Abstract
Background:
Tobramycin, an aminoglycoside antibiotic, is a polar pharmaceutical compound which lacks a UV absorbing chromophore. Due to the absence of a UV absorbing chromophore and high polar nature of this antibiotic, the analysis of such compounds becomes a major challenge.
Objective:
To overcome these problems, a novel method for the determination aminoglycoside tobramycin was developed and validated based on reversed-phase high-performance liquid chromatography (RP-HPLC) with UV detector.
Materials and Methods:
An isocratic mobile phase consists of buffer 0.05 M diammonium hydrogen phosphate, pH adjusted to 10.0 using tetramethyl ammonium hydroxide. Chromatography was carried out at 25°C on a Purosphere RP-8e, 250 mm × 4.6 mm, 5mm. The detection was carried out using variable wavelength UV-Vis detector set at 210 nm. The compounds were eluted isocratically at a steady flow rate of 1.0 mL/min.
Result and Discussion:
Tobramycin retention time was about 9.0 min with an asymmetry factor of 1.4. A logarithmic calibration curve was obtained from 0.47 to 0.71 mg/mL (r > 0.9998). Within-day %RSD was 0.29 (n = 6, 0.60 mg/mL) and between-day %RSD was 0.54 Specificity/ selectivity experiments revealed the absence of interference from excipients, recovery from spiked samples was between 99.0–100.0 percent.
Conclusions:
A HPLC method based on UV detection has been developed and validated for determination of tobramycin from ophthalmic solution. The method is simple, rapid, specific, accurate (error 0.80%), precise (RSD <2.0%) and linear (r2=0.9998). The described method is suitable for routine analysis and quality control of ophthalmic solution containing tobramycin.
Keywords: Amino glycoside, derivatization, isocratic, reverse phase chromatography, tobramycin
INTRODUCTION
Tobramycin is an amino glycoside antibiotic used to treat various types of bacterial infections, particularly Gram-negative infections. It works by binding to a site on the bacterial 30S and 50S ribosome, preventing formation of the 70S complex. As a result, mRNA cannot be translated into protein and cell death ensure. Tobramycin is preferred over gentamicin for Pseudomonas aeruginosa pneumonia due to better lung penetration and bactericidal activity.[1,2]
Like all amino glycosides, tobramycin does not pass through the gastro-intestinal tract, so for systemic use it can only be given intravenously or intramuscularly.[1] A sterile tobramycin ophthalmic solution (eye-drops) with a tobramycin concentration of 0.3% is available in the market.
United states Pharmacopoeia 2008 (USP 31) have described the procedure for assay of raw material and tobramycin ophthalmic solution by high-performance liquid chromatography using derivatization with 2,4-Dinitroflurobenzene and tris (hydroxymethyl aminomethane) reagent, mixture of buffer (2 gm of tris (hydroxymethyl aminomethane) and 20 ml of 1N sulfuric acid) and acetonitrile in the ratio 40:60 v/v as the mobile phase at a flow rate of 1.2 ml/min, and a column (3.9 × 300 mm) that contains L1 packing, with detector wavelength set at 365 nm.[3] 2, 4-Dinitroflurobenzene and tris (hydroxyl methyl aminomethane) reagent are stable for 5 days and 4 hours respectively. Derivatization is carried out at 60°C constant temperature.
In comparison with derivatization, simple reverse-phase chromatographic methods have the advantages of reducing analysis time, enhancing sensitivity and flexibility, and lowering the cost of the instruments and maintenance. The biggest disadvantage of derivatization has been lack of stability. The reaction products are not stable and have a short half-life possibly because of a spontaneous intermolecular rearrangement. Another disadvantage of derivatization is that it reacts with only few functional groups.[4]
The literature survey shows that several methods like HPLC with evaporative light scattering detection, electrochemical detection, LC/MS, HPLC UV-Vis have been reported for the determination of tobramycin with derivatization.[5–20] These reported methods and the USP method are not rapid for assay of tobramycin in ophthalmic solutions. All the reported and official methods are complex, insensitive, and risky. However, as per bibliographical revisions performed, no analytical method has been reported for direct (without derivatization) determination of tobramycin by high-performance liquid chromatography.
The present study was aimed at developing a simple, specific, accurate, and precise HPLC method for determination of tobramycin in commercially available pharmaceutical formulations such as ophthalmic solution, injection, suspensions, and in-house prepared ophthalmic suspension, based on direct UV- detection, in which a 100% buffer was used as a mobile phase to determine the compounds for use in routine quality control applications associated with these ingredients.
The proposed method for the determination of tobramycin in pharmaceutical formulations by HPLC UV detectors is first of its kind without involving derivatization. This information could be very useful for many of the pharmaceutical industries for the determination of this compound and for those who do not have the costly instruments, selective detectors such as a refractive index detector.
MATERIALS AND METHODS
Instrumentation
Integrated high-performance liquid chromatographic systems LC-2010AHT from Shimadzu Corporation (Chromatographic and Spectrophotometric Division, Kyoto, Japan) consisted of a binary gradient system, high-speed auto-sampler, column oven, and UV-Vis detector. A Purospher RP-8e, 250 mm × 4.6 mm, 5mm analytical column from Merck, Germany, was used as a stationary phase. Chromatograms were recorded and integrated on PC installed with LC solution chromatographic software, version 1.22 SP1 (Shimadzu, Kyoto, Japan).
Reference substances, reagents, and chemicals
Working standard of tobramycin was obtained from Chongqing daxin pharmaceuticals laboratories Ltd., China. Diammonium hydrogen phosphate and tetra methyl ammonium hydroxide were purchased from Panreac (Barcelona) Espana. Distilled water was obtained from a Milli-Q system, Millipore, Milford, MA, USA. All the chemicals and reagents were of analytical or reagent grade. Reference standards of tobramycin were obtained from United States Pharmacopoeia Convention, Rockville, MD, USA. Ophthalmic formulations containing tobramycin were developed and manufactured in our research and development laboratory.
Chromatographic conditions
The isocratic mobile phase consisted of 0.05 M diammonium hydrogen phosphate, pH adjusted to 10.0 ± 0.05 using tetramethyl ammonium hydroxide (25% solution in water). The mobile phase was filtered and degassed through a membrane filter of 0.45 μm porosity under vacuum. A Purosphere RP-8e, 250 mm × 4.6 mm, 5 mm analytical column was used as a stationary phase. A constant flow rate of l.0 ml/min was employed throughout the analysis. A variable UV-Vis detector was set at 210 nm. All pertinent analyses were made at 25°C and volume of solution injected on to the column was 50 μl. The mobile phase was used as diluent for standard and sample preparations.
Samples
Test samples were ophthalmic solutions prepared in-house and purchased from the local market with composition 3.0 mg/ml of tobramycin. Other test samples used were accelerated stability samples with similar composition.
Solution preparation
Tobramycin standard solution
Tobramycin standard solutions were prepared by transferring accurately about 66.0 mg of tobramycin working standard equivalent to 60.0 mg of anhydrous tobramycin to a 100 ml volumetric flask. About 70 ml of diluent was added and sonicated for few minutes to solubilize tobramycin. The solution was diluted to volume with the diluent and mixed. The solution was filtered through a 0.45 μm membrane filter and 50 μl was injected.
Estimation from formulations
Five bottles of ophthalmic suspension, containing tobramycin 3.0 mg/ml, were shaken gently and transferred to a glass beaker and mixed. About 5.0 gm of ophthalmic suspension was weighed accurately into a 25 ml volumetric flask, about 10 ml of diluent was added, shaken to disperse the sample, and diluted to volume with diluent and mixed. This solution theoretically contains 0.60 mg/ml of tobramycin. The solution was filtered through a 0.45 μm membrane filter and 50 μl was injected directly on to the column.
Quantitation
Peak areas were recorded for all peaks. Peak areas were taken into account to quantitate the label amount in milligram per ml of ophthalmic suspension by using the following formula:
Tobramycin mg/ml = Ru/Rs × C/100 × 25/W × 1/L × P × D
where Ru is the peak area obtained from tobramycin in the investigation solution; Rs is the peak area obtained from tobramycin in the standard solution; C is the weight (mg) of tobramycin working standard taken to prepare the standard solution; W is the weight (g) of the test sample; P is purity of tobramycin working standard, L is the labeled amount of tobramycin in mg/ml of ophthalmic suspension, and D is the density of the ophthalmic suspension.
RESULTS AND DISCUSSION
Chromatography
Our method development started with the search for the suitable column and mobile phase.
A chromatographic system comprising 0.02 M formic acid:acetonitrile (50: 50 v/v), as a mobile phase at a constant flow rate of l.0 ml/min, silica column, 250 mm × 4.0 mm, 5μm analytical column as a stationary phase, and detector wavelength at 205 nm resulted in no peak elutions even after 60 minutes of run time. The mobile phase consisting of a 0.02 M aqueous potassium ammonium phosphate buffer and acetonitrile in the ratio 50:50, v/v, was tried in isocratic conditions on the Spherisorb ODS-1, 250 mm × 4.6 mm, 5μm to obtain symmetrical peak shapes and clear separation of the signal peaks from the solvent front peaks.
Upon investigation of the two chromatographic systems containing 0.05 M potassium dihydrogen phosphate, pH adjusted to 7.0 using potassium hydroxide, and 0.1 M potassium dihydrogen phosphate, pH adjusted to 6.9 using a potassium hydroxide solution as the mobile phase at a constant flow rate of l.0 ml/min, BioSep SEC-S2000, 300 mm × 7.8 mm and a Purosphere RP-8e, 250 mm × 4.6 mm, 5μm analytical column as a stationary phase and detector wavelength at 205 nm resulted in peak elution at 3.2 minutes and 9.7 minutes respectively, the first investigation resulted in tobramycin peak eluted very close to negative peak (peak from diluent) and the second resulted in a tailing factor as high (>3) as shown Figure 1.
Figure 1.
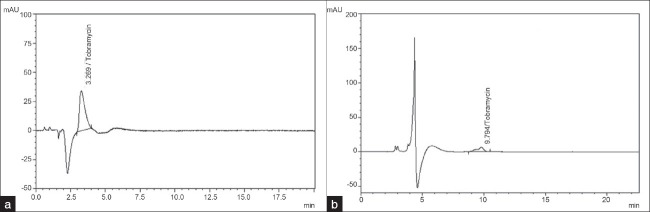
(a) Chromatogram of tobramycin showing fi rst investigation. Chromatographic column: BioSep SEC-S2000, 300 mm × 7.8 mm, mobile phase: 0.05 M potassium dihydrogen phosphate, pH adjusted to 7.0 using potassium hydroxide, flow rate 1 ml/min. Detector wavelength: 205 nm. (b) Chromatogram of tobramycin showing second investigation. Chromatographic column: Purosphere RP-8e, 250 mm × 4.6 mm, 5μm, mobile phase: 0.05 M potassium dihydrogen phosphate, pH adjusted to 7.0 using potassium hydroxide ,flow rate 1 ml/min. Detector wavelength: 205 nm
Further, to develop a suitable and robust LC method for the determination of tobramycin by UV detection, different mobile phases and columns were employed [Table 1] to achieve the best signal response and retention time.
Table 1.
Employed mobile phases, columns and elution time during the investigation of tobramycin

Finally, the mobile phase consisting of water: 0.05 M diammonium hydrogen phosphate, pH adjusted to 10.0 ± 0.05 using tetra methyl ammonium hydroxide (25% solution in water) at a constant flow rate of 1.0 ml/min and detector wavelength set at 210 nm, using a Purosphere RP-8e, 250 mm × 4.6 mm, 5μm column, was found to be appropriate, allowing well signal response of tobramycin.
Optimization of HPLC
The pH of the mobile phase can affect the analyte retention time as well as the detection sensitivity. Figure 2 shows the result of detection response (peak area), efficiency (shown as plate number N/column) and capacity factor of tobramycin at different pHs. The optimal pH 10.0 ± 0.05 was chosen for the determination of tobramycin.
Figure 2.

Effect of pH on (peak area) and effi ciency (shown as plate number N/column) and capacity factor of tobramycin column: Purosphere RP-8e, 250 × 4.6 mm, 5μm, mobile phase: 0.05 M diammonium hydrogen phosphate, pH adjusted to 10 using tetramethyl ammonium hydroxide
Concentration of the buffer is another factor that can alter the ion-pair formation. Figure 3 shows the capacity factor and detection response (peak area) as the concentration of the buffer varied. Response was minimal when less than 0.05 M diammonium hydrogen phosphate was used. This may be due to highly aqueous environment that is unfavorable for ion pairing. Therefore, pH 10.0 and 0.05 M diammonium hydrogen phosphate was chosen for estimation of tobramycin. Typical chromatogram of the test solution is shown in Figure 3.
Figure 3.

A typical chromatogram of test sample by proposed methods column: Purosphere RP-8e, 250 × 4.6 mm, 5μm, mobile phase: 0.05 M diammonium hydrogen phosphate, pH adjusted to 10.0 using tetramethyl ammonium hydroxide, flow rate 1 ml/min, λ set at 210 nm
Method validation
The test method for the determination of tobramycin was validated to include the essential demands of International Conference on Harmonization (ICH) guidelines.[21] Parameters like specificity, linearity, accuracy, precision, range, robustness, and system suitability were examined.
Specificity
No interferences were observed due to the obvious presence of excipients and mobile phase.
Linearity
Peak areas versus concentration in milligram per milliliter were plotted for tobramycin at the concentration range between 80% and 120% of the target level. Tobramycin showed linearity between 0.47 and 0.71 mg/ml with a correlation coefficient (r2) of 0.9998.
Accuracy
Accuracy of the proposed HPLC determination was evaluated from the assay results of the components. Accuracy was done by performing the assay of samples and calculated the peak area responses of different samples by the component recovery method.
Stock solution
A Stock solution was prepared by dissolving accurately weighed portions of about 666 mg of tobramycin in portions of the mobile phase and diluted to produce 100 ml solution.
An appropriate portion of the stock solution was spiked into a blank placebo matrix to produce concentrations of 80, 100, and 120 of the target level. Mean recovery of spiked samples was 99.45 % for tobramycin [Table 2].
Table 2.
Accuracy data (analyte recovery): Tobramycin

Precision
Instrumental precision was determined by six replicate determinations of standard solution and the relative standard deviation was 0.29 for tobramycin.
Method precision or intra-assay precision was performed by preparing six different samples involving different weighing. Each solution was injected in triplicate under same conditions and the mean value of peak area response for each solution was taken. Corrections in area were made for each weight that had been taken to prepare six sample solutions and relative standard deviation of the contents of tobramycin the six sample solutions was calculated. Relative standard deviation was 0.29 for tobramycin.
Intermediate precision was performed by analyzing the samples by two different analysts employing different instruments. The standard solution and six different samples at 100% target level were prepared by each analyst. The relative standard deviation obtained from 12 assay results by two analysts was 0.42 for tobramycin.
Robustness
Robustness of the proposed method was performed by keeping chromatographic conditions constant with the following deliberate variations:
-
(i)
change in the column oven temperature
-
(ii)
change in flow rate from 1.0 ml/min to 1.2 ml/min.
The standard solution was injected six times in replicate for each minor change. System suitability parameters like peak asymmetry, theoretical plates, capacity factor, and relative standard deviation were recorded for each peak and found to be within acceptable limits.
Six test samples at the target concentration level were prepared and analyzed for each change. The percentage difference in assay and relative standard deviations was calculated during each change and found to be ± 0.5% and less than 1.0, respectively. It was noted that slight addition of solvents in the mobile phase affects the method and does not produce results of similar system suitability, as in the proposed method it resulted in poor peak response or no elution of tobramycin peak.
System suitability
System suitability tests were performed to chromatograms obtained from standard and test solutions to check parameters such as column efficiency, peak asymmetry, and capacity factor of tobramycin peaks. Results obtained from six replicate injections of standard solution as per the proposed method are summarized in Table 3.
Table 3.
Comparison of system suitability parameters: Tobramycin

Application of the proposed method
In-house prepared samples, innovator samples, and samples stored at accelerated stability conditions (40°C/25%RH) were evaluated for assay of tobramycin. The method gave reproducible results of assay for all the samples tested for tobramycin. The excipients in the formulation and the probable degradation products of tobramycin as a result of accelerated storage did not interfere with the estimation of the component. The assay of the test samples (RT and accelerated) and innovator samples are summarized in Table 4.
Table 4.
Application of the developed HPLC method to the determination of tobramycin in dosage forms

CONCLUSIONS
A HPLC method based on UV detection has been developed and validated for determination of tobramycin from an ophthalmic solution. The method is simple, rapid, specific, accurate (error 0.80%), precise (RSD <2.0%), and linear (r2=0.9998). The described method is suitable for routine analysis and quality control of the ophthalmic solution and injection containing tobramycin.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bendush CL, Weber R. Tobramycin sulfate: a summary of worldwide experience from clinical trials. J Infect Dis. 1976;134(Suppl):S219–34. doi: 10.1093/infdis/134.supplement_1.s219. [DOI] [PubMed] [Google Scholar]
- 2.Hewitt WL. Gentamycin: Toxicity in perspective. Postgrad Med J. 1974;50:55–9. [PubMed] [Google Scholar]
- 3.USP 31 NF 26. United States Pharmacopoeia. Rockville, MD: U.S. Pharmacopeial Convention, Inc; 2008. pp. 3421–3. [Google Scholar]
- 4.Andrei Medvedovici, Alexandra Farca, Victor David. Derivatization Reactions in Liquid Chromatography for Drug Assaying in Biological Fluids. In: Nelu Grinberg, Eli Grushka., editors. Advances in chromatography. United States: CRC press; 2009. pp. 283–315. [Google Scholar]
- 5.Guo MX, Wrisley L, Maygoo E. Measurement of tobramycin by reversed-phase high-performance liquid chromatography with mass spectrometry detection. Anal Chim Acta. 2006;571:12–6. doi: 10.1016/j.aca.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Feng CH, Lin SJ, Wu HL, Chen SH. Trace analysis of tobramycin in human plasma by derivatization and high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780:349–54. doi: 10.1016/s1570-0232(02)00544-5. [DOI] [PubMed] [Google Scholar]
- 7.Soltés L. Aminoglycoside antibiotics- two decades of their HPLC bioanalysis. Biomed Chromatogr. 1999;13:3–10. doi: 10.1002/(SICI)1099-0801(199902)13:1<3::AID-BMC811>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.Essers L. An automated high-performance liquid chromatographic method for the determination of aminoglycosides in serum using pre-column sample clean-up and derivatization. J Chromatogr. 1984;305:345–52. doi: 10.1016/s0378-4347(00)83348-4. [DOI] [PubMed] [Google Scholar]
- 9.Barends DM, Rutgers A, Van Klingeren B, Hulshoff A. The determination of amino glycoside antibiotics in serum: a comparison of a high performance liquid chromatographic method with a microbiological assay. Pharm Weekbl Sci. 1982;4:104–11. doi: 10.1007/BF01962630. [DOI] [PubMed] [Google Scholar]
- 10.Barends DM, Zwaan CL, Hulshoff A. Micro-determination of tobramycin in serum by high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1981;225:417–26. doi: 10.1016/s0378-4347(00)80289-3. [DOI] [PubMed] [Google Scholar]
- 11.Anhalt JP, Brown SD. High-performance liquid-chromatographic assay of aminoglycoside antibiotics in serum. Clin Chem. 1978;24:1940–7. [PubMed] [Google Scholar]
- 12.Mashat M, Chrystyn H, Clark BJ, Assi KH. Development and validation of HPLC method for the determination of tobramycin in urine samples post-inhalation using pre-column derivatization with fluorescein isothiocyanate. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;869:59–66. doi: 10.1016/j.jchromb.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Attema-de Jonge ME, Bekkers JM, Oudemans-van Straaten HM, Sparidans RW, Franssen EJ. Simple and sensitive method for quantification of low tobramycin concentrations in human plasma using HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;862:257–62. doi: 10.1016/j.jchromb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Clarot I, Storme-Paris I, Chaminade P, Estevenon O, Nicolas A, Rieutord A. Simultaneous quantitation of tobramycin and colistin sulphate by HPLC with evaporative light scattering detection. J Pharm Biomed Anal. 2009;50:64–7. doi: 10.1016/j.jpba.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Ghinami C, Giuliani V, Menarini A, Abballe F, Travaini S, Ladisa T. Electrochemical detection of tobramycin or gentamicin according to the European Pharmacopoeia analytical method. J Chromatogr A. 2007;1139:53–6. doi: 10.1016/j.chroma.2006.10.099. [DOI] [PubMed] [Google Scholar]
- 16.Hanko VP, Rohrer JS. Determination of tobramycin and impurities using high-performance anion exchange chromatography with integrated pulsed amperometric detection. J Pharm Biomed Anal. 2006;40:1006–12. doi: 10.1016/j.jpba.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Megoulas NC, Koupparis MA. Development and validation of a novel HPLC/ELSD method for the direct determination of tobramycin in pharmaceuticals, plasma, and urine. Anal Bioanal Chem. 2005;382:290–6. doi: 10.1007/s00216-004-2948-8. [DOI] [PubMed] [Google Scholar]
- 18.Keevil BG, Lockhart SJ, Cooper DP. Determination of tobramycin in serum using liquid chromatography-tandem mass spectrometry and comparison with a fluorescence polarization assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;794:329–35. doi: 10.1016/s1570-0232(03)00492-6. [DOI] [PubMed] [Google Scholar]
- 19.Lai F, Sheehan T. Enhancement of detection sensitivity and cleanup selectivity for tobramycin through pre-column derivatization. J Chromatogr. 1992;609:173–9. doi: 10.1016/0021-9673(92)80160-v. [DOI] [PubMed] [Google Scholar]
- 20.Statler JA. Determination of tobramycin using high-performance liquid chromatography with pulsed amperometric detection. J Chromatogr. 1990;527:244–6. doi: 10.1016/s0378-4347(00)82108-8. [DOI] [PubMed] [Google Scholar]
- 21. ICH Harmonized Tripartite Guideline, International Conference on Harmonization on Technical Requirements for Registration of Pharmaceuticals for Human Use, Q2A, Text on Validation of Analytical Procedures, Step 4 of the ICH process (1994) and Q2B, Validation of Analytical Procedures: Methodology, Step 4 of the ICH process, ICH Steering Committee (1996) International Conference on Harmonization, Geneva, Switzerland.


