Abstract
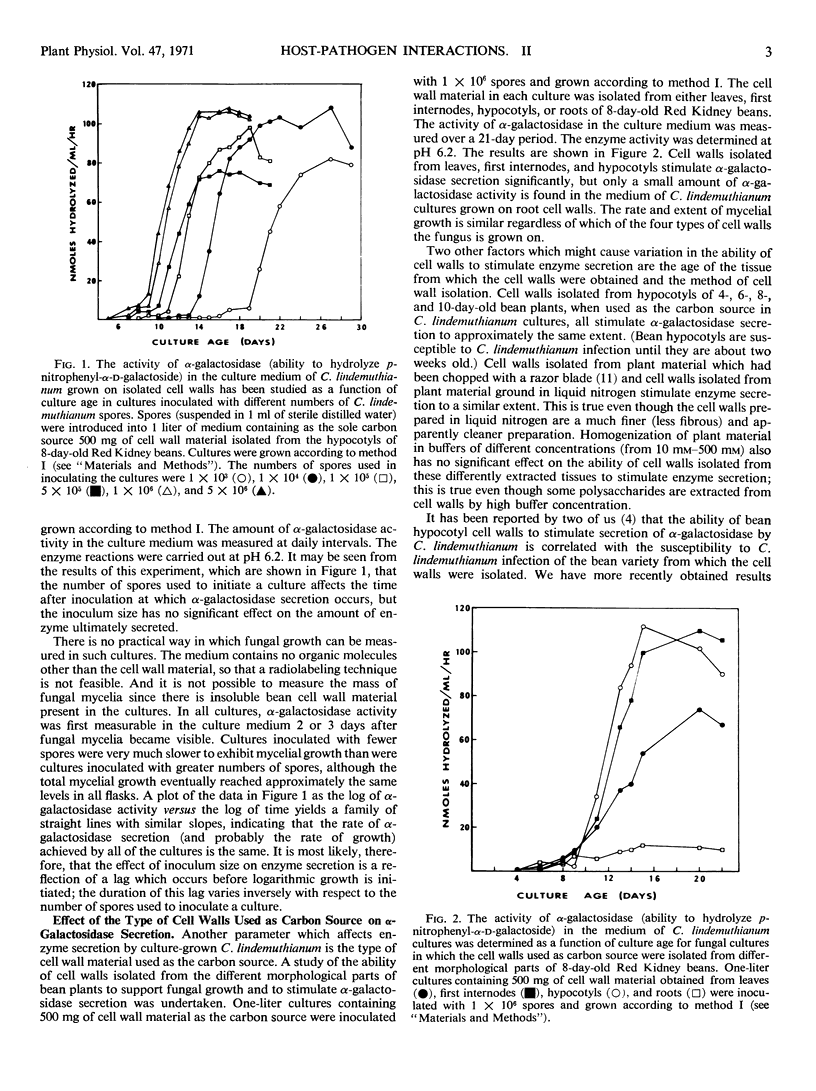
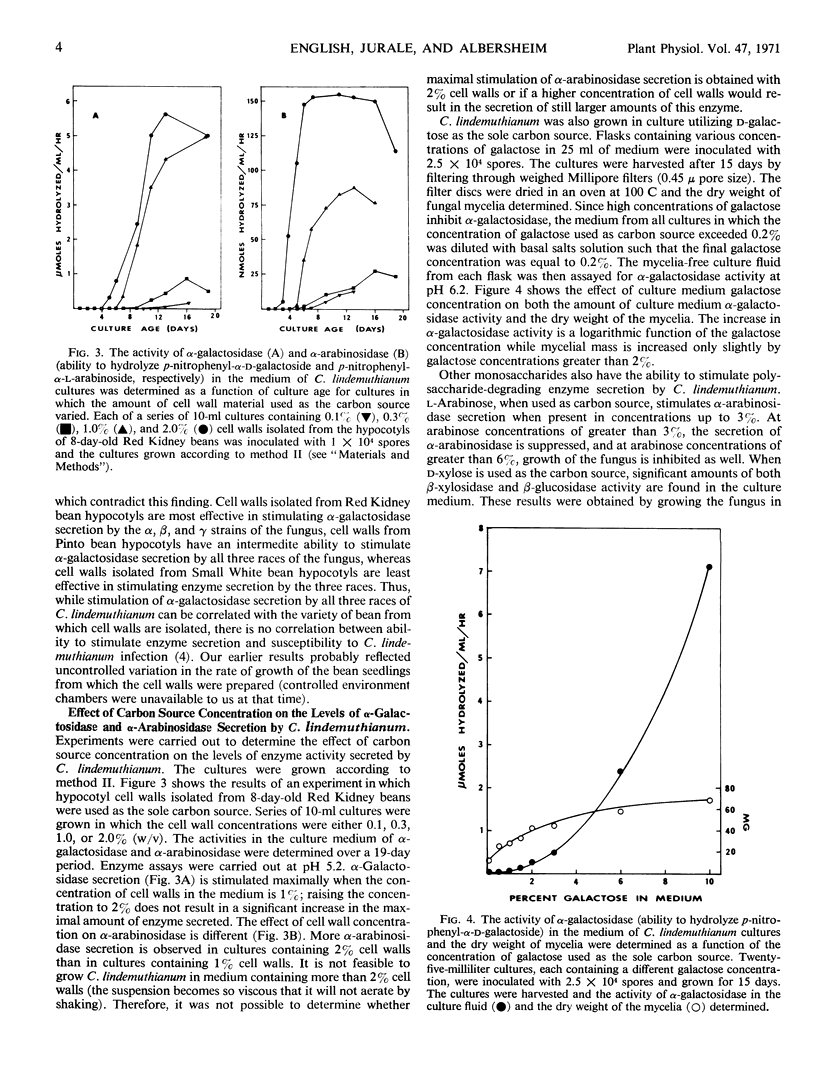
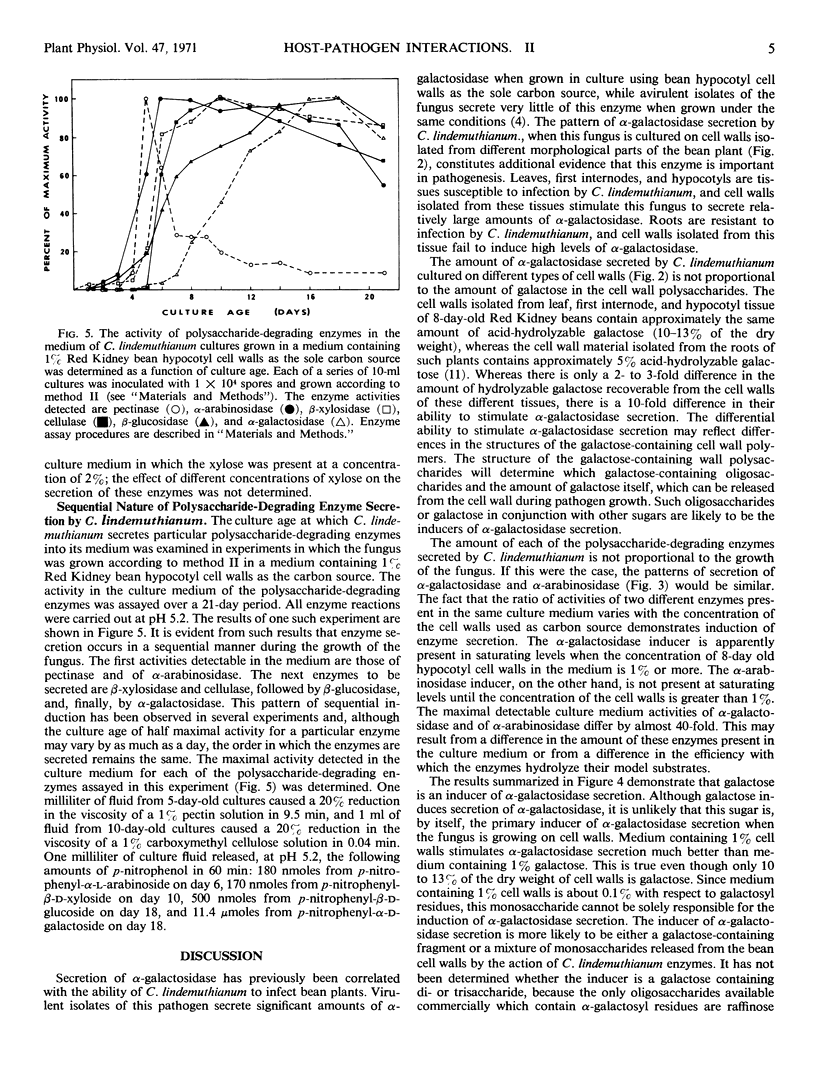
The effect of a number of physiological variables on the secretion of polysaccharide-degrading enzymes by culture-grown Colletotrichum lindemuthianum (Saccardo and Magnus) Scribner was determined. The number of spores used to inoculate cultures grown on isolated bean hypocotyl cell walls affects the time after inoculation at which enzyme secretion occurs, but has no significant effect on the maximal amount of enzyme ultimately secreted. Cell walls isolated from bean leaves, first internodes, or hypocotyls (susceptible to C. lindemuthianum infection), when used as carbon source for C. lindemuthianum growth, stimulate the fungus to secrete more α-galactosidase than do cell walls isolated from roots (resistant to infection). The concentration of carbon source used for fungal growth determines the final level of enzyme activity in the culture fluid. The level of enzyme secretion is not proportional to fungal growth; rather, enzyme secretion is induced. Maximal α-galactosidase activity in the culture medium is found when the concentration of cell walls used as carbon source is 1% or greater. A higher concentration of cell walls is necessary for maximal α-arabinosidase activity. Galactose, when used as the carbon source, stimulates α-galactosidase secretion but, at comparable concentrations, is less effective in doing so than are cell walls. Polysaccharide-degrading enzymes are secreted by C. lindemuthianum at different times during growth of the pathogen on isolated cell walls. Pectinase and α-arabinosidase are secreted first, followed by β-xylosidase and cellulase, then β-glucosidase, and, finally, α-galactosidase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almin K. E., Eriksson K. E. Enzymic degradation of polymers. I. Viscometric method for the determination of enzymic activity. Biochim Biophys Acta. 1967 Jul 11;139(2):238–247. doi: 10.1016/0005-2744(67)90028-9. [DOI] [PubMed] [Google Scholar]
- BARKER S. A., PARDOE G. I., STACEY M., HOPTON J. W. Sequential enzyme induction a new approach to the structure of complex mucoproteins. Nature. 1963 Jan 19;197:231–233. doi: 10.1038/197231a0. [DOI] [PubMed] [Google Scholar]
- English P. D., Albersheim P. Host-Pathogen Interactions: I. A Correlation Between alpha-Galactosidase Production and Virulence. Plant Physiol. 1969 Feb;44(2):217–224. doi: 10.1104/pp.44.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S. I., Lin E. C. Product induction of glycerol kinase in Escherichia coli. J Mol Biol. 1965 Dec;14(2):515–521. doi: 10.1016/s0022-2836(65)80200-5. [DOI] [PubMed] [Google Scholar]
- Karr A. L., Albersheim P. Polysaccharide-degrading Enzymes are Unable to Attack Plant Cell Walls without Prior Action by a "Wall-modifying Enzyme". Plant Physiol. 1970 Jul;46(1):69–80. doi: 10.1104/pp.46.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHLIS L. Effect of certain organic acids on the utilization of mannose and fructose by the filamentous watermold, Allomyces macrogynus. J Bacteriol. 1957 May;73(5):627–631. doi: 10.1128/jb.73.5.627-631.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins D. J., English P. D., Albersheim P. Changes in cell wall polysaccharides associated with growth. Plant Physiol. 1968 Jun;43(6):914–922. doi: 10.1104/pp.43.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins D. J., English P. D., Albersheim P. The specific nature of plant cell wall polysaccharides. Plant Physiol. 1967 Jul;42(7):900–906. doi: 10.1104/pp.42.7.900. [DOI] [PMC free article] [PubMed] [Google Scholar]



