Abstract
Objective:
A simple, precise and accurate isocratic reversed phase (RP) column high-performance liquid chromatographic (HPLC) method has been developed for simultaneous analysis of eprosartan (EPR) and hydrochlorothiazide (HCT) in tablet formulations.
Materials and Methods:
Isocratic RP-HPLC separation was achieved on phenomenex C18 column (250 × 4.6 mm i.d., 5 μm particle size) using mobile phase composed of 0.5% formic acid-methanol-acetonitrile [(80 : 25 : 20 v/v/v) pH, 2.80 ± 0.04] at a flow rate of 1.0 ml/min. The retention time for EPR and HCT was 7.69 ± 0.10 and 4.24 ± 0.09 minutes, respectively. The detection was performed at 272 nm.
Results:
The method was linear in the concentration range of 60-600 μg/ml for EPR and 2.5-25 μg/ml for HCT with a correlation coefficient of 0.9992 and 0.9997, respectively. The repeatability for six samples was 0.53 and 0.61 % RSD for EPR and HCT, respectively. The accuracy (recovery) was found to be in the range of 99.46 to 100.61% for EPR and 99.06 to 100.93% for HCT, respectively.
Conclusions:
The method was validated and successfully used for determination of the drugs in tablets.
Keywords: Eprosartan, high-performance liquid chromatographic, hydrochlorothiazide, tablets
INTRODUCTION
Eprosartan (EPR) {(E)-3-[2-butyl-1-[(4-carboxyphenyl)methyl]-1H-imidazol-5-yl]-2-[(2-thienyl) methyl] propenoic acid} is a highly selective, nonpeptide angiotensin-II antagonist [Figure 1]. The compound has been shown to inhibit angiotensin-II induced vasoconstriction in preclinical species and cause reductions in systolic and diastolic blood pressure at peak effect after dosing in clinical patients.[1] It is currently being developed for the treatment of hypertension as other compounds of the class angiotensin-II receptor antagonists (ARA-II).[2] Hydrochlorothiazide (HCT) {(6-chloro-3,4-dihydro-2H-1,24-benzothiadiazine-7-sulphonamide-1,1-dioxide} is a diuretic drug [Figure 1].[3] The rationale behind this drug combination is that in treatment of hypertension in patients whose blood pressure is not adequately controlled by monotherapy, oral administration of EPR with HCT has been found more effective than use of either drug alone.[4].
Figure 1.

Structure of eprosartan and hydrochlorothiazide
Literature survey revealed that different analytical methods are reported for EPR in pharmaceutical dosage forms and biological samples, which includes LC-MS-MS,[5] capillary zone electrophoresis,[6] micellar electrokinetic capillary chromatography[7] and capillary zone electrophoresis for ARA-II with HCT,[8] analysis of EPR in biological samples by HPLC,[9,10] chemometric method,[11] and densitometry with HCT.[12] There are several reports of the determination of HCT in combination with other ARA – II drugs, including use of HPLC, HPTLC and spectrophotometry.[13–16] So far, to our present knowledge, no validated HPLC method for the simultaneous determination of EPR and HCT in bulk drug and tablets was reported in literature. Therefore, the aim of the proposed method was to develop and validate HPLC method in accordance with International Conference on Harmonization (ICH) guidelines,[17] which can be used successfully to determine EPR and HCT in tablet dosage forms.
MATERIALS AND METHODS
Apparatus
A Shimadzu (Columbia, MD) HPLC instrument (LC-10 ATvp) equipped with UV-Visible detector, manual injector of 20-μl loop and phenomenex (Torrence, CA) Luna C18 column (250 × 4.6 mm i.d., 5 μm particle size) was used; a weighing balance (Acculab ALC-210.4, India) and a sonicator (Enertech Fast clean, India) were used for the study.
Chemicals and reagents
EPR (Batch No. BK6-EP-080) and HCT (Batch No. RF0314) were obtained as gift samples from Dishmann pharmaceuticals Ltd. (Ahmedabad, India) and Unichem Laboratories Ltd. (Mumbai, India). EPR and HCT tablets (600 and 25 mg/tab) were procured from the market. Acetonitrile (HPLC grade, S. D. fine chemicals, Ahmedabad, India) methanol and water (HPLC grade, Finar chemicals Ltd., Ahmedabad, India), Formic acid (HPLC grade, Spectrochem Pvt Ltd., Mumbai, India) and nylon filter (Millipore Pvt. Ltd, Bangalore, India) were used for study.
Chromatographic conditions
HPLC was performed on a Phenomenex Luna C18 column (250 × 4.6 mm i.d., 5 μm particle size). The mobile phase consisted of 0.5% formic acid: methanol : acetonitrile [(80 : 25 : 20 v/v/v) pH, 2.80 ± 0.04]. The mobile phase was filtered through Nylon 0.45 μm, 47 mm membrane filter and was degassed before use. The flow rate was 1.0 ml/min. The determination was carried out at 272 nm and the injection volume was 20 μl. The total run time was 10 minutes.
Preparation of standard solution
Accurately weighed EPR standard (600 mg) and HCT standard (25 mg) was transferred into a 100-ml volumetric flask, and dissolved in and diluted to the mark with methanol to obtain standard stock solution (6000 μg/ml for EPR and 250 μg/ml for HCT). The stock solution was serially diluted with mobile phase to get linearity range of 60-600 μg/ml for EPR and 2.5-25 μg/ml for HCT.
Selection of wavelength
EPR and HCT stock solution (0.2 ml) was transferred into a 10 ml volumetric flask. The volume was adjusted to the mark with mobile phase and scanned over 200 to 400 nm. Wavelength 272 nm was selected for the study where HCT has good absorbance as EPR and HCT present in the tablets as ratio of 24: 1 [Figure 2].
Figure 2.
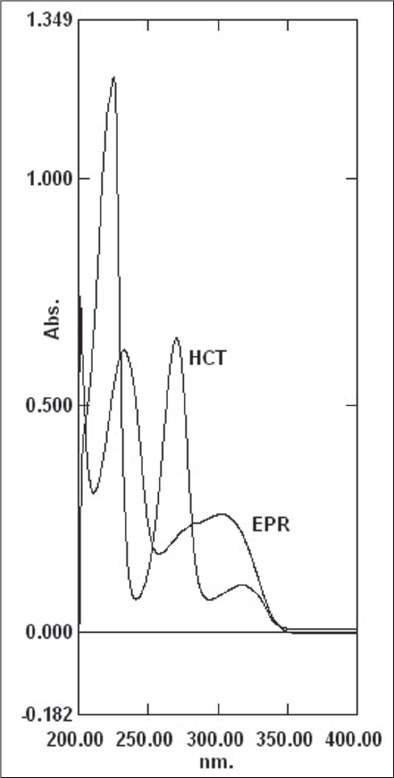
Overlay UV spectrum of EPR and HCT
Preparation of sample solution
Twenty tablets (each containing 600 mg of EPR and 25 mg HCT) were weighed accurately and powdered finely. The powder equivalent to 600 mg of EPR and 25 mg of HCT was transferred in a 100 ml volumetric flask; 60 ml methanol was added, sonicated for 20 minutes, and diluted up to the mark with methanol. The solution was filtered through 0.45 μm nylon filter paper. An aliquot (0.3ml) was transferred into a 10 ml volumetric flask and was diluted up to mark with mobile phase to obtain sample stock solution (180 and 7.5 μg/ml for EPR and HCT).
Method validation
Linearity
Aliquots (0.1, 0.2, 0.3, 0.4, 0.5 and 1 ml) from the stock solution (equivalent to 60, 120, 180, 240, 300 and 600 μg/ml for EPR and 2.5, 5, 7.5, 10, 12.5 and 25 μg/ml) were transferred in a series of 10ml volumetric flasks and diluted to the mark with mobile phase. An aliquot (20 μl) of each solution was injected under the operating chromatographic conditions as described earlier. Calibration curve was constructed by plotting peak areas vs. concentrations, and the regression equation was calculated. Each response was average of five determinations.
Intermediate precision (reproducibility)
The intraday and interday precisions of the proposed method were determined by estimating the corresponding response three times on the same day and on three different days over a period of 1 week for three different concentrations of EPR (60, 120 and 180 μg/ml) and HCT (2.5, 5 and 7.5μg/ml). The results are reported in terms of relative standard deviation (RSD).
Method precision (repeatability)
The repeatability was checked by repeatedly injecting (n = 6) solutions of EPR (120 μg/ml) and HCT (5 μg/ml).
Accuracy
Accuracy was determined by calculating recovery of EPR and HCT by the standard addition method. Known amounts of sample solutions (120 μg/ml of EPR and 5 μg/ml of HCT) were spiked with three different concentrations of standard solutions (60, 120, 180 μg/ml for EPR and 2.5, 5, 7.5 μg/ml for HCT). Each solution was injected in triplicate and the percentage recovery was calculated by measuring peak areas and fitting these values into the regression equation of the calibration curves.
Sensitivity
Limit of detection (LOD) and limit of quantification (LOQ) of the drug were calculated using the following equations according to ICH guidelines.[17]
LOD = 3.3 × σ / S
LOQ = 10 × σ / S
Where, σ is the standard deviation of the response and S is the standard deviation of slope of the regression equation.
System suitability test parameters
System suitability tests are used to verify that the resolution and repeatability of the system were adequate for the analysis intended. The parameters used in this test were asymmetry of the chromatographic peak resolution, theoretical plates and tailing factor.
Determination of eprosartan and hydrochlorothiazide in tablets
The validated method was used for the analysis of EPR and HCT in their combined tablets (Brand A).
RESULTS
We used several mobile phases in trying to accomplish good separation of EPR and HCT. Chromatographic conditions were optimized with a view to develop an assay method for EPR and HCT. The analytical conditions were selected after testing the different parameters such as organic solvents for mobile phase, mobile phase compositions, pH and other chromatographic conditions. Our preliminary trials using different combination of mobile phases of water with methanol and acetonitrile did not give good peak shape, optimum retention time and good resolution of peaks. Satisfactory results were obtained with the mobile phase consisting of 0.5% formic acid : methanol : acetonitrile (80 : 25 : 20 v/v/v, pH, 2.80 ± 0.04) [Figure 3]. The retention time of EPR and HCT was 7.69 ± 0.10 and 4.24 ± 0.09 minutes, respectively.
Figure 3.
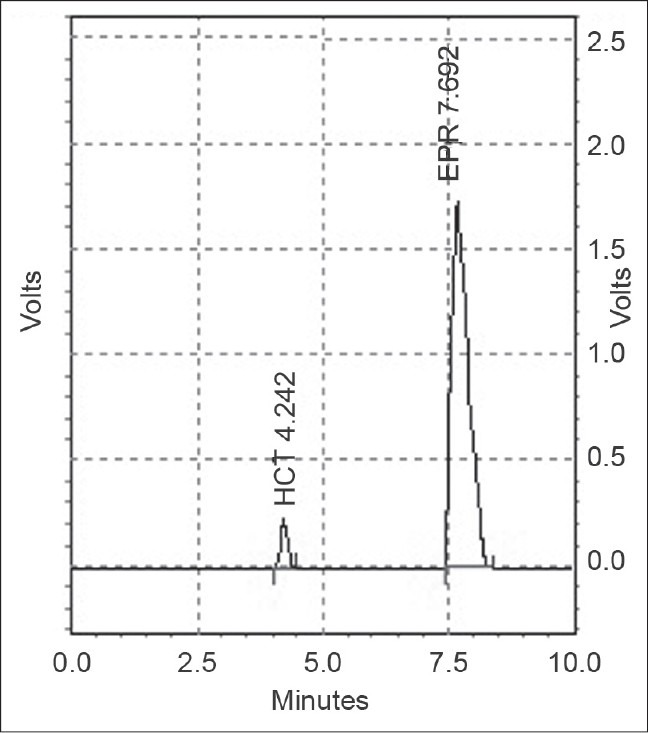
HPLC chromatogram of EPR and HCT (180 and 7.5 μg/ml; tablet dosage form at 272 nm)
Method validation
Linearity
Response to EPR and HCT was linear in the concentration ranges 60–600 μg/ml and 2.5–25 μg/ml, respectively. The regression equations for EPR and HCT (n = 6) were y = 35727x + 119429 and y 101589x + 27807 for EPR and HCT, respectively, where y is response and x the amount chromatographed. The correlation coefficients were 0.9992 and 0.9997 respectively, over these concentration ranges.
Sensitivity
The LOQ and LOD for EPR were 0.0288 and 0.0872 μg/ml, respectively. For HCT, the values were 0.0139 and 0.0460 μg/ml, respectively.
Precision and repeatability
The results for intraday & interday precision studies and repeatability are listed in Table 1.
Table 1.
Summary of validation parameters for the proposed method

Accuracy and system suitability parameters
The results for the accuracy study are shown in Table 2. The recovery was found in the range of 99.46 to 100.61% for EPR and 99.06 to 100.93% for HCT, indicating the method accuracy. System suitability parameters are listed in Table 3.
Table 2.
Accuracy data for analysis of EPR and HCT
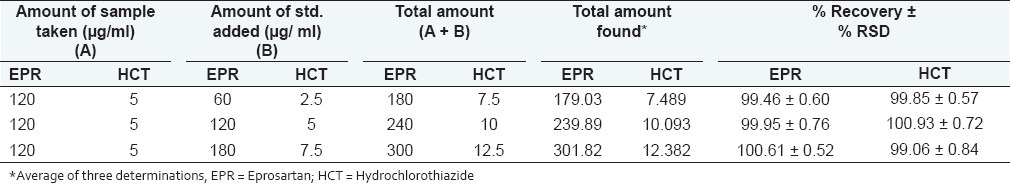
Table 3.
System-suitability test parameters for EPR and HCT

Determination of eprosartan and hydrochlorothiazide in combined tablets
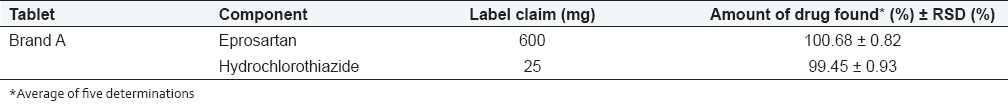
The validated method was successfully applied to analysis of EPR and HCT in their combined tablets (Brand A). The results obtained for EPR and HCT were comparable with the corresponding labelled amounts [Table 4].
Table 4.
Analysis of eprosartan and hydrochlorothiazide in combined tablet dosage form
DISCUSSION
A new analytical method has been developed to determine EPR and HCT in their combined pharmaceutical dosage form. The developed method was proved to be simple, rapid, accurate and precise. There is no interference of any excipients in the determination of EPR and HCT in tablets and the method can be successfully applied for routine quality control analysis of EPR and HCT tablets.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.McClellan KJ, Balfour JA. Eprosartan. Drugs. 1998;55:713–8. doi: 10.2165/00003495-199855050-00011. [DOI] [PubMed] [Google Scholar]
- 2.13th ed. USA: Merck; 2001. Chapman & Hall, The Merck Index – An Encyclopedia of Chemicals, Drugs and Biologicals; p. 1692. [Google Scholar]
- 3.Meredith PA. Angiotensin II receptor antagonists alone and combined with hydrochlorothiazide: Potential benefits beyond the antihypertensive effect. Am J Cardiovasc Drugs. 2005;5:171–83. doi: 10.2165/00129784-200505030-00004. [DOI] [PubMed] [Google Scholar]
- 4.Plosker GL, Foster RH. Eprosartan: A review of its use in the management of hypertension. Drugs. 2000;60:177–201. doi: 10.2165/00003495-200060010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Ferreirós N, Dresen S, Alonso RM, Weinmann W. Validated quantitation of angiotensin ii receptor antagonists (ARA-II) in human plasma by liquid-chromatography-tandem mass spectrometry using minimum sample clean-up and investigation of ion suppression. Ther Drug Monit. 2007;29:824–34. doi: 10.1097/FTD.0b013e31815d0f66. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez L, Akesolo U, Jimenez RM, Alonso RM. Application of capillary zone electrophoresis to the screening of some angiotensin II receptor antagonists. Electrophoresis. 2002;23:223–9. doi: 10.1002/1522-2683(200202)23:2<223::AID-ELPS223>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Hillaert S, De Beer TRM, De Beer JO, Van den Bossche W. Optimization and validation of a micellar electrokinetic chromatographic method for the analysis of several angiotensin-II receptor antagonists. J Chromatogr A. 2003;984:135–46. doi: 10.1016/s0021-9673(02)01832-0. [DOI] [PubMed] [Google Scholar]
- 8.Hillaert S, Van den Bossche W. Simultaneous determination of hydrochlorothiazide and several angiotensin-II-receptor antagonists by capillary electrophoresis. J Pharm Biomed Anal. 2003;31:329–39. doi: 10.1016/s0731-7085(02)00643-x. [DOI] [PubMed] [Google Scholar]
- 9.Lundberg DE , Jr, Person CR, Knox S, Cyronak MJ. Determination of SK& F 108566 (Teveten) in human plasma by reversed-phase high-performance liquid chromatography. J Chromatogr B. 1998;707:328–33. doi: 10.1016/s0378-4347(97)00598-7. [DOI] [PubMed] [Google Scholar]
- 10.Ferreiros N, Iriate G, Alonso RM, Jimenez RM, Ortiz E. Validation of solid phase extraction-high performance liquid chromatographic method for the determination of eprosartan in plasma. J chromatogr A. 2006;1119:309–14. doi: 10.1016/j.chroma.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 11.Ferreirós N, Iriarte G, Alonso RM, Jiménez RM. MultiSimplex and experimental design as chemometric tools to optimize a SPE-HPLC-UV method for the determination of eprosartan in human plasma samples. Talanta. 2006;69:747–56. doi: 10.1016/j.talanta.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 12.Patel HU, Suhagia BN, Patel CN. Simultaneous analysis of Eprosartan and Hydrochlorothiazide in tablets by high-performance thin-layer chromatography with ultraviolet absorption densitometry. Acta Chromatogr. 2009;21:319–26. [Google Scholar]
- 13.Sagirli O, Onal A, Toker SE, Sensoy D. Simultaneous HPLC analysis of olmesartan and hydrochlorothiazide in combined tablets and in vitro dissolution studies. Chromatographia. 2007;66:213–8. [Google Scholar]
- 14.Satana E, Altinay S, Göğer NG, Ozkan SA, Sentürk Z. Simultaneous determination of valsartan and hydrochlorothiazide in tablets by first-derivative ultraviolet spectrophotometry and LC. J Pharm Biomed Anal. 2001;25:1009–13. doi: 10.1016/s0731-7085(01)00394-6. [DOI] [PubMed] [Google Scholar]
- 15.Bebawy LI, Abbas SS, Fattah LA, Refaat HH. Application of firstderivative, ratio derivative spectrophotometry, TLC-densitometry and spectrofluorimetry for the simultaneous determination of telmisartan and hydrochlorothiazide in pharmaceutical dosage forms and plasma. Farmaco. 2005;60:859–67. doi: 10.1016/j.farmac.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Bhat LR, Godge RK, Vora AT, Damle MC. A Validated RPHPLC Method for Simultaneous Determination of Telmisartan and Hydrochlorothiazide in Pharmaceutical Formulation. J Liq Chromatogr Relat Technol. 2007;30:3059–67. [Google Scholar]
- 17.ICH Guidelines Q2B, Technical Requirements for Registration of Pharmaceuticals for Human Use Guideline on validation of Analytical Procedures-Methodology, Geneva, Switzerland; 1996.


