to carry disease-associated mutations in ACTN4. Several of the findings seem to be unique to ACTN4-mediated disease and enlighten our understanding of the pathogenesis of kidney disease associated with mutations in this gene.
We obtained renal biopsy material from members of four families, all of whom carried distinct FSGS-causing ACTN4 mutations. Twelve patients with confirmed (heterozygous) mutations in ACTN4 for whom biopsy material was available for review were identified (Table 1). The patients (six female, six male) ranged in age from 16 to 56 yr, with a mean age of 30 yr at the time of biopsy. Proteinuria or nephrotic syndrome was the indication for biopsy or documented at the time of biopsy in 11 of the 12 patients; hematuria was the indication for biopsy in the remaining patient (FSX-233). Renal failure was documented in two patients (FSA-192, FGCI-111) at the time of biopsy. We analyzed all of this material using standard histologic and ultrastructural approaches and immunostaining for α-actinin-4. For comparison, we also obtained biopsies from patients with other glomerular diseases affecting the podocyte, from archived material available at Brigham and Women's Hospital.
Table 1.
Histopathologic findings in biopsies from patients with ACTN4 mutationsa
| Patient Data |
Glomerular Histopathology |
Histopathologic Features of Segmental Sclerosing Lesions |
Glomerular Immunofluorescence |
Tubulointerstitial and Vascular Histopathology |
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID No. | Age (yr) | Gender | SCr (mg/dl) | Urine Prot. (g/d) | Total Glom. | GS (% [n]) | SS (% [n]) | GH (% [n]) | Mes. Expan. | No. Examined | Perihilar Lesion | Tip Lesion | Cellular Lesion | Coll. Les. | Columbia Class. | Hyalin | Reabs. Gran. | Juxtamed. | IgA | IgG | IgM | C3 | C1q | Fibrinogen | Interstitial Fibrosis/Tubular Atrophy (%) | Tubular Distension/Cysts | Tubular Reabs. Gran. | Foam Cells | Interstitial Inflammation | Hyaline Casts | Vascular Sclerosis |
| FSX-233 | 23 | M | 16 | 0 | 6(1) | 13(2) | 1+ | 1 | 1/1 | 0/1 | 1 | 0 | Cellular | 0 | 0 | 1/1 | 0 | 0 | 0 | 0 | 0 | 15 | 1+ | 0 | 0 | 1+ | 0 | 0 | |||
| FSA-1622 | 26 | M | Proteinuria | 15 | 7(1) | 20(3) | 20(3) | 1+ | 3 | 1/1 | 1/1 | 0 | 0 | NOS | 1 | 2 | 2/3 | 1+ DSPM | 1+ DSPM | 1+ DSPM | 1+ DSPM | 1+ DSPM | 0 | 10 | 1+ | 0 | 0 | 1+ | 0 | 2+ | |
| FSX-17 | 33 | F | Normal | Proteinuria | 15 | 7(1) | 13(2) | 0 | 1+ | 2 | 2/2 | 0/2 | 0 | 0 | Perihilar | 2 | 1 | 1/2 | 2+ DGM | 0 | 0 | 0 | 2+ DGM | 30 | 0 | 0 | 0 | 2+ | 0 | 2+ | |
| FGCI-1111 | 20 | F | 8.9 | 39 | 8(3) | 16(6) | 8(3) | 1+ | 6 | 3/4 | 1/4 | 0 | 0 | Perihilar | 2 | 2 | 2/2 | 0 | 0 | 3+ FSPM | 3+ FSPM | 3+ FSPM | 1+ FSPM | 10 | 1+ | 1+ | 0 | 1+ | 1+ | 1+ | |
| FSXL-201 | 20 | M | 0.9 | 1.2 | 24 | 8(2) | 21(5) | 0 | 0 | 0b | 0 | 0 | 2+ FS | 2+ FS | 0 | 10 | 0 | 0 | 0 | 1+ | 0 | 0 | |||||||||
| FSX-18 | 29 | M | Normal | 2.5 | 39 | 12(5) | 3(1) | 0 | 0 | 1 | 1/1 | 0/1 | 0 | 0 | Perihilar | 1 | 0 | 0/0 | 1+ DSP | 0 | 0 | 0 | 1+ DSP | 10 | 0 | 0 | 0 | 1+ | 0 | 1+ | |
| FSA-192 | 32 | M | 3.2 | Proteinuria | 12 | 16(2) | 16(2) | 0 | 1+ | 0b | 30 | 0 | 0 | 0 | 1+ | 1+ | 0 | ||||||||||||||
| FSX-14 | 40 | M | Moderate | 52 | 19(10) | 2(1) | 2(1) | 1+ | 1 | 0/0 | 0/0 | 0 | 0 | NOS | 0 | 0 | 1/1 | 1+ DGM | 0 | 2+ FGPM | 2+ FGPM | 0 | 40 | 0 | 0 | 0 | 2+ | 1+ | 2+ | ||
| FSX-12 | 56 | F | Normal | Proteinuria | 25 | 20(5) | 8(2) | 0 | 1+ | 0b | 0 | 0 | 1+ FPM | 1+ FPM | 0 | 10 | 0 | 0 | 1+ | 1+ | 1+ | 2+ | |||||||||
| FSX-16 | 35 | F | Normal | 6.7 | 40 | 20(8) | 5(2) | 0 | 0 | 2 | 0/0 | 0/0 | 0 | 0 | NOS | 2 | 0 | 0/0 | 0 | 0 | 0 | 0 | 0 | 15 | 0 | 0 | 0 | 1+ | 0 | 1+ | |
| FGCI-111 | 29 | F | 4.8 | 6.6 | 40 | 53(21) | 15(6) | 20(8) | 2+ | 5 | 2/2 | 0/2 | 0 | 0 | NOS | 2 | 4 | 0/0 | 0 | 0 | 3+ FSPM | 3+ FSPM | 3+ FSPM | 0 | 50 | 2+ | 2+ | 0 | 2+ | 2+ | 0 |
| FGCI-1112 | 16 | F | Proteinuria | 19 | 74(14) | 16(3) | 10(2) | 2+ | 2 | 0/0 | 0/0 | 0 | 0 | NOS | 2 | 1 | 0/0 | 0 | 0 | 3+ DSPM | 3+ DSPM | 2+ FSPM | 3 + DSPM | 60 | 1+ | 2+ | 1+ | 2+ | 1+ | 0 | |
Patient data presented in order of increasing proportion of globally sclerosed glomeruli. SCr, serum creatinine concentration; Urine Prot., descriptive or quantitative assessment of urine protein at time of biopsy; Total Glom, total number of glomeruli in biopsy; GS, global sclerosis; SS, segmental sclerosis; GH, glomerular hypertrophy. Mesangial expansion (Mes. Expan.) graded on a scale of 0 (no involvement) to 4+ (severe involvement). Number of perihilar and tip lesions and lesions involving juxtamedullary glomeruli presented as fraction of number of glomeruli for which this could be determined on the basis of the presence of morphologic landmarks; other characteristics of segmentally sclerosing lesions expressed as total number exhibiting the described characteristic. Coll. Les., collapsing lesion. Columbia Class., Columbia FSGS classification system variant (see references1,2); Reabs. Gran., protein reabsorption granules in epithelial cells; Juxtamed., juxtamedullary glomeruli. Immunofluorescence findings derived from description in written pathology reports; findings graded on a scale of 0 (no involvement) to 4+ (severe involvement). Codes for immunofluorescence findings: D, diffuse; F, focal; G, global; S, segmental; P, peripheral capillary wall; M, mesangial. Interstitial fibrosis/tubular atrophy expressed as percentage of tissue involved; all other tubulointerstitial findings graded on a scale of 0 (no involvement) to 4+ (severe involvement). Missing entries indicate no data available.
No segmentally sclerosed glomeruli observed in specimen available for examination.
All of the biopsies from the patients with ACTN4 mutations showed the diagnostic glomerular lesion of segmental sclerosis (Table 1, Supplemental Figures 1 and 2). Other findings in the biopsies were also in keeping with a diagnosis of FSGS, including the lack of glomerular proliferative lesions, the presence in many cases of focal and segmental glomerular IgM and C3 deposition and mesangial expansion, and the extent of parenchymal atrophy and fibrosis in rough proportion to the number of sclerosed glomeruli in each biopsy (Table 1, Supplemental Figures 1 and 2). There was some variation among the biopsies in the extent of chronic damage and vascular sclerosis (Table 1). In almost every case in which the orientation of the involved glomerulus could be determined (10 of 11 glomeruli), glomeruli with segmental sclerosing lesions exhibited perihilar sclerosis, usually accompanied by hyalinosis (Table 1, Supplemental Figures 1 and 2). At least three of the 12 cases would be classified as “FSGS, perihilar variant” according to the Columbia classification system; one additional case (FGCI-111) approached but did not meet the formal criteria for this classification.2 With the exception of one case that would be classified as “FSGS, cellular variant” by virtue of one cellular lesion observed in the biopsy (FSX-233), the remaining cases would be characterized as “FSGS, not otherwise specified.”
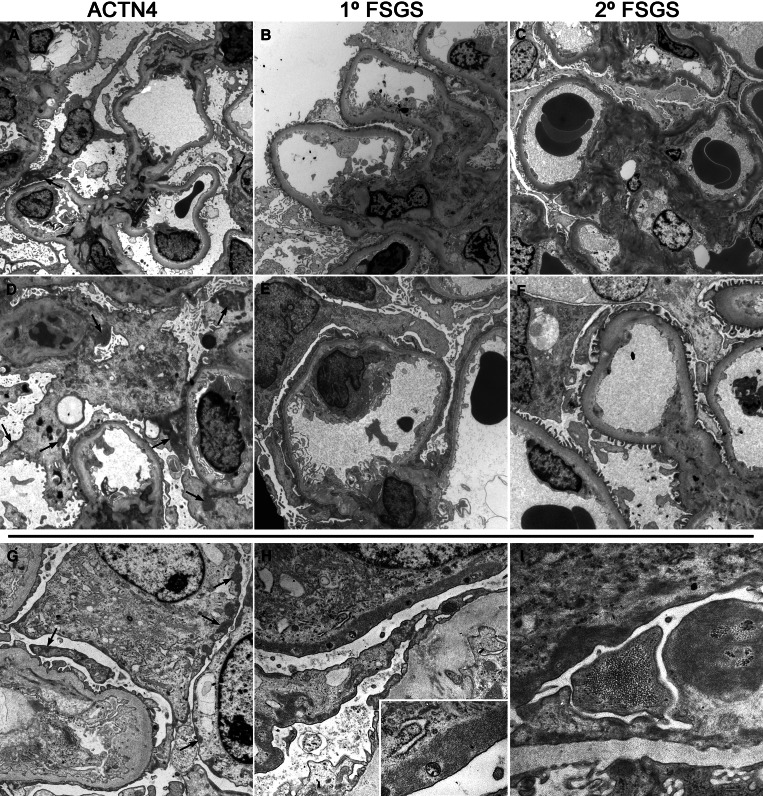
Electron microscopy material was available for five of the 12 biopsies from ACTN4 mutants. For comparison at the ultrastructural level, we also examined four biopsies each from patients with documented primary (“idiopathic”) FSGS and postadaptive FSGS (i.e., FSGS secondary to structural and functional adaptations). The biopsies from ACTN4 mutants all exhibited features of moderate podocyte injury, the extent of which was generally intermediate between that seen in the primary and postadaptive FSGS cases (Table 2, Figure 1). Varying degrees of foot process effacement and irregularity were present in all cases, as well as varying degrees of basal cytoskeletal condensation adjacent to the glomerular basement membrane (GBM; Table 2, Figure 1). Other features of podocyte injury including microvillous degeneration, vacuolization, and electron-dense lysosome accumulation were also seen (Table 2, Figure 1); however, these changes tended to be segmental in distribution in the postadaptive FSGS and ACTN4 mutant biopsies (scores of 1+ or 2+) and more diffuse in the primary FSGS biopsies (Table 2, Figure 1). The extent of foot process alteration was somewhat greater in the two ACTN4 mutant biopsies exhibiting the most chronic damage, FGCI-111 and FGCI-1112.
Table 2.
Glomerular ultrastructural findings in biopsies from patients with ACTN4 mutations, along with findings in biopsies exhibiting features of primary FSGS and postadaptive FSGSa
| ID No. | Podocytes |
GBM |
Endothelium |
Mesangium |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FP Effacement | FP Irreg. | FP Actin Condens. | μ-Villi | Vacuoles | ED AGG | GRAN | Thickening | Irreg. | DC | Subepi. EDD | Subendo. EDD | Swelling | Loss of Fenestrae | Matrix Expan. | Cellular Expan. | EDD | |
| FSA-1622 | 2+ | 2+ | 1+ | 2+ | 2+ | 1+ | 0 | 1+ | 1+ | 0 | 0 | 0 | 1+ | 1+ | 1+ | 1+ | 1+ |
| FGCI-1111 | 2+ | 2+ | 2+ | 1+ | 3+ | 3+ | 0 | 0 | 1+ | 1+ | 1+ | 1+ | 1+ | 1+ | 1+ | 1+ | 2+ |
| FSA-192 | 2+ | 2+ | 2+ | 2+ | 1+ | 1+ | 0 | 2+ | 1+ | 1+ | 0 | 0 | 1+ | 1+ | 2+ | 2+ | 1+ |
| FGCI-111 | 3+ | 1+ | 3+ | 2+ | 2+ | 3+ | 2+ | 0 | 1+ | 1+ | 0 | 0 | 1+ | 1+ | 1+ | 1+ | 1+ |
| FGCI-1112 | 3+ | 2+ | 2+ | 3+ | 2+ | 2+ | 0 | 1+ | 1+ | 2+ | 0 | 2+ | 2+ | 2+ | 1+ | 1+ | 1+ |
| 1FSGS-1 | 3+ | 3+ | 2+ | 3+ | 2+ | 0 | 0 | 1+ | 1+ | 2+ | 0 | 1+ | 2+ | 2+ | 3+b | 3+b | 0 |
| 1FSGS-2 | 3+ | 3+ | 2+ | 2+ | 1+ | 0 | 0 | 0 | 0 | 1+ | 0 | 0 | 1+ | 1+ | 2+ | 2+ | 0 |
| 1FSGS-3 | 2+ | 3+ | 1+ | 2+ | 2+ | 0 | 0 | 0 | 1+ | 1+ | 0 | 1+ | 1+ | 1+ | 2+ | 2+ | 2+ |
| 1FSGS-4 | 4+ | 4+ | 3+ | 3+ | 1+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1+ | 1+ | 0 |
| 2FSGS-1 | 1+ | 2+ | 2+ | 1+ | 0 | 0 | 0 | 0 | 0 | 1+ | 0 | 0 | 1+ | 1+ | 2+ | 2+ | 1+ |
| 2FSGS-2 | 1+ | 1+ | 1+ | 1+ | 1+ | 0 | 0 | 0 | 1+ | 1+ | 0 | 0 | 1+ | 1+ | 1+ | 1+ | 0 |
| 2FSGS-3 | 1+ | 1+ | 1+ | 1+ | 1+ | 0 | 0 | 1+ | 0 | 1+ | 0 | 0 | 2+ | 2+ | 1+ | 2+ | 0 |
| 2FSGS-4 | 2+ | 1+ | 1+ | 2+ | 2+ | 0 | 0 | 1+ | 2+ | 1+ | 0 | 0 | 2+ | 2+ | 2+ | 1+ | 0 |
All findings graded on a scale of 0 (no involvement) to 4+ (severe involvement). 1FSGS, primary FSGS; 2FSGS, postadaptive FSGS; FP, foot process; ED AGG, electron-dense intracytoplasmic aggregates; GRAN, intracytoplasmic electron-dense lysosomes (reabsorption granules); DC, double contours; EDD, electron-dense deposits.
No history of diabetes in this patient.
Figure 1.
Electron micrographs of kidney biopsies from patients with ACTN4 mutations and biopsies exhibiting features of primary and postadaptive FSGS. (A and D) Patients with ACTN4 mutations (ACTN4). There is extensive podocyte injury, including segmental but extensive foot process effacement and morphologic irregularity, microvillous degeneration and cytoplasmic vacuolization, and intracytoplasmic cytoskeletal condensation adjacent to the GBM. Large aggregates of electron-dense material are present in the podocyte cytoplasm (arrows), associated with the cell membrane of the podocyte cell bodies and major processes, occasionally extending into major processes from areas of foot process effacement and cytoskeletal matting. The GBM is segmentally thickened and shows segmental subendothelial redundancy (double contours), associated with loss of endothelial fenestrations. An electron-dense lysosome and an occluded capillary loop are also noted in D. (B and E) Patients with primary FSGS (1° FSGS). There is severe podocyte injury, including diffuse foot process effacement with intracytoplasmic cytoskeletal condensation adjacent to the GBM, an increase in cytoplasmic organelles, and microvillous degeneration. Segmental areas of endothelial swelling are noted. The mesangium is mildly expanded, without electron-dense deposits. Although the basal cytoskeletal filament condensation in podocytes is prominent, electron-dense cytoplasmic aggregated material is not seen elsewhere in the podocyte cytoplasm. (C and F) Patients with postadaptive FSGS (2° FSGS). There are few segmental areas of podocyte foot process effacement and irregularity. The GBM shows segmental areas of wrinkling and redundancy (double contours). No electron-dense cytoplasmic aggregated material is noted in podocyte cytoplasm. (G through I) High-magnification electron micrographs from ACTN4 mutant biopsies showing intracytoplasmic electron-dense aggregated material within podocytes (arrows), including podocyte cell bodies (G and H), and major processes. In one case, short filamentous substructure is apparent in the material associated with the cell body (H, inset). In another case, the aggregated material exhibits a fine granular substructure with intervening accumulations of loosely aggregated coarse granular material (I). Magnifications: ×1600 in A through C; ×2400 in D through F; ×4000 in G; ×7200 in H; ×14,400 in H inset; ×12,000 In I.
Changes to the GBM were noted in almost all cases regardless of the disease etiology and included thickening and irregularity of the lamina densa and focal double-contour formation (Table 2, Figure 1). Endothelial cells exhibited mild features of injury in almost all cases, including segmental swelling and loss of fenestrations. All biopsies examined ultrastructurally revealed mild to moderate expansion of the mesangium by matrix and cells, although scattered small and ill-defined electron-dense deposits were more commonly seen in the mesangium of the ACTN4 mutant biopsies (Table 2, Figure 1).
A distinctive ultrastructural feature of podocyte injury seen in all five of the ACTN4 biopsies, not seen in any of the primary or postadaptive FSGS biopsies, consisted of irregularly aggregated electron-dense material in the podocyte cytoplasm (Table 2, Figure 1). The aggregated material was seen more prominently in the biopsies from family FGCI, a family who also exhibited earlier disease onset. This material was usually seen in podocyte major processes or cell bodies and was almost always associated with the cell membrane (Figure 1). These cytoplasmic aggregates were also occasionally noted to be more closely associated with the GBM in areas of foot process effacement, distinguishable from basal condensations of cytoskeletal filaments by their large size and irregular shape (Figure 1). Similar material was not clearly observed in other cell types in the kidney, including endothelial, mesangial, tubular epithelial, and interstitial cells. The aggregates, ranging from <500 nm up to 5 μm in greatest dimension, exhibited a fine granular to short filamentous substructure, interspersed with occasional accumulations of loose, coarsely granular material (Figure 1).
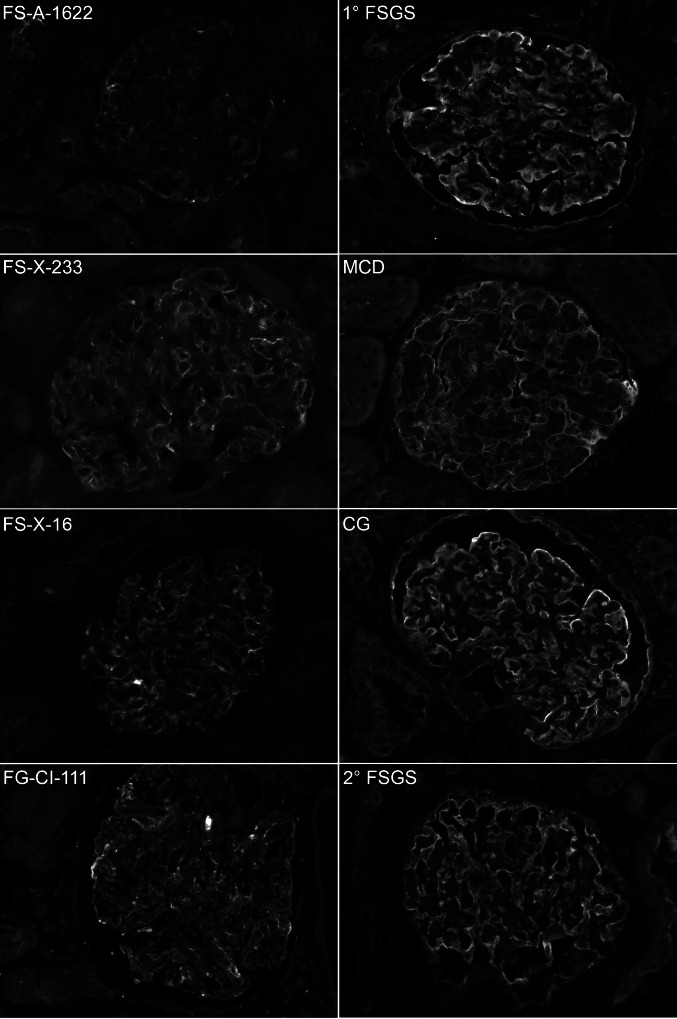
Indirect immunofluorescence was performed on a subset of biopsies from patients with ACTN4 mutations for which material was available, using antibodies against a conserved domain of α-actinin-4 (present in both wild-type and mutant proteins). For comparison, staining was also performed on a set of biopsies from patients with primary FSGS, postadaptive FSGS, minimal-change disease, and collapsing glomerulopathy. Indirect immunofluorescence for α-actinin-4 revealed an irregular and occasionally coarse granular or clumped distribution of α-actinin-4 in glomerular capillary walls of patients with ACTN4 mutations (Figure 2). Biopsies from patients with primary FSGS, postadaptive FSGS, minimal-change disease, and collapsing glomerulopathy did not show this pattern of staining but instead showed a smooth, continuous linear staining pattern in the glomerular capillary walls (Figure 2).
Figure 2.
Epifluorescence microscopy of kidney biopsies from patients who had ACTN4 mutations and from patients who had other glomerular diseases affecting the podocyte. Biopsies were treated with antibodies against a conserved epitope of α-actinin-4 protein and FITC-conjugated secondary antibody. Biopsies from patients with mutations in ACTN4 (left) show segmental weak linear and irregular granular staining in the glomerular peripheral capillary wall. Biopsies from patients with other diseases affecting the podocyte (right) show uniform linear staining in the glomerular peripheral capillary wall. MCD, minimal-change disease; CG, collapsing glomerulopathy. Magnification, ×300.
The biopsies from patients with ACTN4 mutations examined here all revealed light microscopic features that are characteristic of FSGS; however, there was a considerable degree of morphologic heterogeneity at the light microscopic level. Some of this heterogeneity was associated with the extent of chronic damage present in each sample, with extent of glomerulosclerosis ranging from 6 to 90% of glomeruli and extent of tubular atrophy and interstitial fibrosis ranging from 10 to 60% involvement of the sample. There was also a variety of morphologic subtypes of segmental sclerosing lesions noted, leading to a corresponding variety of classifications represented, according to the Columbia University classification scheme (see Table 1, Supplemental Figures S1 and S2). Such morphologic heterogeneity and/or lack of specificity, particularly with regard to glomerular lesions, has been observed in other studies of familial FSGS.3,7–10 It is not clear why such lesional heterogeneity occurs, although it is plausible that either genetic modifiers or environmental factors may play a role. The presence of such morphologic heterogeneity, seen across the spectrum of familial FSGS, underscores the need for more specific indicators that may be used on a routine basis.
It is auspicious, then, that the ACTN4 mutant biopsies did demonstrate unique morphologic features seen by electron microscopy and immunofluorescence microscopy for α-actinin-4 protein, which may be of diagnostic relevance and which support our current understanding of the mechanism of glomerular injury in these patients. One of these features is the presence of cytoplasmic electron-dense aggregates in podocytes. Strikingly similar aggregated electron-dense material is seen in podocytes of mouse models that express mutant Actn4.5,11 In mice, this aggregated material has been demonstrated to be composed, at least in part, of actin and (mutant) α-actinin-4. The significance of this material to podocyte function or dysfunction is not clear. One possibility stems from recent evidence that mutant α-actinin-4 exhibits increased actin-binding affinity.4,12 It is conceivable that altered α-actinin-4 binding affinity for actin could lead to a shift in equilibrium between bound and unbound actin, such that bound actin accumulates (perhaps slowly) in the podocyte cytoplasm. Over time, this material may accumulate to form the ultrastructurally visible aggregates, which ultimately contribute to podocyte damage or increased susceptibility to injury through interference with normal structure and function. Features of actin organization that are specific to the podocyte may predispose this cell type to the aggregation and damage. This possibility could help to explain the tendency for aggregated material to be more pronounced in family FGCI, because this could reflect a difference in the binding properties of the distinct mutant forms of α-actinin-4 present in specific families.13 Alternatively, the aggregated material may simply be a variably occurring by-product of the molecular processes that ultimately result in podocyte injury and that happens to be detectable at the ultrastructural level. This possibility could reconcile the variable occurrence of these aggregates in the material examined here.
The other distinctive characteristic of the ACTN4 mutant biopsies is the irregular granular staining pattern of α-actinin-4 in the glomerular capillary walls observed by indirect immunofluorescence. This finding seems to be uniquely associated with the presence of mutant α-actinin-4, because a uniform linear pattern was seen in the glomeruli in other diseases affecting the podocyte. This irregular staining pattern may suggest cytoskeletal disorganization that is present even in intact glomeruli. The scattered granular structures may represent the correlate at the light microscopic level of the aggregated cytoplasmic material seen ultrastructurally. Although α-actinin-4 immunostaining is not performed as part of a routine kidney biopsy workup, this finding suggests that it may be diagnostically useful in selected cases.
As discussed, our findings are consistent with our current understanding of the mechanism of ACTN4-mediated kidney disease; however, one particular feature of the ACTN4 mutant biopsies may present new insights into this disease process. Several of the ACTN4 mutant biopsies exhibited perihilar segmental sclerosing lesions, including three biopsies that would be classified as “FSGS, perihilar variant” according to the Columbia classification system (see Table 1 and Supplemental Figures S1 and S2). The perihilar lesion, characterized by hyalinosis and sclerosis involving the vascular pole, may be seen in primary or “idiopathic” forms of FSGS and is not etiologically specific; however, this lesion is a common feature of postadaptive glomerulosclerosis, which develops as a consequence of adaptive responses to conditions that are known to increase hemodynamic stress on the glomerulus, such as obesity or a reduction in renal mass.1 In these instances, FSGS is accompanied by glomerular hypertrophy (“glomerulomegaly”). The pathogenesis of postadaptive glomerulosclerosis is thought to be initiated by damage to the podocyte occurring when glomerular capillary pressures become elevated. Such a mechanism suggests mechanical damage to the podocyte, perhaps through excessive stretch or tension.14,15 In several patients with ACTN4 mutations, perihilar lesions were observed to occur without a significant reduction in functional mass. Furthermore, glomerular hypertrophy was not uniformly seen in the biopsies from ACTN4 mutants (Table 1). This might suggest that the podocytes of ACTN4 mutants are less “mechanically robust” and perhaps over time develop injury in a similar manner to that seen in postadaptive glomerulosclerosis but without the requisite increase in glomerular capillary pressures and compensatory glomerular hypertrophy. In other words, even normal glomerular capillary pressures may be sufficient to cause podocyte damage in ACTN4 mutants.
In conclusion, we have presented a detailed description of pathologic findings in a set of biopsies from patients who were found to carry disease-associated mutations in ACTN4. Our study reveals in these biopsies morphologic findings that seem to be unique to ACTN4-mediated disease, including electron-dense cytoplasmic aggregates in podocytes, and an irregular immunostaining pattern for α-actinin-4. The unique morphologic features seen in these biopsies may be of diagnostic value for identifying patients and families who harbor ACTN4 mutations. In addition, these findings resemble features observed in mouse models and shed new light on the pathogenesis of kidney disease associated with mutations in the ACTN4 gene. Thus, this rare but increasingly understood etiologic form of FSGS may serve as a prototype in the effort to unify the morphologic appearance of FSGS with disease etiology. This and similar studies will ultimately contribute to a refined approach to the treatment and determination of prognosis of the many etiologic forms of FSGS.
CONCISE METHODS
Material Reviewed
Biopsy materials, including stained and unstained light microscopic slides, Kodochrome slides, paraffin- and epoxy-embedded tissue, prepared electron microscopy grids, electron micrographs, and corresponding pathology reports, were obtained retrospectively from the case files at Brigham and Women's Hospital, as well as from outside institutions, according to Brigham and Women's Hospital institutional review board guidelines.
Light Microscopy and Routine Immunofluorescence
Light microscopic specimens from 12 patients were available for examination but varied among the 12 cases. In four cases, the available material was less extensive than that originally evaluated to render a pathologic diagnosis (e.g., “recuts” or exhausted paraffin blocks were provided). In these instances, information provided in the original pathology reports supplemented the data obtained through our review of the provided light microscopic material. When paraffin-embedded tissue was available, additional 4-μm sections were cut and stained with hematoxylin and eosin, periodic acid-Schiff, Jones's methenamine silver, and trichrome stains. All histochemically stained tissue sections on all available microscopic slides, regardless of the particular stain used, were examined and evaluated to generate a standard histopathologic description of the kidney biopsy. The description of glomerular histopathology included total number of glomeruli in one microscopic section, type and number of glomerular lesions (including global sclerosis, segmental sclerosis, and glomerular hypertrophy), and presence of mesangial expansion (graded 0 to 4+). All segmental sclerosing lesions were characterized in detail, including determination of the segmental location of the lesion in the glomerular tuft, and assignment of morphologic subtype (e.g., perihilar, tip, cellular, collapsing). The presence of perihilar and tip lesions, as well as identification of juxtamedullary glomeruli, was reported only when appropriate anatomic landmarks were present. The proportion of kidney parenchyma involved by tubular atrophy and interstitial fibrosis was estimated as a percentage of the parenchyma involved. Other tubulointerstitial and vascular histopathologic findings were graded on a scale from 0 to 4+ and included the presence of tubular distension and microcysts, tubular epithelial protein reabsorption granules, interstitial foam cells, interstitial inflammation, hyaline casts, and arterial or arteriolar sclerosis. Although immunofluorescence studies were performed for 11 of 12 of the biopsies as part of the original pathologic workup, none of the original immunofluorescence slides was available for review; therefore the description of immunofluorescence findings in the original pathology reports was used to obtain the data presented here.
Electron Microscopy
Epoxy-embedded tissue blocks originally prepared for ultrastructural examination were available from three biopsies, all from family FGCI. In these cases, semithin sections were cut from these blocks at 1 μm and stained with toluidine blue for light microscopic examination. Subsequently, ultrathin sections were cut from selected blocks at 80 nm, mounted on 200 mesh copper grids, treated with uranyl acetate and lead citrate, and examined in a JEOL 1010 transmission electron microscope (Tokyo, Japan) to obtain representative electron micrographic images of kidney ultrastructure. Previously collected electron micrographic prints were available from two additional biopsies from family FSA. The other two families represented in this study (FSX and FSXL) reside outside the United States in regions where electron microscopy is not routinely performed; therefore, no electron microscopy material was available from these two families.
For each biopsy, scores were assigned from 0 (no involvement) to 4+ (severe involvement) for four categories of ultrastructural changes indicative of glomerular injury: (1) Abnormalities of the podocyte, including foot process effacement, morphologic irregularity of foot process cross-sections, intracytoplasmic actin condensation at the basal foot processes in areas of effacement, microvillous degeneration, vacuolization of the cell body, the presence of electron-dense intracytoplasmic aggregates, and increased numbers of electron-dense lysosomes (protein reabsorption granules) and other cytoplasmic organelles; (2) abnormalities of the GBM, including GBM thickening, irregularities of GBM contour, subendothelial basement membrane redundancy (“double contouring”), subepithelial electron-dense deposits, and subendothelial electron-dense deposits; (3) abnormalities of the endothelium, including cell swelling and loss of fenestrations; and (4) abnormalities of the mesangium, including matrix expansion and increase, cellular expansion and increase, and electron-dense deposits.
Indirect Immunofluorescence for α-Actinin-4
Unstained sections of formalin-fixed, paraffin-embedded material available from biopsies of four patients with ACTN4 mutations, as well as from biopsies of four patients with other forms of glomerular disease involving the podocyte, were used for indirect immunofluorescence for α-actinin-4. Sections were initially processed for antigen retrieval, then treated with antibodies against α-actinin-4 (rabbit polyclonal; gift of Dr. Alan Beggs, Children's Hospital, Boston, MA), followed by a FITC-conjugated secondary antibody. Stained sections were evaluated and photomicrographed using epifluorescence microscopy.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health research grant R01DK054931 (to M.P.); National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Career Development Award K08DK073091 (to J.H.); and an International Society of Nephrology Fellowship (to M.A.).
We thank Dr. Mark Haas, Stephanie Herbert, Andi Ramirez, Dr. José Carlos Rodriguéz Pérez, Andrea Uscinski, and Dr. Ivàn Villegas for assistance in obtaining the biopsy materials reviewed for this study. We also thank A. Shahsafaei (Department of Pathology, Brigham and Women's Hospital) for technical assistance with indirect immunofluorescence studies, Dr. A. Flores-Vargas for assistance with translation of pathology reports, Dr. A. Beggs (Children's Hospital, Boston, MA) for the gift of anti–α-actinin-4 antibodies, and Dr. H. Rennke (Brigham and Women's Hospital) for helpful advice and encouragement.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.D'Agati V: Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol 23 :117 –134,2003 [DOI] [PubMed] [Google Scholar]
- 2.D'Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43 :368 –382,2004 [DOI] [PubMed] [Google Scholar]
- 3.Barisoni L, Schnaper HW, Kopp JB: A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2 :529 –542,2007 [DOI] [PubMed] [Google Scholar]
- 4.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodríguez-Pérez JC, Allen PG, Beggs AH, Pollak MR: Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24 :251 –256,2000 [DOI] [PubMed] [Google Scholar]
- 5.Yao J, Le TC, Kos CH, Henderson JM, Allen PG, Denker BM, Pollak MR: α-Actinin-4-mediated FSGS: An inherited kidney disease caused by an aggregated and rapidly degraded cytoskeletal protein. PLoS Biol 2 :787 –794,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Möller CC, Pollak MR, Reiser J: The genetic basis of human glomerular disease. Adv Chronic Kidney Dis 13 :166 –173,2006 [DOI] [PubMed] [Google Scholar]
- 7.Conlon PJ, Butterly D, Albers F, Rodby R, Gunnells JC, Howell DN: Clinical and pathologic features of familial focal segmental glomerulosclerosis. Am J Kidney Dis 26 :34 –40,1995 [DOI] [PubMed] [Google Scholar]
- 8.Vats A, Nayak A, Ellis D, Randhawa PS, Finegold DN, Levinson KL, Ferrell RE: Familial nephrotic syndrome: Clinical spectrum and linkage to chromosome 19q13. Kidney Int 57 :875 –881,2000 [DOI] [PubMed] [Google Scholar]
- 9.Rana K, Isbel N, Buzza M, Dagher H, Henning P, Kainer G, Savige J: Clinical, histopathologic, and genetic studies in nine families with focal segmental glomerulosclerosis. Am J Kidney Dis 41 :1170 –1178,2003 [DOI] [PubMed] [Google Scholar]
- 10.Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, Zalewski I, Imm A, Ruf EM, Mucha B, Bagga A, Neuhaus T, Fuchshuber A, Bakkaloglu A, Hildebrandt F: Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol 15 :722 –732,2004 [DOI] [PubMed] [Google Scholar]
- 11.Henderson JM, Al-Waheeb S, Weins A, Dandapani SV, Pollak MR: Mice with altered α-actinin-4 expression have distinct morphologic patterns of glomerular disease. Kidney Int 73 :741 –750,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weins A, Schlondorff JS, Nakamura F, Denker BM, Hartwig JH, Stossel TP, Pollak MR: Disease-associated mutant α-actinin-4 reveals a mechanism for regulating its F-actin-binding affinity. Proc Natl Acad Sci USA 104 :16080 –16085,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weins A, Kenlan P, Herbert S, Le TC, Villegas I, Kaplan BS, Appel GB, Pollak MR: Mutational and biological analysis of α-actinin-4 in focal segmental glomerulosclerosis. J Am Soc Nephrol 16 :3694 –3701,2005 [DOI] [PubMed] [Google Scholar]
- 14.Kriz W, Elger M, Nagata M, Kretzler M, Uiker S, Koeppen-Hageman I, Tenschert S, Lemley KV: The role of podocytes in the development of glomerular sclerosis. Kidney Int 45 :S64 –S72,1994 [PubMed] [Google Scholar]
- 15.Rennke HG: How does glomerular epithelial cell injury contribute to progressive glomerular damage? Kidney Int 45 :S58 –S63,1994 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.