Abstract
Background:
The main objective was to develop and validate the UV-spectrophotometric method for the estimation of terbinafine hydrochloride in bulk and pharmaceutical formulations as per ICH guidelines
Materials and Methods:
A simple, rapid, accurate, and economical UV-spectrophotometric method has been developed for the estimation of terbinafine hydrochloride from bulk and pharmaceutical formulation.
Results:
The λmax of terbinafine hydrochloride in water was found to be 283 nm. The drug follows linearity in the concentration range 5–30 μg/ml with a correlation coefficient value of 0.999. The proposed method was applied to pharmaceutical formulation and % amount of drug. estimated was 99.19% and was found to be in good agreement with the label claim. The accuracy of the method was checked by recovery experiment performed at three different levels, i.e., 80%, 100%, and 120%. The % recovery was found to be in the range of 98.54– 99.98%. The low values of % RSD are indicative of the accuracy and reproducibility of the method. The precision of the method was studied as an intraday; interday variations, and repeatability. The % RSD value < 2 indicates that the method is precise. Ruggedness of the proposed method was studied with the help of two analysts.
Conclusion:
The above method was a rapid tool for routine analysis of terbinafine hydrochloride in the bulk and in the pharmaceutical dosage form.
Keywords: Quantitative determination, terbinafine hydrochloride, UV, validationic
INTRODUCTION
Terbinafine hydrochloride,[1] (E)-N-(6,6-dimethyl-2-hepten-4-ynyl)-N-methyl-1-naphthalene methanamine hydrochloride [Figure 1], is a new potent antifungal agent of allylamine class that selectively inhibits fungal squalene epoxidase. The drug is indicated for both oral and topical treatment of mycoses.[2,3] Terbinafine hydrochloride is not yet official in any pharmacopeia, where, only few analytical methods have been reported for its determination in pharmaceutical formulations and biological fluids. Such methods include HPLC,[4–10] colorimetry,[11] electrochemistry,[12] and solvent meting method.[13]
Figure 1.

Chemical structure of terbinafine hydrochloride
Among the various methods available for the determination of drugs, spectrophotometry continues to be very popular, because of their simplicity, specificity, and low cost. This study presents a new spectrophotometric method for the determination of terbinafine hydrochloride phosphate in bulk and pharmaceutical formulations. Accordingly, the objective of this study was to develop and validate the UV-spectrophotometric method for the estimation of terbinafine hydrochloride in bulk and pharmaceutical formulations as per ICH guidelines.[14]
MATERIALS AND METHODS
Materials
Terbinafine hydrochloride was a gift sample from Dr. Reddys Lab, Hyderabad. All chemicals and reagents used were of analytical grade and purchased from Qualigens Fine Chemicals, Mumbai, India.
Preparation of standard stock solution
Accurately weighed 10 mg of terbinafine hydrochloride was transferred to a 100 ml volumetric flask, dissolved in 20 ml distilled water by shaking manually for 10 min. The volume was adjusted with the same up to the mark to give the final strength, i.e. 100 μg/ml.
Selection of wavelength for analysis of terbinafine hydrochloride
Appropriate volume 0.5 ml of standard stock solution of terbinafine hydrochloride was transferred into a 10 ml volumetric flask, diluted to a mark with distilled water to give concentration of 5 μg/ml. The resulting solution was scanned in the UV range (200–400 nm). In spectrum terbinafine hydrochloride showed absorbance maximum at 283 nm [Figure 2].
Figure 2.
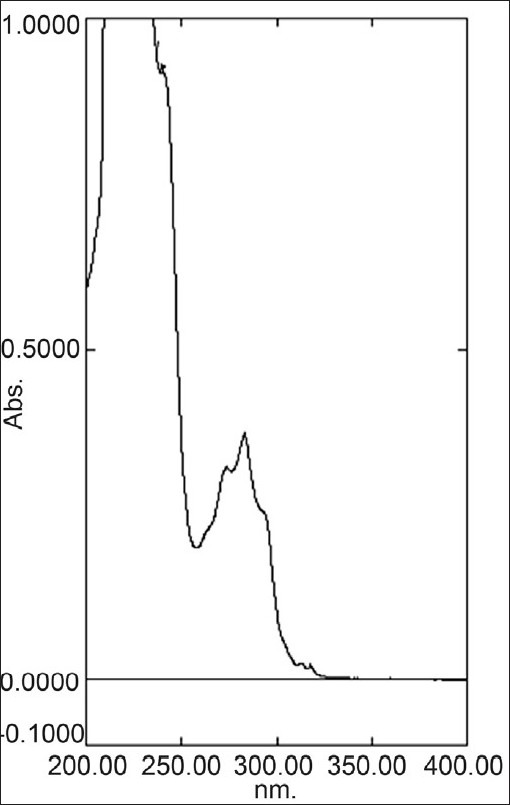
UV spectrum of terbinafine hydrochloride at 283 nm
Validation of the method
The method was validated in terms of linearity, accuracy, precision, and ruggedness.
Linearity study
Different aliquots of terbinafine hydrochloride in the range 0.5–3 ml were transferred into series of 10 ml volumetric flasks, and the volume was made up to the mark with distilled water to get concentrations 5, 10, 15, 20, 25, and 30 μg/ml, respectively. The solutions were scanned on a spectrophotometer in the UV range 200–400 nm. The spectrum was recorded at 283 nm. The calibration plot was constructed as concentration vs. amplitude [Figure 3].
Figure 3.
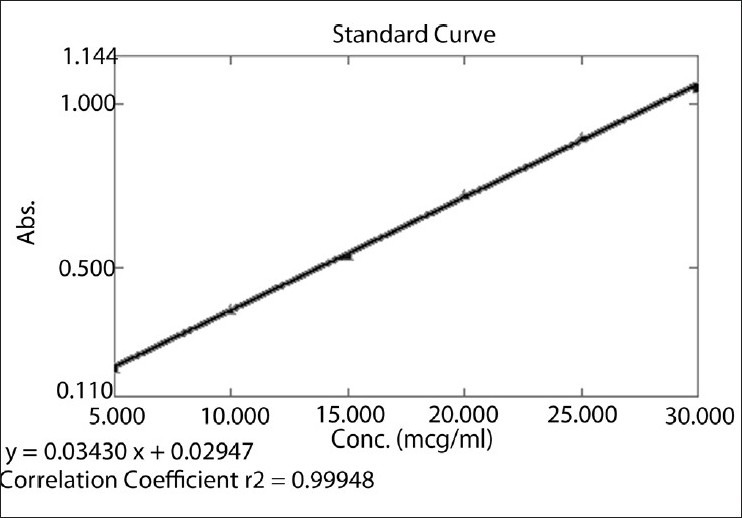
Calibration curve of terbinafine hydrochloride at 283 nm
Accuracy
To the preanalysed sample solutions, a known amount of standard stock solution was added at different levels, i.e. 80%, 100%, and 120%. The solutions were reanalyzed by the proposed method.
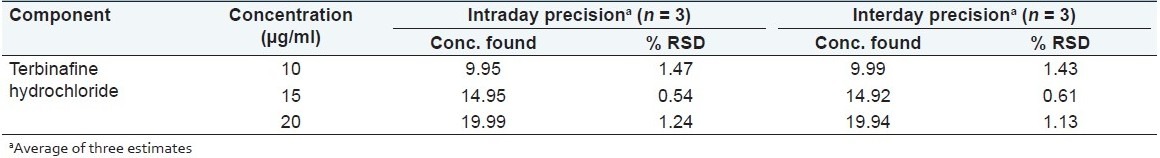
Precision
Precision of the method was studied as intraday and interday variations. Intraday precision was determined by analyzing the 10, 15 and 20 μg/ml of terbinafine hydrochloride solutions for three times in the same day. Interday precision was determined by analyzing the 10, 15, and 20 μg/ml of terbinafine hydrochloride solutions daily for 3 days over the period of week.
Sensitivity
The sensitivity of measurements of terbinafine hydrochloride by the use of the proposed method was estimated in terms of the limit of quantification (LOQ) and limit of detection (LOD). The LOQ and LOD were calculated using equation LOD = 3.3 × N/B and LOQ = 10 × N/B, where ‘N’ is standard deviation of the peak areas of the drugs (n = 3), taken as a measure of noise, and ‘B’ is the slope of the corresponding calibration curve.
Repeatability
Repeatability was determined by analyzing 20 μg/ml concentration of terbinafine hydrochloride solution for six times.
Ruggedness
Ruggedness of the proposed method is determined for 20 μg/ml concentration of terbinafine hydrochloride by analysis of aliquots from a homogenous slot by two analysts using same operational and environmental conditions.
Determination of terbinafine hydrochloride in bulk
Accurately weighed 10 mg of terbinafine hydrochloride was transferred into a 100 ml volumetric flask containing 20 ml distilled water, and the volume was made up to the mark using the same. Appropriate volume 0.6 ml of this solution was transferred to a 10 ml volumetric flask, and the volume was adjusted to the mark using distilled water. The resulting solution was scanned on a spectrophotometer in the UV range 200–400 nm. The concentrations of the drug were calculated from linear regression equations.
Application of the proposed method for pharmaceutical formulation
For analysis of commercial formulation 5 ml of terbinafine hydrochloride eye drop solution was taken in a 100 ml volumetric flask and the volume was made up to the mark with distilled water to give 100 μg/ml concentration. From this 2 ml was taken and transferred to a 10 ml volumetric flask and the volume was made up to the mark with distilled water to give 20 μg/ml concentration. It was scanned on a spectrophotometer in the UV range 200–400 nm. The spectrum was recorded at 283 nm. The concentrations of the drug were calculated from the linear regression equation.
RESULTS AND DISCUSSION
Method validation
The proposed method was validated as per ICH guidelines. The solutions of the drugs were prepared as per the earlier adopted procedure given in the experiment.
Linearity studies
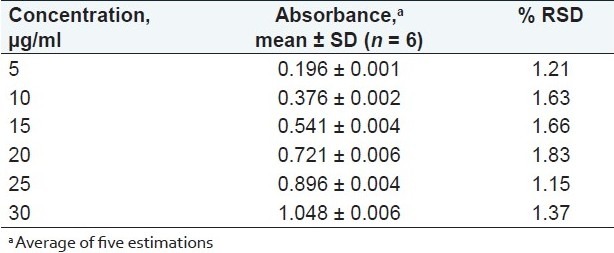
The linear regression data for the calibration curves showed good linear relationship over the concentration range 5–30 μg/ml for terbinafine hydrochloride [Figure 4]. Linear regression equation was found to be Y = 0.0343X + 0.0294 (r2 = 0.999). The result is expressed in Table 1.
Figure 4.
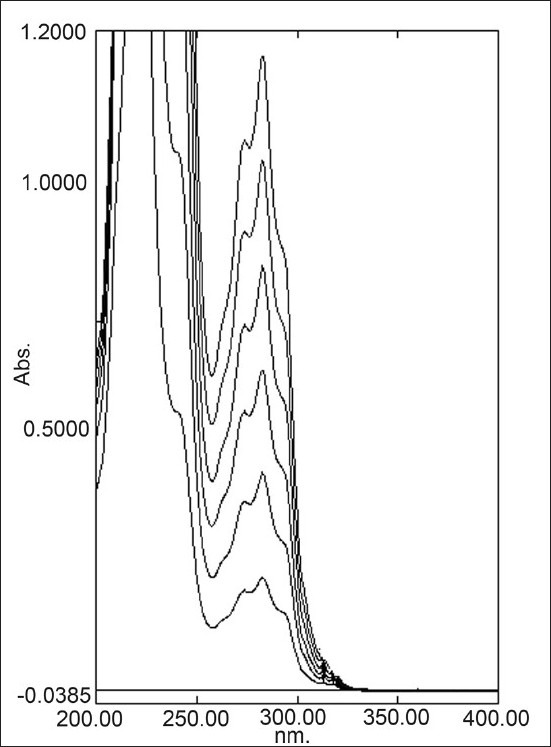
Overlain spectra of terbinafine hydrochloride (5-30 μg/ml) at 283 nm
Table 1.
Linearity study of terbinafine hydrochloride
Accuracy
The solutions were reanalyzed by the proposed method; results of recovery studies are reported in Table 2 which showed that the % amount found was between 98.54% and 99.98% with % RSD > 2.
Table 2.
Recovery studies

Precision
The precision of the developed method was expressed in terms of % relative standard deviation (% RSD). These results show reproducibility of the assay. The % RSD values found to be less than 2 that indicate this method precise for the determination of both the drugs in formulation [Table 3].
Table 3.
Precision studies
Sensitivity
The linearity equation was found to be Y = 0.0384X + 0.0314. The LOQ and LOD for terbinafine hydrochloride were found to be 0.42 μg and 1.30 μg, respectively.
Repeatability
Repeatability was determined by analyzing 20 μg/ml concentration of terbinafine hydrochloride solution for six times and the % amount found was between 98% and 102% with % RSD < 2 [Table 4].
Table 4.
Repeatability studies

Ruggedness
The peak area was measured for same concentration solutions, six times. The results are in the acceptable range for both the drugs. The results are given in Table 5. The result showed that the % RSD was less than 2%.
Table 5.
Ruggedness studies

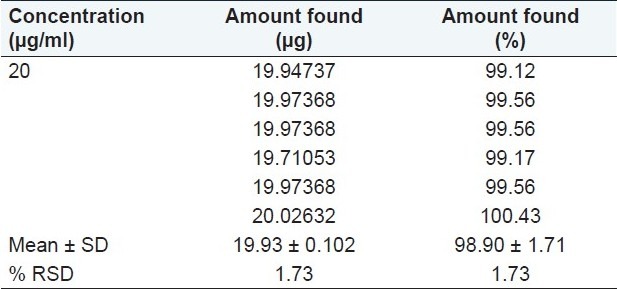
Determination of terbinafine hydrochloride in bulk
The concentrations of the drug were calculated from linear regression equations. The % amount found was between 99.12% and 100.43% [Table 6].
Table 6.
Analysis of Terbinafine hydrochloride in bulk
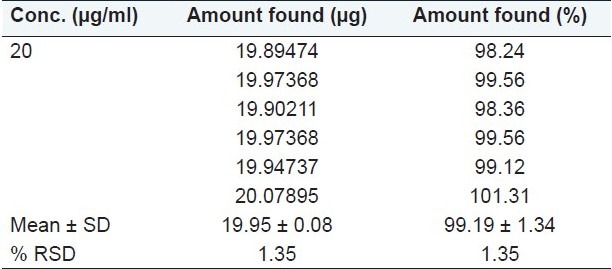
Application of the proposed method for pharmaceutical formulation
The spectrum was recorded at 283 nm. The concentrations of the drug were calculated from the linear regression equation. The % amount found was between 98.24% and 101.31% [Table 7].
Table 7.
Analysis of formulation
CONCLUSION
This UV-spectrophotometric technique is quite simple, accurate, precise, reproducible, and sensitive. The UV method has been developed for quantification of terbinafine hydrochloride in tablet formulation. The validation procedure confirms that this is an appropriate method for their quantification in the formulation. It is also used in routine quality control of the formulations containing this entire compound.
ACKNOWLEDGMENTS
The authors are thankful to the Principal and Management, R.C. Patel Institute of Pharmaceutical Education and Research, Shirpur (M.S.), India, for providing the required facilities to carry out this research work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Budavari S. The Merck Index. 12th ed. New Jersey, USA: Merck and Co., Inc; 1996. p. 1564. [Google Scholar]
- 2.Martindale. The Complete Drug Reference. 32nd ed. Great Britain: Council of Royal Pharmaceutical Society of Great Britain; 1999. pp. 367–87. [Google Scholar]
- 3.Delgodo JN, Remers WA. Text book of Organic Medicinal and Pharmaceutical Chemistry. 9th ed. Philadelphia: J. B. Liipincott Company; 1991. Wilson and Gisvold's; pp. 145–53. [Google Scholar]
- 4.Gonecalves-codoso S, Schapoval EE. High performance chromatographic assay of terbinafine hydrochloride in tablets and creams. J Pharm Biomed Anal. 1999;19:809–12. doi: 10.1016/s0731-7085(98)00119-8. [DOI] [PubMed] [Google Scholar]
- 5.Ji XF, Yu KY, Zhang W. Determination of terbinafine hydrochloride, chlorohexidine and triameinolone acetonide acetate in compound ointment by reversed phase HPLC. Chin J Pharm Anal. 1997;17:363–5. [Google Scholar]
- 6.Yagnesh MH, Melachlan AJ. Determination of terbinafine in tissues. Biomed Chromatogr. 2000;14:261–8. doi: 10.1002/1099-0801(200006)14:4<261::AID-BMC983>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 7.Zehender H, Denouel J, Roy M, Le-Saux L, Schuab P. Simultaneous determination of terbinafine and five metabolites in human plasma and urine by high performance liquid chromatography using online solid phase extraction. J Chromatogr B. 1995;664:347–55. doi: 10.1016/0378-4347(94)00483-l. [DOI] [PubMed] [Google Scholar]
- 8.Denouel J, Keller HP, Schaub P, Delborde C, Humbert H. Determination of terbinafine and its desmethyl metabolites in human plasma by high performance liquid chromatography. J Chromatogr B. 1995;663:353–9. doi: 10.1016/0378-4347(94)00449-f. [DOI] [PubMed] [Google Scholar]
- 9.Schatz F, Haberl H. Analytical methods for the determination of terbinafine and its metabolites in human plasma, milk and urine. Arzneim-Forsch. 1989;39:527–32. [PubMed] [Google Scholar]
- 10.Brigol N, Bakhtiyar R, Dou L, Majumdar T, Tse FL. Quantitative analysis of terbinafine in human and minipig plasma by liquid chromatography tandem mass spectrometry. Rapid Commun Mass-Spctrom. 2000;14:141–9. doi: 10.1002/(SICI)1097-0231(20000215)14:3<141::AID-RCM856>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.Cardoso SG, Schapoval EE. UV spectrophotometry and non aqueous determination of terbinafine hydrochloride in dosage forms. J AOAC Int. 1999;82:380–3. [PubMed] [Google Scholar]
- 12.Arranz A, Fernandez S, Moreda JM, Cid A, Arranz JF. Voltametric behavior of the antimycotic terbinafine at the hanging mercury drop electrode. Anal Chim Acta. 1997;351:97–103. [Google Scholar]
- 13.Arun Prasad K, Narayanan N, Rajalakshami G. Preparation and evaluation of solid dispersion of terbinafine hydrochloride. Int J Pharm Sci Rev Res. 2010;3:130–4. [Google Scholar]
- 14.ICH-Guidelines Q2 (R1), Validation of Analytical Procedures: Text and Methodology. 2005 [Google Scholar]


