Abstract
Three simple, economical, precise, and accurate methods are described for the simultaneous determination of Tenofovir disoproxil fumarate (TE) and Emtricitabine (EM) in combined tablet dosage form. The first method is ratio derivative spectra, second is first-order derivative spectrophotometry and third is absorption corrected method. The amplitudes at 271.07 and 302.17 nm in the ratio derivative method, 224.38 and 306.88 nm in the first order derivative method were selected to determine Tenofovir disoproxil fumarate (TE) and Emtricitabine (EM), respectively, in combined formulation. Beer's law is obeyed in the concentration range of 3-21 μg/ml for TE and 2-14 μg/ml for EM for first two methods and range for third method was 6-30 μg/ml of TE and 4-20 μg/ml of EM. The percent assay for commercial formulation was found to be in the range 98.91%–101.72% for both the analytes by the proposed three methods. Absorption corrected method was successfully applied to carry out dissolution study of commercial tablet formulation by using USP II dissolution test apparatus. The methods were validated with respect to linearity, precision, and accuracy. Recoveries by proposed methods were found in the range of 99.06 %-101.34 % for both the analytes.
Keywords: Absorbance corrected, derivative spectrophotometry, Emtricitabine, ratio derivative spectrophotometry, tablet dosage form, Tenofovir disoproxil fumarate
INTRODUCTION
Tenofovir (TE);9[(R)2[[bis[[(isopropoxycarbonyl)oxy]methoxy]phosphinyl]methoxy] propyl and Emtricitabine (EM); 5-fluro-1-(2R, 5S)-[2-9hydroxymethyl]-1,3-oxathiolan-5-yl both are the antiviral agents, acts as the nucleoside reverse transcriptase enzyme inhibitors. These are the nucleoside analogues which are phosphorylated by host cell enzyme to give 5-triphosphate derivative. This moiety competes with the equivalent host cellular triphosphate substrates for pro viral DNA synthesis by viral reverse transcriptase which is viral RNA-dependent DNA polymerase. Eventually, the incorporation of the 5-triphosphate moiety into the growing viral DNA chain results in chain termination. Mammalian α--DNA polymerase is relatively resistant to the effect.
Emtricitabine is potent and selective against HIV types I and II and hepatitis B virus. Tenofovir is active against a variety of drug resistant HIV-I strains. Recently, the combination of TE and EM has demonstrated significantly greater HIV RNA suppression compared to the combination of zidovudine and lamivudine.[1–2]
Several analytical methods that have been reported for the individual determination of TE in biological fluids and pharmaceutical formulations which include liquid chromatography coupled with spectrofluorimetric, UV, and mass spectroscopy detection.[3–8] For EM several analytical methods have been reported for its individual analysis which includes chiral liquid chromatography, liquid chromatography with UV detection[1,8–11] Few bioanalytical methods are reported for combination of TE and EM which includes liquid chromatography with PDA and UV detection.[12] There are also few spectrophotometric methods reported in the literature for the simultaneous estimation of EM and TE in combined dosage form but no derivative spectroscopic, ratio derivative and simple absorbance corrected methods were reported.[13,14] This derivative spectroscopic technique offers various advantages over the conventional absorbance methods such as the discrimination of the sharp spectral features over the large bands and the enhancement of the resolution of overlapping spectra. Therefore, the aim of this study was to develop and validate ratio derivative, first derivative spectroscopic methods, and simple absorbance corrected method for the determination of TE and EM in tablet dosage form. The proposed methods were optimized and validated as per the International conference on harmonization (ICH) guidelines.[15]
MATERIALS AND METHODS
Instrumentation
An UV-visible double beam spectrophotometer (Varian Cary 100) with 10 mm matched quartz cells was used. All weighing were done on electronic balance (Model Shimadzu AUW-220D).
Reagents and chemicals
Pure drug sample of TE, %purity 99.86 and EM, % purity 99.92 was kindly supplied as a gift sample by Emcure Pharmaceutical Pvt. Ltd. Pune. These samples were used without further purification. Tablet used for analysis was TENVIR-EM (Batch No. X81241) manufactured by Cipla Ltd. Goa, India containing TE 300 mg and EM 200 mg per tablet.
Method A: Ratio derivative
The method involves dividing the spectrum of mixture by the standardized spectra of each of the analyte and deriving the ratio to obtain spectrum that is dependent of concentration of analyte used as a divisor. Using appropriate dilutions of standard stock solution, the two solutions were scanned separately. The ratio spectra of different TE standards at increasing concentrations were obtained by dividing each with the stored spectrum of the standard solution of EM (8 μg/ml) as shown in [Figure 1a] and the first derivative of these spectra traced, are illustrated in [Figure 1b]. Wavelength 271.07 nm was selected for the quantification of TE in TE + EM mixture. The ratio and ratio derivative spectra of the solutions of EM at different concentrations were obtained by dividing each with the stored standard spectrum of the TE (12 μg/ ml) [Figure 2a and b, respectively]. Wavelength 302.17 nm was selected for the quantification of EM in TE + EM mixture. Measured analytical signals at these wavelengths were proportional to the concentrations of the drugs. Calibration curves were prepared from the measured signals at the selected wavelength and concentration of the standard solutions. The amount of TE and EM in tablets was calculated by using following equations.[16]
Figure 1.
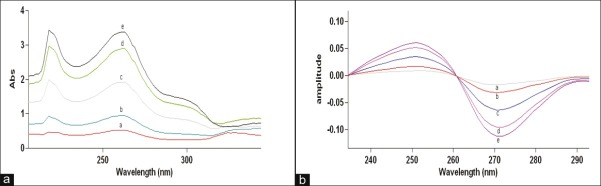
Ratio spectra (a) and first derivative of the ratio spectra (b) of (a) 3, (b) 6, (c) 12, (d) 18, and (e) 21 μg/ml solution of TE when 8 μg/ ml solution of EM is used as divisor.
Figure 2.
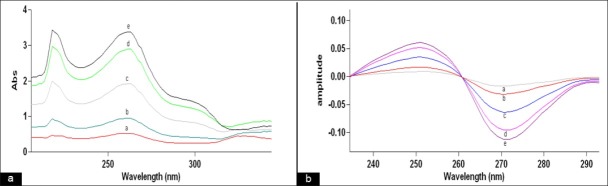
Ratio spectra (a) and first order derivative of the ratio spectra (b) of (a) 2, (b) 4, (c) 8, (d) 12, and (e) 14 μg/ml solution of EM when 12 μg/ml solution of TE is used as divisor.
At 271.07 nm: CTE = d/dλ [ATE/AEM] – Intercept (C)/ Slope (m), (1)
At 302.17 nm: CEM = d/dλ [AEM/ATE] – Intercept (C) / Slope (m). (2)
Method B: Derivative method
The method involves obtaining the first derivative spectrum of the series of the solution of mixtures of TE + EM in ascending and descending concentration. From the observations of the derivative spectrum, derivative amplitudes responsible for TE and EM were selected and wavelength for each amplitude was noted. These wavelengths were further confirmed by checking the first order derivative amplitude of the mixed standard solutions of these drugs in the given ratio. Mixed standard solutions were prepared in the range of 3-21 TE (and 2-14 μg/ml for EM) were used for the study. Wavelengths 306.88 and 224.38 nm were selected for the quantification of TE in TE + EM mixture and EM in TE + EM mixture, respectively [Figure 3].[17]
Figure 3.
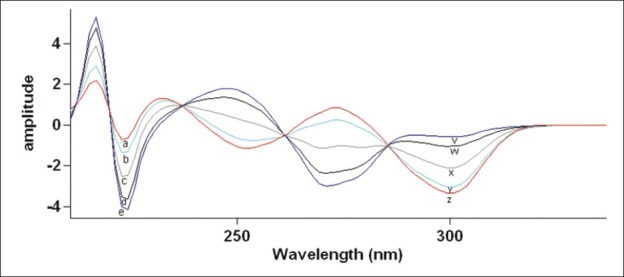
First order derivative spectra of (a) 3, (b) 6, (c) 12 (d) 18, and (e) 21 μg/ml solution of TE and (v) 2, (w) 4, (x) 8, (y) 12 and (z) 14 μg/ml solution of EM in methanol.
Method C: Absorption corrected method
The value of λmax of EM and TE was determined by scanning the drug solution in the range 200- 400 nm at 0.5 band width and 600 nm/min scan speed and was found to be at 293.38 and 270.29 nm, respectively. EM also showed absorbance at 270.29 nm, while TE did not show any interference at 293.38 nm [Figure 4]. To construct Beer's plot for EM and TE, stock solutions of 1000 μg/ml of both the drugs were prepared in 0.1N HCl and working standard dilutions were made in 0.1N HCl using stock solution of 1000 μg/ml. Also Beer's plot was constructed for EM and TE in solution mixture at different concentration (4:6, 8:12, 12:18, 16:24, 20:30 μg/ml) levels. Both the drugs followed linearity individually and in mixture within the concentration range 4-20 μg/ml and 6-30 μg/ml for EM and TE, respectively.
Figure 4.
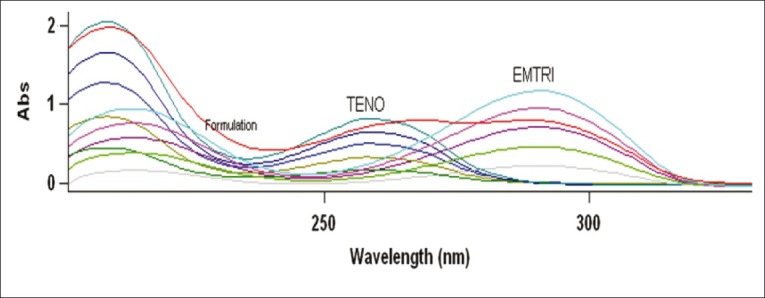
Simple overlay spectra of EM (4-20 μg/ml) and TE (6-30 μg/ml) in 0.1N HCl with formulation (12 + 18 μg/ml) of EM and TE, respectively.
Determination of absorption factor at selected wavelengths
EM and TE solution in 0.1N HCl of known concentrations were scanned against blank on spectrophotometer. The value of absorption factor was found to be 0.52. Quantitative estimation of EM and TE was carried out using following equations:
Corrected Absorbance of TE at 270.29 nm = Abs270.29 (TE + EM) – [(abs270.29 (EM)/ abs293.38 (EM)] × abs293.38 (EM) or
Corrected Absorbance of TE at 270.29 nm = abs270.29 (TE + EM) – 0.52 × abs293.38 (EM) where; abs: Absorption value at given wavelengths.
Preparation of standard stock solutions and calibration curve
Standard stock solutions of pure drug containing 1000 μg/ml of TE and EM were prepared separately by dissolving 100 mg of pure TE and EM in a 100 ml volumetric flask and volume was made up to the mark with methanol for method A and B and with 0.1N HCl for method C. The working standard solutions of these drugs were obtained by dilution of the respective stock solution in methanol and 0.1N HCl for proposed methods. Derivative amplitudes of spectrum, by using the above mentioned procedures, were used to prepare calibration curves for both the drugs. Beer's law obeyed in the concentration range of 3-21 μg/ml for TE and 2-14 μg/ml for EM by method A and B whereas 6-30 μg/ml for TE and 4-20 μg/ml for EM by method C.
Preparation of sample stock solution and formulation analysis
To assay by using method A and B, 20 tablets were weighed accurately and a quantity of tablet powder equivalent to 100 mg of TE (66.66 mg of EM) was weighed and dissolved in the 80 ml of methanol with the aid of ultrasonication for 5 min and solution was filtered through Whatman paper No. 41 into a 100 ml volumetric flask. Filter paper was washed with methanol, adding washings to the volumetric flask and volume was made up to the mark with methanol. The solution was suitably diluted with methanol to get required final concentration of TE (12μg/ml) and EM (8μg/ml). Above procedure was repeated for method C using 0.1 N HCl instead of methanol to get solution containing TE, 18 μg/ml and EM, 12 μg/ml.
Recovery studies
The accuracy of the proposed methods were checked by recovery studies, by addition of standard drug solution to preanalyzed sample solution at three different concentration levels (80%, 100%, and 120%) within the range of linearity for both the drugs. The basic concentration level of sample solution selected for spiking of the drugs standard solution was 12 μg/ml of TE and 8 μg/ml of EM for method A and B, whereas 18 μg/ ml of TE and 12 μg/ml of EM for method C.
Precision of the method
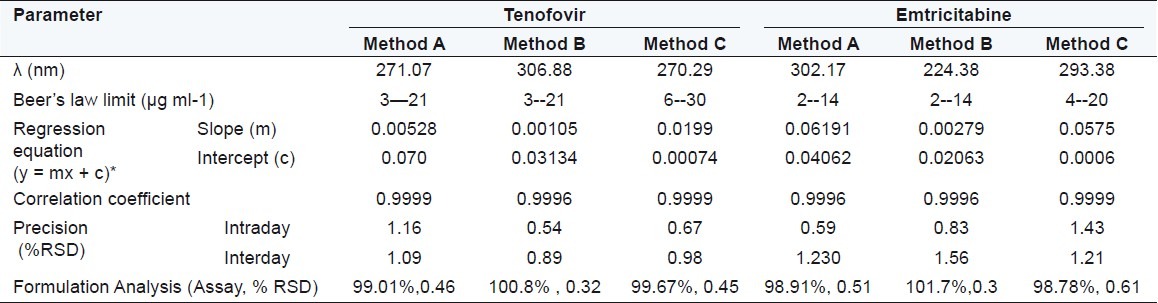
To study intraday precision, methods were repeated five times in a day and for interday methods were repeated on five different days and the average %RSD was found to be always less than 2. These values shown in Table 1 confirm the intraday and interday precision.
Table 1.
Optical characteristics and results of formulation analysis
Dissolution study
The dissolution study was carried out for the above combination and was validated. A calibrated dissolution apparatus (USP II) was used with paddles at 50 r/min and bath temperature was maintained at 37 ± 1°C. Nine hundred milliliter freshly prepared and degassed 0.1N HCl solution was used as the dissolution medium. Six tablets were evaluated and dissolution sample were collected at 5, 10, 15, 20, 25, 30, 35, 40, and 45 min interval. At each time point, a 5 ml sample with replacement was removed from each sample, filtered through a Nylon filter (0.45 μm, 25 mm), 1.0 ml of filtrate was diluted to 10 ml with 0.1N HCl and analyzed by absorption corrected method, and percentage release of EM and TE was calculated by using Eqs. (3) and (4), respectively,
EM % release = (CEM × 900×10×100)/(1000×200), ……. (3)
TE % release = (CTE × 900×10×100)/ (1000×300). ……. (4)
RESULTS AND DISCUSSIONS
The method A involves dividing the spectrum of mixture into the standardized spectra for each of the analyte and deriving the ratio to obtain spectra that is independent of analyte concentration used as divisor.
Under experimental conditions described, calibration curve, assay of tablets and recovery studies were performed. A critical evaluation of proposed method was performed by statistical analysis of data where slope, intercept, correlation coefficient is shown in Table 1. As per the ICH guidelines, the method validation parameters checked were linearity, accuracy and precision. Beer's law is obeyed in the concentration range of 3-21 μg/ml for TE and 2-14 μg/ml for EM by method A and B whereas 6-30 μg/ml for TE and 4-20 μg/ml for EM by method C, with correlation coefficient >0.999 for both the drugs. The proposed methods were also evaluated by the assay of commercially available tablets containing TE and EM (n = 5). The results of formulation analysis are presented in Table 1. For TE, the recovery study results ranged from 99.58% to 101.34% with %RSD values ranging from 0.52% to 1.92% for the proposed methods. For EM, the recovery results ranged from 99.06% to 100.45%, with %RSD values ranging from 0.19% to 1.23% for the proposed methods. Results of recovery studies are shown in Table 2. The accuracy and reproducibility is evident from the data as results are close to 100 % and standard deviation is low. Percentage release during dissolution study was always greater than 80% within 45 min for both drugs for tablet formulation under study [Figure 5].
Table 2.
Result of recovery studies
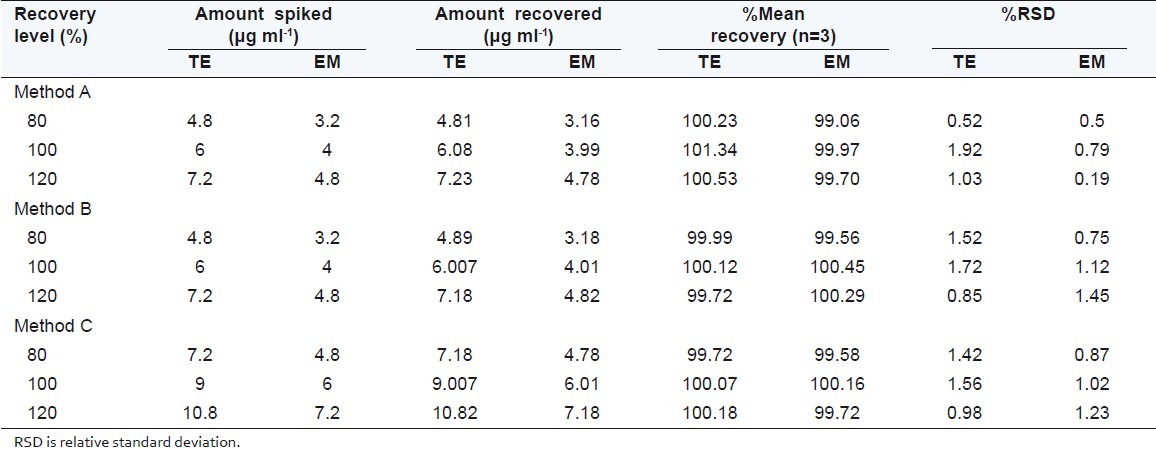
Figure 5.
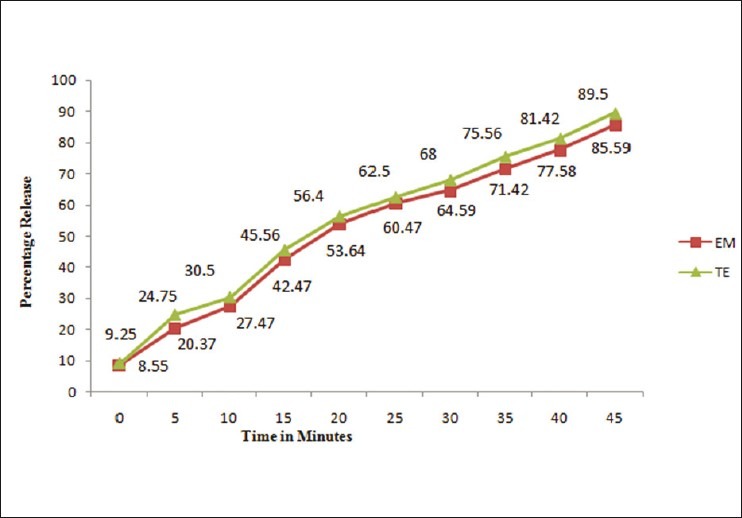
Dissolution profile of TE and EM tablet formulation by absorbance corrected method.
CONCLUSION
The validated spectrophotometric methods employed here proved to be simple, economical, precise and accurate. Thus, it can be used as IPQC test and for routine simultaneous determination of TE and EM in tablet dosage form. Absorption corrected method can be used to carry out dissolution study in combination tablet formulation.
ACKNOWLEDGMENTS
The authors wish to express their gratitude to Emcure Pharmaceuticals Pvt. Ltd. Pune, India, for the sample of pure Tenofovir disoproxil fumarate and Emtricitabine. The authors are also thankful to the management of MAEER's Maharashtra Institute of Pharmacy for providing necessary facilities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gomes NA, Vaidya VV, Pudage A, Joshi SS, Parekh SA. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) method for simultaneous determination of tenofovir and emtricitabine in human plasma and its application to a bioequivalence study. J Pharm Biomed Anal. 2008;48:918–26. doi: 10.1016/j.jpba.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Rang HP, Dale MM, Ritter JM, Flower RJ. Rang And Dale's Pharmacology. 6th ed. China: Churchill Livingstone, Elsevier; 2007. p. 685. [Google Scholar]
- 3.Jullien V, Treéluyer J, Pons G, Rey E. Determination of tenofovir in human plasma by high-performance liquid chromatography with spectrofluorimetric detection. J Chromatogr B. 2003;785:377–81. doi: 10.1016/s1570-0232(02)00933-9. [DOI] [PubMed] [Google Scholar]
- 4.Bezy V, Morin P, Couerbe P, Leleu G, Agrofoglio L. Simultaneous analysis of several antiretroviral nucleosides in rat-plasma by high-performance liquid chromatography with UV using acetic acid/hydroxylamine buffer Test of this new volatile medium-pH for HPLC-ESI-MS/MS. J Chromatogr B. 2005;82:132–43. doi: 10.1016/j.jchromb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Delahunty T, Bushman L, Fletcher CV. Sensitive assay for determining plasma tenofovir concentrations by LC/MS/MS. J Chromatogr B. 2005;830:6–12. doi: 10.1016/j.jchromb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Barkil ME, Gagnieu M, Guitton J. Relevance of a combined UV and single mass spectrometry detection for the determination of tenofovir in human plasma by HPLC in therapeutic drug monitoring. J Chromatogr B. 2007;854:192–97. doi: 10.1016/j.jchromb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Sentenac S, Fernandez C, Thuillier A, Lechat P, Aymard G. Sensitive determination of tenofovir in human plasma samples using reversed-phase liquid chromatography. J Chromatogr B. 2003;793:317–24. doi: 10.1016/s1570-0232(03)00333-7. [DOI] [PubMed] [Google Scholar]
- 8.Delahunty T, Bushman L, Fletcher CV. Sensitive assay for determining plasma tenofovir concentrations by LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830:6–12. doi: 10.1016/j.jchromb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Cass QB, Watanabe CS, Rabi JA, Bottari PQ, Costa MR, Nascimento RM, et al. Polysaccharide-based chiral phase under polar organic mode of elution in the determination of the enantiomeric purity of emtricitabine an anti-HIV analogue nucleoside. J Pharm Biomed Anal. 2003;33:581–/87. doi: 10.1016/s0731-7085(03)00339-x. [DOI] [PubMed] [Google Scholar]
- 10.Notari S, Bocedi A, Ippolito G, Narciso P, Pucillo LP, Tossini G, et al. Simultaneous determination of 16 anti-HIV drugs in human plasma by high-performance liquid chromatography. J Chromatogr B. 2006;831:258–66. doi: 10.1016/j.jchromb.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Rezk NL, Crutchley RD, Kashuba AD. Simultaneous quantification of emtricitabine and tenofovir in human plasma using high-performance liquid chromatography after solid phase extraction. J Chromatogr B. 2005;822:201–08. doi: 10.1016/j.jchromb.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Nirogi R, Bhyrapuneni G, Kandikere V, Mudigonda K, Komarneni P, Aleti R, et al. Simultaneous quantification of a non-nucleoside reverse transcriptase inhibitor efavirenz, a nucleoside reverse transcriptase inhibitor emtricitabine and a nucleotide reverse transcriptase inhibitor tenofovir in plasma by liquid chromatography positive ion electrospray tandem mass spectrometry. J Biomed Chromatogr. 2009;23:371–81. doi: 10.1002/bmc.1125. [DOI] [PubMed] [Google Scholar]
- 13.Ghorpade SA, Sali MS, Kategaonkar AH, Patel DM, Choudhari VP, Kuchekar BS. Simultaneous determination of emtricitabine and tenofovir by area under curve and dual wavelength spectrophotometric method. J Chil Chem. Soc. 2010;54:331–33. [Google Scholar]
- 14.Choudhari VP, Ingale KD, Barhate A, Kale AN, Bobade CD, Kuchekar BS. Development and validation of Simultaneous and Isoabsorptive UV -Spectrophotometric methods for Tenofovir and Emtricitabine in Pharmaceutical Formulations. J Pharm Res. 2010;9:11–13. [Google Scholar]
- 15.Geneva: Switzerland; 1996. ICH-Q2B Validation of Analytical Procedures: Methodology International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. [Google Scholar]
- 16.Nikam AD, Pawar SS, Gandhi SV. Estimation of paracetamol and aceclofenac in tablet formulation by ratio spectra derivative spectroscopy. Indian J Pharm Sci. 2008;70:635–37. doi: 10.4103/0250-474X.45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannucci C, Bertini J, Cocchini A, Perico A, Salvagnini F, Triolo A. Simultaneous determination of otilonium bromide and diazepam by first-derivative spectroscopy. Indian J Pharm Sci. 1992;81:1175–78. doi: 10.1002/jps.2600811209. [DOI] [PubMed] [Google Scholar]


