Abstract
Purpose:
This study was designed to develop and validate two simple, rapid, and economical UV-spectrophotometric and the first-order derivative methods using the area under curve method for estimation of diacerein in bulk and in capsule formulation.
Materials and Methods:
In this study, hydrotrophic solution of 8 M urea and 0.5 M potassium citrate were employed as the solubilizing agent to solubilize a poorly water-soluble drug, diacerein. In the UV-spectrophotometry method, two wavelengths 252.0 nm and 266.2 nm and in the first-order derivative spectrophotometric methods two wavelengths 259.4 nm and 274.2 nm in 8 M urea and two wavelengths 247.8 nm and 267.4 nm in the UV-spectrophotometry method and in the first-order derivative spectrophotometric methods two wavelengths 259.2 nm and 274.2 nm in 0.5 M potassium citrate were selected for determination of areas.
Results:
Hydrotrophic agents used did not interfere in spectrophotometric analysis of diacerein. Diacerein followed linearity in the concentration range of 2–12 μg/mL with a coefficient correlation of 0.999 for both methods.
Conclusion:
The amount of drugs estimated by both proposed methods are in good accord with label claim. The % RSD value in recovery, precision, and ruggedness studies are found to be less than 2 indicate that the method is accurate, precise, and rugged.
Keywords: Diacerein, derivative spectroscopy, hydrotropic solubilization, potassium citrate, urea
INTRODUCTION
Diacerein chemically is 4,5-bis(acetyloxy)-9,10-dioxo-2-anthracene carboxylic acid.[1–2] It is a selective inhibitor of interleukin-1 having a protective effect on granuloma-induced cartilage breakdown by a reduction in the concentrations of proinflammatory cytokines. It is used in the symptomatic treatment of osteoarthritis.[3] The literature survey revealed many analytical methods includes UV-spectroscopic methods,[4–7] RP-HPLC,[8–12] and HPTLC methods[13] for the estimation of diacerein in bulk, pharmaceutical formulation, and in biological samples. The spectrophotometric methods available for diacerein in literature reveal the use of dimethyl acetamide (DMA)[14] and dimethyl sulfoxide (DMSO)[15] to solubilize diacerein. Drawbacks of organic solvents include their higher cost, toxicity, and pollution. In addition, diacerein is poorly soluble in water. Therefore, hydrotropic solution may be a suitable alternative to exclude the use of organic solvents. Special systems are required to solubilize poorly water-soluble drugs. Hydrotropy is one of such techniques. Hydrotropy may be defined as a phenomenon in which the water solubility of poorly water-soluble compounds may be increased several folds by the use of diverse groups of hydrophilic solutes called hydrotropes.[16] Hydrotropes commonly used includes sodium benzoate, sodium acetate, sodium salicylate, nicotinamide, urea, trisodium citrate, sodium ascorbate, piperazine, caffeine, potassium citrate, etc.[17] Hydrotropic agents have been observed to enhance the solubility of various substances in water.[18] The mixed hydrotropic solubilization process is used to increase the solubility of poorly water-soluble drugs by using blends of hydrotropic agents. There was a miraculous synergistic effect on enhancement in solubility of a poorly water-soluble drug by mixing two hydrotropic agents.[19] In the literature, several methods were reported for estimation of poorly water-soluble drugs using hydrotropic solubilizing agents.[20–22] Therefore, it was thought useful to utilize this hydrotropic solution to extract out the drug from fine powder of tablets to carry out spectrophotometric estimation.
The objective of the present investigation is to develop simple, precise, and accurate UV-spectrophotometric and first-order derivative methods for determination of diacerein in bulk and in the capsule dosage form using hydrotropic solubilizing agents. The developed methods were validated as per the ICH guidelines.[23–25]
MATERIALS AND METHODS
Chemicals
Diacerein supplied as a gift sample by Macleods Pharmaceuticals Ltd., Mumbai. Capsule formulation (Orcerein) was purchased from an Indian market, containing Diacerein 50 mg.
Instrumentation
A UV-visible spectrophotometer (1700 Shimadzu, software UV Probe 2.21) with a spectral bandwidth of 1 nm was employed for all spectroscopic measurements, using a pair of 10 mm matched quartz cells.
Selection of solvent
Urea and potassium citrate solution were selected as a solvent for developing spectral characteristics of a drug. The selection was made after assessing the solubility in different hydrotropic solvents.
Preparation of stock standard solutions
Stock standard solutions of diacerein were prepared by dissolving 10 mg in a 100 mL volumetric flask containing 25 mL (8 M urea) solution and the volume was made up to the mark with water and again 10 mg diacerein was dissolved in a 100 mL volumetric flask containing 25 mL 0.5 M potassium citrate solution and the volume was made up to the mark with water to obtain concentrations 100 μg/mL each. After appropriate dilutions, 10 μg/mL diacerein was scanned in the UV-region, i.e. 400–200 nm. Diacerein showed λmax 258.2 nm in urea solution and 257.4 nm in potassium citrate solution.
Area under curve method
The area under curve method is used when a broad spectrum of the drug is obtained. From the spectrum of diacerein, the area under the curve in the range of 252.0-266.2 nm for the zero-order spectra [Figure 1] and 259.4-274.2 nm for first-order derivative spectra [Figure 2] were selected for determination of areas using urea solution. In the case of potassium citrate solution, two wavelengths 247.80 and 267.40 nm in the UV spectrophotometric method [Figure 3] and in the first-order derivative spectrophotometric method two wavelengths 259.2 nm and 274.2 nm [Figure 4] were selected for determination of areas.
Figure 1.
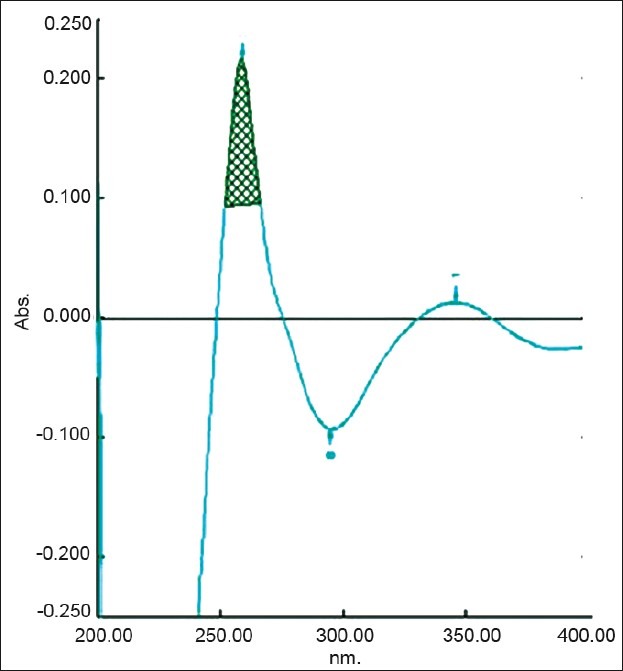
Zero-order spectra of diacerein showing λmax 258.2 nm in 8 M urea
Figure 2.
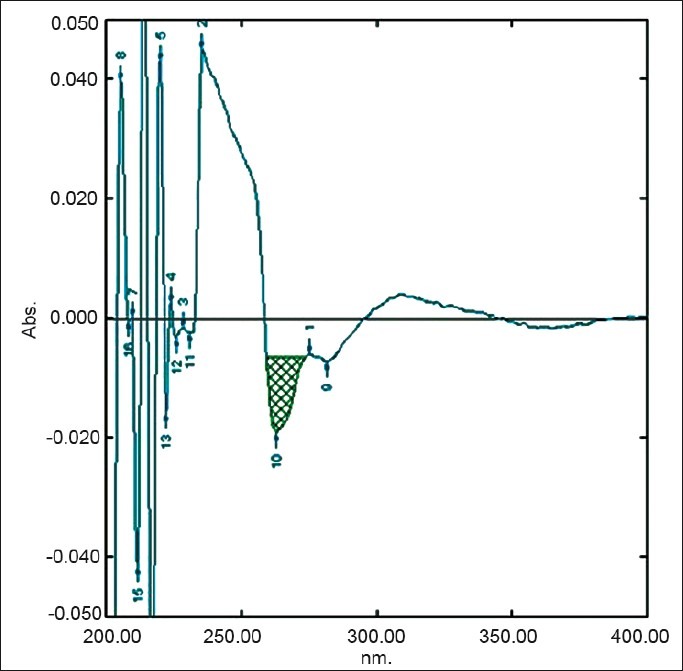
First derivative spectra of diacerein showing amplitude at 263.2 nm in 8 M urea
Figure 3.
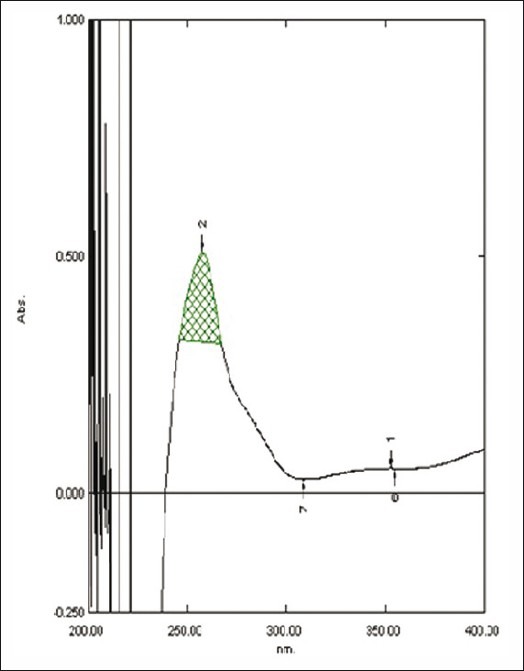
Zero-order spectra of diacerein showing λmax 257.4 nm in 0.5 M potassium citrate
Figure 4.
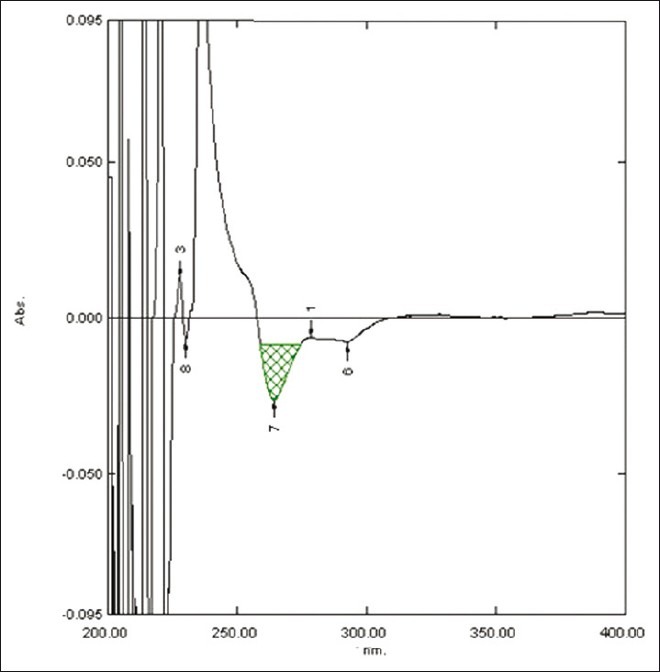
First derivative spectra of diacerein showing amplitude at 264.2 nm in 0.5 M potassium citrate
Study of linearities curve
An appropriate volume of diacerein in the range of 0.2-1.2 mL were transferred into six separate 10 mL volumetric flasks, from standard stock solutions of urea and potassium citrate and the volume was made up to mark with water to obtain concentration of 2-12 μg/mL each. The area of diacerein was measured at selected wavelengths, and calibration curves were plotted as concentration versus area.
Analysis of marketed formulation
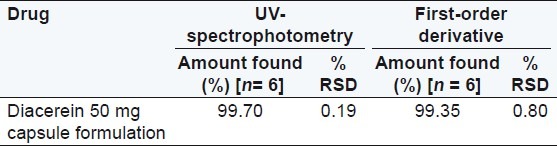
An accurately weighed capsule content equivalent to 10 mg of diacerein was transfer into a 100 mL volumetric flask containing 25 mL (8 M urea) solution and the volume was made up to the mark with water, similarly 10 mg equivalent capsule content was transferred in a 100 mL volumetric flask containing 25 mL (0.5M potassium citrate) solution and again the volume was made up to the mark with water. This stock solution was filtered through a 0.45 μm Whatmann filter paper. An appropriate volume of solution was further diluted with water to obtain the concentration 6 μg/mL of diacerein. The area of this solution was measured at selected wavelengths and the concentration of the drug was determined using a linear regression equation. The analysis procedure was repeated for six times with capsule formulation. The results of analysis of the capsule formulation are reported in Tables 1 and 2.
Table 1.
Results of the assay in 8 M urea
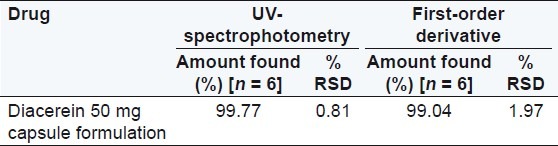
Table 2.
Results of the assay in 0.5 M potassium citrate
Recovery study
The accuracy of the method was studied at three different levels, i.e. 80%, 100% and 120% levels. To the pre-analyzed sample solution (4 μg/mL of diacerein), a known amount of drug standards of diacerein was added. The solutions were reanalyzed by the proposed method.
RESULTS AND DISCUSSION
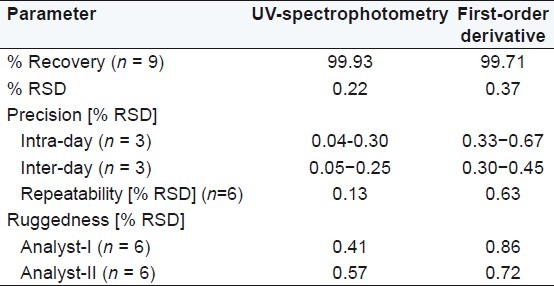
Diacerein showed absorbance maximum at 258.2 nm in the UV-spectrophotometric and 263.2 nm amplitude in first-order derivative spectra in urea solution and 257.4 nm in UV-spectrophotometric and 264.20 nm amplitude in first-order derivative spectra in potassium citrate solution. Diacerein followed linearity in both methods in the concentration range of 2-12 μg/mL at their respective wavelengths in zero-order and first-order spectra with the linear regression equations Y = 0.1228X + 0.2289 in the UV-spectroscopic method (r2 = 0.9993) [Figure 5] and Y = 0.0196X + 0.0483 in the first-order derivative method (r2 = 0.999) [Figure 6] in urea solution, and Y = 0.2428X + 0.4553 in the UV-spectroscopic method (r2 = 0.9996) [Figure 7] and Y = 0.0216X + 0.0189 in the first-order derivative method (r2 = 0.9992) [Figure 8] in potassium citrate solution. The amount of the drug estimated by using these two methods was found to be in good agreement with the label claim, which indicates that there was no interference from the excipients commonly present in the capsule formulation. These methods were validated for accuracy, precision, and ruggedness. These methods were found to be accurate indicated by low values (<2) of % RSD. The precision of the methods were studied as repeatability, intra-day and inter-day precision. The % RSD values for precision studies of diacerein were found to be < 2, which indicates that the methods to be precise. Both these methods are simple, economical, and rapid and can suitably be used for determination of diacerein in bulk and in capsule formulation. The results are shown in Tables 3 and 4.
Figure 5.
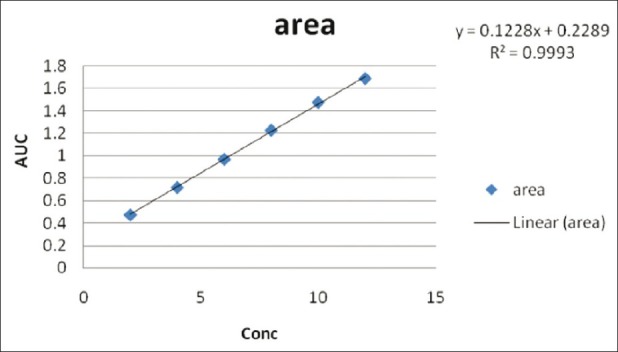
Linear regression equations Y = 0.1228X + 0.2289 in the UV- spectroscopic method (r2 = 0.9993) in 8M urea
Figure 6.
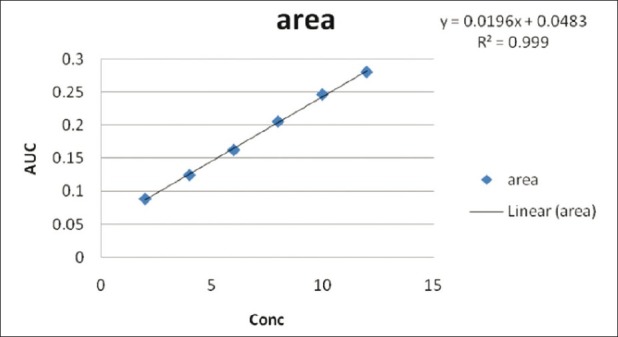
Linear regression equation Y=0.0196X + 0.0483 in the first-order derivative method (r2 =0.999) in 8M urea
Figure 7.
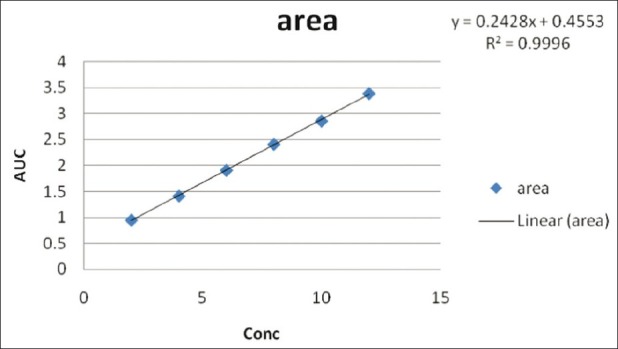
Quantitative estimation of diacerein in bulk and in capsule formulation: Linear regression equations Y = 0.2428X + 0.4553 in the UV-spectroscopic method (r2 = 0.9996) in 0.5 M potassium citrate
Figure 8.
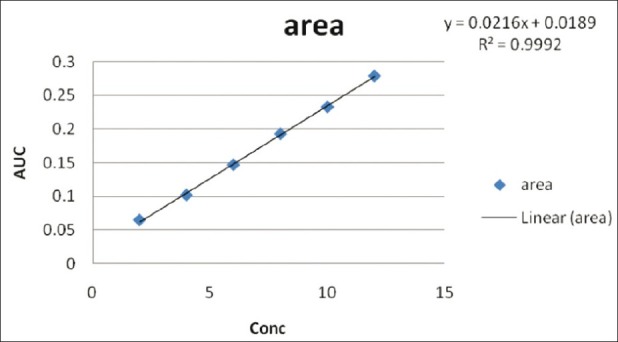
Linear regression equation Y = 0.0216X + 0.0189 in the first-order derivative method (r2 = 0.9992) in 0.5 M potassium citrate
Table 3.
Summary of validation parameters in 8 M urea
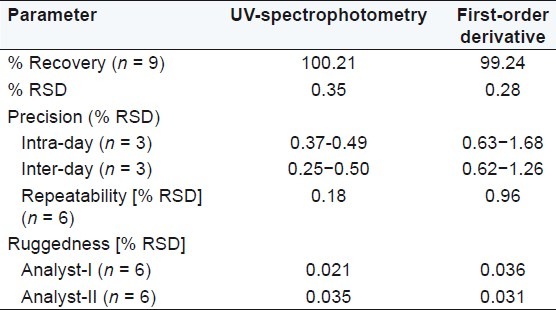
Table 4.
Summary of validation parameters in 0.5 M potassium citrate
CONCLUSION
The developed UV-spectrophotometric and first-order derivative methods for determination of diacerein were found to be simple, accurate, precise, and economical. These methods were proved to be rugged and can be used for routine analysis of diacerein in bulk and in capsule formulation.
ACKNOWLEDGEMENTS
The authors are thankful to H.R. Patel Institute of Pharmaceutical Education and Research, Shirpur (M.S.), India, for providing the required facilities to carry out this research work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.14th ed. Whitehouse Station, New Jersey, USA: Merck Research Laboratories; 2006. The Merck Index – An Encyclopedia of Chemicals, Drugs and Biologicals; p. 2980. [Google Scholar]
- 2.Vol. 2. Ghaziabad: Indian Pharmacopoeial Convention; 2010. Indian Pharmacopoeia, Govt. of India, Ministry of Health and Family Welfare; pp. 1191–3. [Google Scholar]
- 3.Tamura T, Shirai T, Kosaka N, Ohmori K, Takafumi K. Pharmacological studies of diacerein in animal models of inflammation, arthritis and bone resorption. Eur J Pharmacol. 2002;448:81–7. doi: 10.1016/s0014-2999(02)01898-8. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande MM, Gondane SJ, Mahajan MP, Deshpande AS, Sawant SD. Estimation of Diacerein by UV Spectrophotometry in Bulk and formulation. Int J Chem Anal Sci. 2011;2:1–2. [Google Scholar]
- 5.Narade S, Patil S, Surve S, Shete D, Pore Y. Simultaneous UV spectrophotometric method for the determination of diacerein and aceclofenac in tablets. J Pharm Sci Res. 2010;2:137–42. [Google Scholar]
- 6.Nyola NK, Kalra N. Spectrophotometric determination of diacerin in bulk and pharmaceutical formulation. Int J Pharm Bio Sci. 2010;1:202–7. [Google Scholar]
- 7.Sreejith KR, Premalatha K. Novel spectrophotometric methods for estimation of diacerein from formulations. Int J Res Pharm Biomed Sci. 2011;2:992–9. [Google Scholar]
- 8.Lalithaa KG, Venkatachalam T, Srinivasanb R, Kalaiselvi P, Kannappanc N. A simple HPLC method for quantitation of diacerein in tablet dosage Form. Euras J Anal Chem. 2010;5:81–8. [Google Scholar]
- 9.Gandhi SP, Dewani MG, Borole TC, Damle MC. Development and validation of stability indicating HPLC method for determination of diacerein and aceclofenac as bulk drug and in tablet dosage form. Int J Res Pharm Chem. 2011;1:799–806. [Google Scholar]
- 10.Jagadeeswaran M, Gopal N, Sivakumar T. Development and validation of a RP-HPLC method for simultaneous determination of diacerein and aceclofenac in tablet dosage form. Res Pharm Bio Chem Sci. 2010;1:418–24. [Google Scholar]
- 11.Kannappan N, Madhukar A, Srinivasan R, Srinivas RL, Naveen Kumar CH, Mannavalan R. Analytical method development and validation of diacerein tablets by RP-HPLC. Int J Chem Tech Res. 2010;2:143–8. [Google Scholar]
- 12.Sekar V, Jayaseelan S, Udhaya Kumar E, Subash N, Prakash M, Perumal P. Development and validation of RP-HPLCmethod for the simultaneous estimation of diacerein and aceclofenac in dosage form. Int J Chem Tech Res. 2010;2:168–71. [Google Scholar]
- 13.Chitlange SS, Pawbake GR, Mulla AI, Wankhede SB. Stability-indicating HPTLC method for estimation of diacerein in pharmaceutical dosage form. Res Pharm Bio Chem Sci. 2010;1:226–34. doi: 10.1080/10826068.2010.525410. [DOI] [PubMed] [Google Scholar]
- 14.Gupta KR, Samrit VE, Thakur V, Hemke AT. UV-spectrophotometric estimation of diacerein in pharmaceutical formulation. J Chem Pharm Res. 2010;2:467–72. [Google Scholar]
- 15.Sivakumar R, Nallasivan PK, Saranya KC, Sam Solomon WD, Akelesh T, Venkatnarayanan R. Visible spectrophotometric estimation of diacerein in bulk and pharmaceutical dosage forms. Pharm Anal. 2010;2:414–6. doi: 10.4103/0975-1483.71631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuberg C. Hydrotropy. Biochemische Zeitschrift. 1916;76:107–76. [Google Scholar]
- 17.Coffman RE, Kildsig D. Hydrotropic solubilization- mechanistic studies. Pharm Res. 1996;13:1460–3. doi: 10.1023/a:1016011125302. [DOI] [PubMed] [Google Scholar]
- 18.Maheshwari RK. Mixed hydrotropy in spectrophotometric analysis of aceclofenac. Indian Pharm. 2007;64:67–9. [Google Scholar]
- 19.Jain NK, Agrawal RK, Singhai AK. Formulation of aqueous injection of carbamazepine. Pharmazie. 1990;45:221. [PubMed] [Google Scholar]
- 20.Pareek V, Tambe SR, Bhalerao SB. Role of different hydrotropic agents in spectrophotometric and chromatographic estimation of Cefixime. Int J Pharm Bio Sci. 2010;1:1–10. [Google Scholar]
- 21.Chandra M, Sharma S. Determination and validation of UVSpectrophotometric method for estimation of Paracetamol and Diclofenac Sodium in tablet dosage forms using hydrotropic solubilizing agents. Int J Pharm Tech Res. 2011;3:244–7. [Google Scholar]
- 22.Jain N, Jain R, Jain A, Pandey SP, Jain DK. Spectrophotometric method development and validation for quantitative estimation of amlodipine besylate in bulk drug and their dosage forms by using hydrotropic agent. Euras J Anal Chem. 2010;5:212–7. [Google Scholar]
- 23.ICH, Q2A: Text on Validation of Analytical Procedures, International Conference on Harmonization. 1994 Oct [Google Scholar]
- 24.ICH, Q3B: Validation of Analytical Procedures: Methodology, International Conference on Harmonization. 1996 Nov [Google Scholar]
- 25.ICH Harmonized Tripartite Guideline, Q2 (R1): Validation of Analytical Procedures: Text and Methodology. 2005 Nov [Google Scholar]


