Abstract
Background:
A new, simple, selective, precise, and stability-indicating high-performance thin-layer chromatographic method has been established for analysis of itraconazole (ITZ) in the bulk drug and in pharmaceutical formulations. Separation was achieved on aluminium plate precoated with silica gel 60F254 using Toluene : Chloroform : Methanol [5 : 5 : 1.5 (v/v)] as mobile phase. Densitometric analysis was performed at 260 nm.
Result:
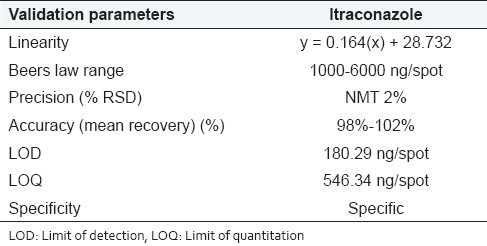
Compact bands of ITZ were obtained at Rf 0.52 ± 0.02. Linearity (R2 = 0.9978), limit of detection (180.29 ng/band), limit of quantification (546.34 ng/band), recovery (98–102%), and precision (≤0.51%) were satisfactory. Drug was not degraded under neutral and alkaline hydrolysis, UV and photolytic degradation, under-elevated temperature, and humidity. ITZ is degraded under acidic hydrolysis and oxidative condition; the degraded products were well resolved from individual bulk drug response. Developed method can effectively resolve drug from its excipients in capsule dosage form. The specificity of the method was confirmed by peak purity of resolved peak.
Conclusion:
The method can be applicable for routine analysis of ITZ in pharmaceutical formulation as stability-indicating. Because the method can effectively separate the drug from its degradation products as well as excipients, it can be used as a stability-indicating method.
Keywords: Degradation, method validation, stability
INTRODUCTION
Itraconazole (ITZ) is a potent triazole antifungal agent that is prescribed to patients with fungal infections. The drug is given orally in capsule form and as liquid oral form. The IUPAC nomenclature of the drug is as follows: (2R,4S)-rel-1-(butan-2-yl)-4-{4-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-ioxolan-4-yl]-methoxy}-phenyl)-piperazin-1-yl]phenyl}-4,5-dihydro-1H-1,2,4-triazol-5-one [Figure 1].[1–3].
Figure 1.
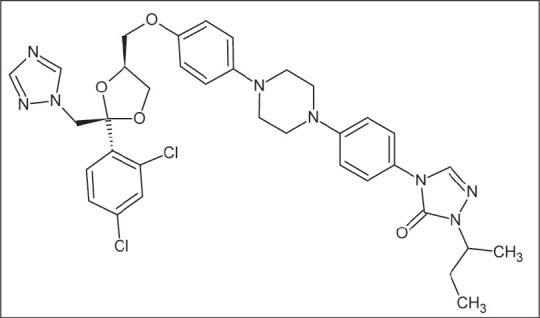
Chemical structure of ITZ
ITZ is used orally in the form of capsule for treatment of dermatophyte infections, on occurrence of superficial fungal infections and in systemic fungal infections. For quality control and stability testing of ITZ in pharmaceutical formulations, limited methods have been published. Spectrofluorimetry method has been published for assay of ITZ in raw material and in dosage forms.[4] RP-HPLC method is published for determination of ITZ in human plasma.[5–7] Chromatographic separation in this method was performed on an octadecylsilane column using fluorescence detector.[5] However, it has the disadvantage of being time-consuming. All these studies have further emphasized the need to perform rapid and sensitive quality-control analysis of pharmaceutical formulations containing ITZ. High-performance thin-layer chromatographic (HPTLC) method for analysis of ITZ in pharmaceutical formulations has not been reported yet. Because of rapidity, selectivity, sensitivity, and overall versatility, development of precise and validated HPTLC methods for quality control of drugs received much more attention.[8] The objective of this study was to develop and validate a rapid, simple, sensitive, and reproducible HPTLC method for quantification of ITZ in the bulk drug and in pharmaceutical formulation that can be applicable as stability-indicating. The developed stability-indicating HPTLC method has been validated according to ICH Q2 (B) guideline.[9]
MATERIALS AND METHODS
ITZ was provided as a gift sample by Akums Drugs and Pharma. Ltd. Drug was used without any further purification. All other reagents used for experimentation were of analytical reagent grade. Chemicals used for this experiment were Toluene, Methanol, Chloroform, NaOH, HCl, and H2O2 purchased from Finar reagents, Ahmedabad. Itaspor capsules were used as marketed formulation of ITZ.
Instrumentation
Chromatographic separation of drug was performed on Merck TLC plate precoated with silica gel 60 F254 (10*10 cm with 250 mm thickness of layer) from E. Merck, Germany. The samples were applied onto the plates as a band width of 4 mm using desaga 100 μl sample syringe (Hamilton, Switzerland) with applicator (Desaga). Linear ascending development was carried out in a twin through glass chamber (10*10 cm). Densitometric analysis was performed with a Desaga TLC scanner operated by ProQuant software (Version 1.06). Electronic balance (Acculab) was used for weighing purpose.
Preparation of solutions
Standard stock solution
Standard stock solution of ITZ was prepared by dissolving 50 mg of drug in 50 ml methanol to obtain 1 000 μg/ml stock solution and further diluted to get final concentration of 100 μg/ml. This solution was applied to TLC plate precoated with silica gel 60 F254 as band of 4 mm at a distance of 20 mm from x-axis and 15 mm from y-axis. It was developed in the chamber using optimized mobile phase consisting of Toluene : Chloroform : Methanol in the ratio of 5 : 5 : 1.5 v/v. The plate was developed up to 90 cm and dried in the air and scanned at 260 nm.
Sample solution
To measure ITZ content of capsule (label claim 100 mg ITZ per capsule, Itaspor capsules), 20 capsules were weighed. The mean weight was determined. A weight of the powder equivalent to 100 mg ITZ was transferred to a 100-ml volumetric flask containing 50 ml methanol and the mixture was sonicated for 30 minutes, then diluted to 100 ml with methanol (1 000 μg/ml). The solution was filtered and 1 ml of filtered solution was diluted five-fold to furnish a concentration of 200 μg/ml. This solution (20 μl, 4 000 ng/band) was applied to a TLC plate which was developed and scanned as described above. The analysis was repeated in triplicate. The possibility of interference of excipients with the analysis was also examined.
Forced degradation studies
Forced degradation of drug substance was carried out under photolytic, UV degradation, elevated temperature and humidity, acid/base/neutral hydrolytic and oxidative conditions. After the degradation, 6 000 ng ITZ per spot was applied.
Acid degradation
For acid degradation study, 10 mg of drug was weighed accurately and transferred into 10 ml volumetric flask. 1N methanolic HCl was added and made up to the mark. The solution was refluxed for 2 hour at 75°C. After this, it was neutralized using 1N NaOH and from this solution, an appropriate dilution was made and 6 000 ng/spot was applied.
Alkali degradation
For alkali degradation study, 10 mg of drug was weighed accurately and transferred into 10 ml volumetric flask. 1N Methanolic NaOH was added and made up to the mark. The solution was refluxed for 2 hour at 75°C. After this, it was neutralized using 1N HCl and from this solution an appropriate dilution was made and 6 000 ng/spot was applied.
Neutral degradation
For neutral degradation study, 10 mg of drug was weighed accurately and transferred into 10 ml volumetric flask. After that, volume was made up to the mark with methanol and water (80 : 20 v/v). The solution was refluxed for 1 hour at 75°C. After this, an appropriate dilution was made and 6 000 ng/spot was applied.
Oxidation degradation
For oxidative degradation study, 10 mg of drug was weighed accurately and transferred into 10 ml volumetric flask. 1 ml of 30% hydrogen peroxide was added and made up to the mark with methanol. After this, an appropriate dilution was made and 6 000 ng/spot was applied.
Photolytic degradation
For photolytic degradation study, 10 mg of drug was weighed accurately and kept into sunlight for 3 days (8 hours/day), then transferred into 10-ml volumetric flask and volume was made up to the mark with methanol. After this, an appropriate dilution was made and 6 000 ng/spot was applied.
UV degradation
For UV degradation study, 10 mg of drug was weighed accurately and kept inside UV chamber for 40 hours at 254 nm. Then, it was transferred into 10-ml volumetric flask and volume was made up to the mark with methanol. After this, an appropriate dilution was made and 6 000 ng/spot was applied.
Degradation under elevated temperature and humidity
For degradation under elevated temperature and humidity study, 10 mg of drug was weighed accurately and kept inside humidity chamber for 7 days at 40°C and 75% relative humidity. Then, it was transferred into 10-ml volumetric flask and volume was made up to the mark with methanol. After this, an appropriate dilution was made and 6 000 ng/spot was applied.
Thermal degradation
For thermal degradation study, 10 mg of drug was weighed accurately and kept inside hot air oven for 3 days at 80°C. Then, it was transferred into 10-ml volumetric flask and volume was made up to the mark with methanol. After this, an appropriate dilution was made and 6 000 ng/spot was applied.
Validation of analytical method
The developed HPTLC method for the estimation of ITZ was validated for specificity, linearity, precision and accuracy, limit of detection (LOD), and limit of quantitation (LOQ) according to ICH guidelines. It was established that products of degradation do not interfere with the peak response of the drug.
Linearity of response
To demonstrate the linearity, concentrations of 1 000 to 6 000 ng were applied to TLC plate and densitograms were developed. The graph was constructed between concentrations vs peak area and treated by regression analysis.
Accuracy and precision
The accuracy of an analytical method describes the closeness of mean test results obtained by the method to the true value (concentration) of the analyte. The precision of an analytical method describes the closeness of individual measures of an analyte when the procedure is applied repeatedly to multiple aliquots of a single homogeneous volume. Accuracy and precision were determined by replicate analysis of samples containing known amounts of the analyte. For reproducibility and accuracy, standard solution at 50%, 100%, and 150% concentrations were prepared.
Intraday precision and accuracy were studied by three replicate measurements at three concentration level in one day. Interday precision was conducted during routine operation of system over a period of three consecutive days. Statistical evaluation like relative standard deviation of drug at different concentrations of three replicates application was calculated to determine intraday and interday variation.
Specificity
Specificity of a method was determined by analyzing drug and forcefully degraded products. Purity of drug peak was ascertained by analyzing the spectrum at peak start, max position, and at peak end. The peak purity was determined by ProQuant software.
Limit of detection and limit of quantitation
The LOD and LOQ were obtained by calculating using the standard formula as per ICH guidelines. LOD = 3.3σ/s and LOD = 10σ/s. Where, σ is standard deviation of response and S is the slope of calibration curve.
RESULTS
Development of the optimum mobile phase
TLC procedure was optimized with a view to develop a stability-indicating assay method. The standard of drug was spotted on the TLC plates and developed in different systems. Different mobile phase were tried to resolve drug and degradation products. The optimum result was obtained with Toluene : Chloroform : Methanol in the ratio of 5 : 5 : 1.5 v/v. The chamber was saturated with mobile phase at room temperature. Developed mobile phase gives Rf of ITZ of 0.52+0.02. The representative chromatogram is given in Figure 2.
Figure 2.
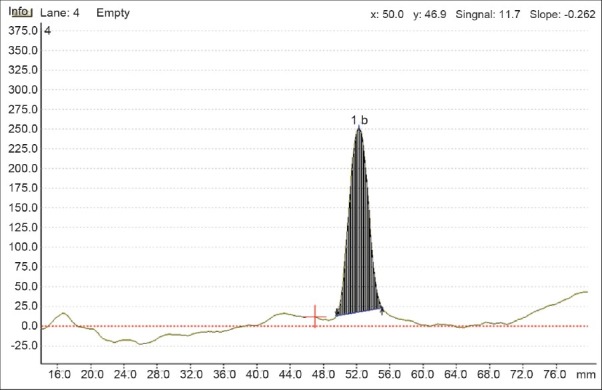
Representative densitogram of ITZ (6000 ng/spot)
Validation of developed stability-indicating method
Linearity
The response of the drug was found to be linear in the concentration range of 1 000 to 6 000 ng/band for ITZ with correlation coefficient of 0.9978. The linear regression equation obtained was y = 0.164 (x) + 28.732. The representative chromatogram is given in Figure 3.
Figure 3.
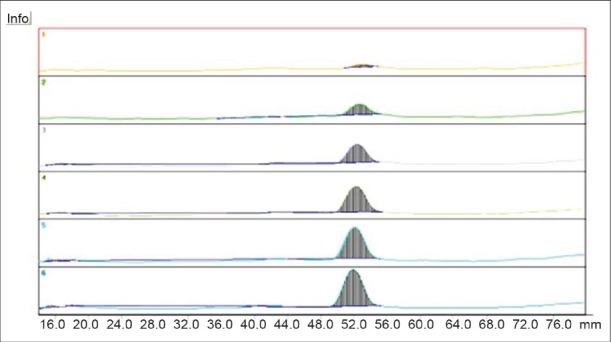
Overlain densitogram of ITZ for concentration of 1000-6000 ng/spot
Precision
The relative standard deviation value for intraday precision study was found to be 0.51% and for interday precision was found to be not more than 0.34% for ITZ. This reveals that method is precise.
Accuracy
Good recoveries between 98.86 and 100.43% were obtained at each level of added concentrations. The results obtained (n = 3 for each 80%, 100%, 120% level) indicated as the mean recovery were between 98 to 102% for ITZ.
Limit of detection
The LOD as calculated by standard formula as given in ICH guidelines was found to be 180.29 ng/band.
Limit of quantitation
The LOQ as calculated by standard formula as given in ICH guidelines was found to be 546.34 ng/spot.
Specificity
The specificity of the method was ascertained by peak purity profiling studies. The purity of drug peak was ascertained by analyzing spectrum at peak start, peak end, and peak max, which showed no interference of any other excipients or impurities in peak of ITZ.
Analysis of marketed formulation
Experimental results from analysis of the amount of ITZ in capsules were in good agreement with the label claims, suggesting that there was no interference from any of the excipients normally present in the capsules. The drug content was found to be 99.86 ± 0.28%. ITZ capsules were analyzed using the proposed procedure; the results obtained are summarized in Table 1.
Table 1.
Results of analysis of pharmaceutical formulation

The validation summary is given in Table 2.
Table 2.
Summary of validation parameters
Degradation behavior
HPTLC study of ITZ under different stress conditions suggested the following degradation behavior.
Hydrolytic studies
Acidic condition
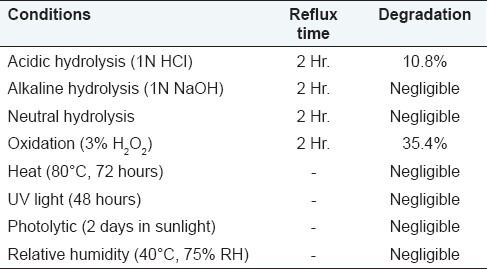
ITZ was degraded under acidic with reflux condition (1N HCl for 2 hours at 75°C). Two additional degradation products were formed having Rf 0.40 and 0.75. The drug was degraded 10.2%.
Alkaline condition
ITZ showed negligible degradation under alkaline hydrolysis (1N NaOH for 2 hours at 75°C) with reflux condition. No additional significant peak was observed and peak area of drug showed negligible decrease.
Neutral (water) condition
ITZ showed negligible degradation under neutral hydrolysis with reflux condition. No additional significant peak was observed and peak area of drug showed negligible decrease.
Oxidative studies
ITZ showed significant degradation upon treatment with 3% H2O2. One additional degradation product was formed having Rf 0.23. The drug was degraded 35.4% [Figure 4].
Figure 4.
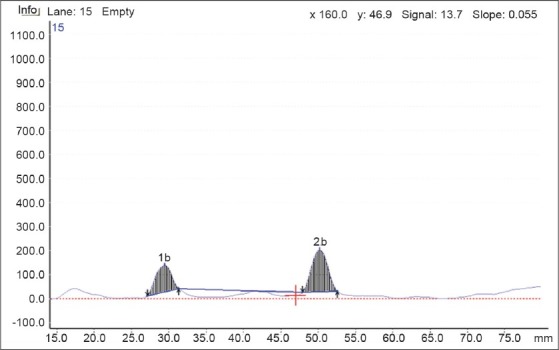
Representative densitogram of ITZ under oxidative condition
Thermal stress (dry heat), photolytic and degradation under humidity studies
Under dry heat (80°C, 72 hours) and under elevated temperature and humidity (40°C, 75%RH), studies did not give any additional peaks and peak area of drug remains almost same. This indicates stability of drug under heat and humidity. Exposure of sunlight (3 days) and UV light (40 hours) does not give any additional peak and minor change in peak area of drug. The forced degradation study results are summarized in Table 3.
Table 3.
Summary of forced degradation study results
DISCUSSION
There have been no reported HPTLC methods for analysis of ITZ in dosage forms. The objective of work was to develop simple, rapid, and sensitive HPTLC method for quantification of ITZ in raw materials and pharmaceutical formulations. The main criteria for development of successful analytical method for determination of ITZ are that the method should be free from interference from excipients and simple enough for routine use in quality control. Initially, the pure drug was applied to TLC plates and chromatographed with different mobile phases. When used alone, toluene and methanol were able to chromatograph the drug on the TLC plate, but the bands were highly diffused. Thereafter, toluene, chloroform, and methanol in different ratios were tried. When the Toluene : Chloroform : Methanol [5 : 5 : 1.5 (v/v)] was used, tailing was significantly reduced. Finally, the optimum mobile phase Toluene : Chloroform : Methanol [5 : 5 : 1.5 (v/v)] resulted in a sharp, well-defined symmetrical peak for ITZ at Rf 0.52 ± 0.02, as shown in Figure 2.
The UV spectrum of ITZ showed that λmax of ITZ is 262 nm, but detection was performed at 260 nm because selectivity was better. Densitometric analysis at 260 nm improved the detection sensitivity and minimized interference. Chromatograms obtained from bulk ITZ were compared with chromatograms obtained from formulations (capsules) to assess the specificity and selectivity of the procedure. No peaks were observed at or near the Rf of ITZ, indicating the high selectivity of the HPTLC method.
The total analytical run-time using this method was also much shorter than the reported run-time in a previous study. This HPTLC assay also has the advantage of simplicity and convenience. The chromatograms and drug recovery indicated that degradation of ITZ had not occurred in the capsule formulations. However, no additional peaks were observed under neutral and alkaline hydrolysis, UV and photolytic degradation, degradation under elevated temperature and humidity. As a result of acidic decomposition of ITZ, the chromatogram of ITZ solution contained two additional peaks (degradation product peaks) with the ITZ peak. As a result of oxidative decomposition of ITZ, the chromatogram of ITZ solution contained one additional peak (degradation product peak) with the ITZ peak. The method also seems to be stability-indicating, because the degradation product of acidic hydrolysis having Rf 0.40 and 0.75 and degradation product of oxidation having Rf 0.23 were well resolved from the drug peak (Rf 0.52) with significantly different Rf values. These results indicated that this HPTLC method is suitable for routine analysis of pharmaceutical dosage forms.
CONCLUSIONS
From the above study, we can conclude that ITZ undergoes degradation in acidic hydrolysis and oxidative conditions. All degradation products formed are well resolved from the drug response. Peak purity reveals that peak of degradation products were not interfering response of drug. It is of potential value for analysis of ITZ in the bulk drug and in commercial formulations. The developed method is simple, accurate, specific, and precise and it can be proposed for routine analysis of drug in presence of degradation products and excipients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rang HP, Dale MM, Ritter JM, Flower RJ. Pharmacology. 6th Ed. New Delhi: Elsevier Publication House; 2003. pp. 666–71. [Google Scholar]
- 2.Joel GH. Goodman and Gilman's the Pharmacological basis of therapeutics. 10th Ed. New York: McGraw Hill Publishers; 2001. pp. 356–62. [Google Scholar]
- 3.Tripathi KD. Essential of Medical Pharmacology. 5th Ed. New Delhi: Jaypee Brothers’ Medical Publishers; 2003. pp. 245–9. [Google Scholar]
- 4.El-Enany N, El-Sherbilny D, Belal F. Spectrofluorimetric determination of itraconazole in dosage forms and spiked human plasma. J Chin Chem Soc. 2007;54:375–82. [Google Scholar]
- 5.Al-Rawithi S, Hussein R, Al-Moshen I, Raines D. Determination of itraconazole and hydroxyitraconazole in plasma by high-performance liquid chromatography with fluorescence detection. Ther Drug Monit. 2001;23:445–8. doi: 10.1097/00007691-200108000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Khoschsorur G, Frueehwirth F, Zelzer S. Isocratic High performance liquid chromatographic method with UV detection for simultaneous determination of voriconazole and itraconazole and its hydroxyl metabolite in human serum. Antimicrob Agents Chemother. 2005;49:3569–71. doi: 10.1128/AAC.49.8.3569-3571.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivatsan V, Dasgupta A, Kale P, Datla R, Soni D, Patel M, et al. Simultaneous determination of itraconazole and hydroxy-itraconazole in human plasma by high-performance liquid chromatography. J Chromatogr A. 2004;1031:307–13. doi: 10.1016/j.chroma.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 8.Sethi PD. HPTLC – Quantitative Analysis of Pharmaceutical formulations. 1st Ed. New Delhi: CBS Publishers and Distributors; 1996. pp. 3–35. [Google Scholar]
- 9. ICH [Validation of Analytical Procedures: Text and Methodology (Q2B)], International Conference on Harmonization of Technical Requirements for Registration of Pharmaceutical for Human use. Geneva: ICH, Geneva, Switzerland. 1997 and August 2002.


