Abstract
Introduction:
A simple, rapid and sensitive liquid chromatography-tandem mass spectrometric assay method has been developed and fully validated for simultaneous quantification of losartan and its active metabolite, losartan carboxylic acid, and amlodipine in human plasma. Irbesartan was used as an internal standard.
Materials and Methods:
The analytes were extracted from human plasma samples by solid-phase extraction technique using Oasis HLB cartridges, (Waters Corporation, Mumbai, India). The reconstituted samples were chromatographed on a C18 column by using an 85:15, v/v mixture of methanol and 0.1% v/v formic acid as the mobile phase at a flow rate of 1.0 mL/min. A detailed validation of the method was performed as per the FDA guidelines.
Results:
The calibration curves obtained were linear (r ≥ 0.99) over the concentration range of 0.5-1000 ng/mL for losartan and for its active metabolite losartan acid and 0.05-10.1 ng/mL for amlodipine. The results of the intra- and inter-day precision and accuracy studies were well within the acceptable limits.
Conclusions:
A run time of 2.5 min for each sample made it possible to analyze more than 300 plasma samples per day. The proposed method was found to be applicable to clinical studies.
Keywords: Amlodipine, losartan, losartan acid, liquid chromatography-tandem mass spectrometric method, pharmacokinetics
INTRODUCTION
The renin-angiotensin-aldosterone system has a key function in the pathogenesis of hypertension, making blockade of this system an ideal target for antihypertensive therapy. All known clinical effects of angiotensin II, including vasoconstriction, aldosterone release, and augmented catecholamine release, are mediated by the AT1-type angiotensin II receptor.[1] Losartan potassium is the first orally active, nonpeptide antagonist of the angiotensin II subtype 1 receptor.[2] Losartan undergoes substantial first-pass metabolism by cytochrome P450 and results in biotransformation of the major active metabolite, losartan acid. Following oral administration, losartan is rapidly absorbed, reaching maximum concentrations 1–2 h post administration.[3] Losartan and its active metabolite, losartan acid, selectively and specifically block the binding of angiotensin II to the AT1 receptor found in many tissues. The active metabolite, losartan acid, is 10–40 times more potent than losartan.[4–6]
Amlodipine, a third-generation dihydropyridine calcium antagonist, is prescribed for the treatment of angina and hypertension. Oral doses of 5 to 10 mg QD are effective in the treatment of mild to moderate hypertension and stable angina pectoris.[7–10] Amlodipine is well tolerated and does not appear to cause some of the undesirable effects often associated with other cardiovascular agents. It has a long elimination half-life and a large volume of distribution. It has been reported that low plasma concentrations are achieved after oral administration of amlodipine.[11]
A combination of antihypertensive agents can better control blood pressure and reduce the number and severity of side effects than a monotherapy. Amlodipine and losartan fixed dose combinations have been demonstrated in numerous clinical trails to be highly effective in lowering blood pressure, and suggest that the combined use might be more effective in treating hypertension than a monotherapy.[12,13] One such combination available in the market is Amlopress-Z, (Cipla Limited, Mumbai, India) which is a combination of amlodipine and losartan in a single pill.
As per the literature, several liquid chromatography-tandem mass spectrometric (LC-MS/MS) methods have been reported for the determination of losartan along with its active metabolite, losartan acid, and amlodipine individually in biological samples.[14–23] To date, no LC-MS/MS method has been reported for the simultaneous determination of losartan, losartan acid, and amlodipine in human plasma. In the present paper, a simple, rapid, and reproducible validated method has been proposed for simultaneous quantification of losartan, losartan acid, and amlodipine concentrations in human plasma without compromising the sensitivity reported earlier for each drug. Indeed, in the present paper, we have achieved a higher sensitivity (2 folds) for losartan and losartan acid.
MATERIALS AND METHODS
Reagents and chemicals
The reference samples of losartan (99.60%), losartan carboxylic acid (99.20%), amlodipine (99.20%), and irbesartan (99.70%) were purchased form Neucon Pharma Limited, (Goa, India). Chemical structures are presented in Figure 1. HPLC grade of acetonitrile and methanol were purchased form J.T Baker, (Phillipsburg, NJ, USA). Analytical grade formic acid was purchased from Merck Ltd (Mumbai, India). The control human plasma sample was procured from Cauvery Diagnostics and Blood Bank, (Secunderabad, India).
Figure 1.
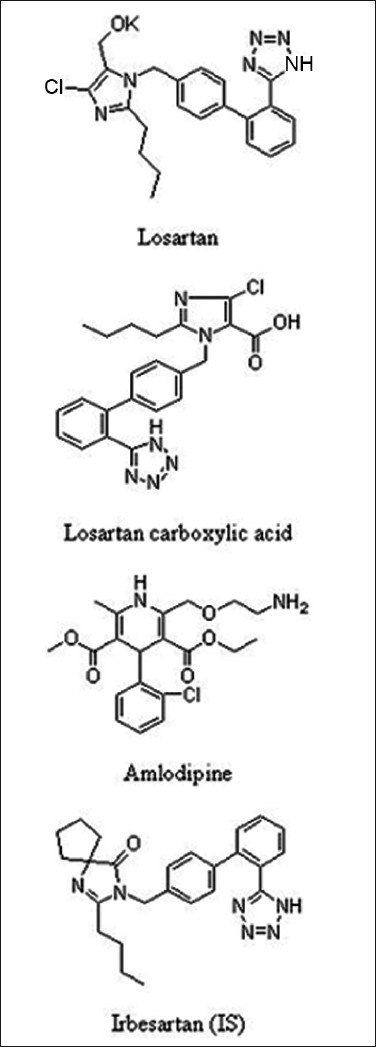
Chemical structures of losartan, losartan carboxylic acid, amlodipine and irbesartan (internal standard [IS])
Instrumentation and chromatographic conditions
An HPLC system (Shimadzu, Kyoto, Japan), consisting of a binary LC-20AD prominence pump, an auto sampler (SIL-HTc), and a solvent degasser (DGU-20A3), was used for the study. Aliquots of the processed samples (15 μL) were injected into the Zorbax XDB-Phenyl column (75 mm × 4.6 mm; 3.5 micron particle size; Agilent Technologies, Santa Clara, CA, USA), which was kept at room temperature (25°C). The isocratic mobile phase, a 85:15, v/v mixture of methanol and 0.1% v/v formic acid was delivered at 1.0 mL/min. Detection was performed by an Applied Biosystems MDS Sciex API-4000, (Foster City, CA, USA) mass spectrometer in positive ionization mode.
Preparation of stock solutions of analytes and internal standard
Stock solutions of losartan, losartan acid, amlodipine, and IS were prepared separately by dissolving in methanol at 1 mg/mL concentration. Working solutions with different concentrations were prepared by dilution of stock with diluent (methanol and water; 50:50%, v/v).
Preparation of calibration curve standards and quality control samples
Calibration samples were prepared by spiking 950 μL of control human plasma with the appropriate working solution of each analyte (25 μL combined dilution of losartan, losartan acid, and 25 μL of amlodipine). Calibration samples were made at concentrations of 1000, 800, 600, 402, 201, 101, 10.5, 1.00, and 0.50 ng/mL for losartan and for losartan acid, and 10.10, 8.08, 6.06, 4.00, 2.00, 1.00, 0.50, 0.10, and 0.05 ng/mL for amlodipine. Quality control samples for losartan, losartan acid, and amlodipine were prepared at concentrations of 858, 854, 8.49 (higher quality control, HQC), 515, 512, 5.09 (middle quality control 2, MQC2), 124, 123, 1.22 (middle quality control 1, MQC1), 1.55, 1.54, 0.15 (lower quality control, LQC) and 0.52, 0.51, 0.05 (lower limit quality control, LLOQ QC) ng/mL, respectively.
Sample processing
A 200-μL aliquot of human plasma sample was mixed with 25 μL of the IS working solution (2000 ng/mL of irbesartan). To this, 200 μL of extraction buffer (0.5% formic acid in water) was added after vortex mixing for 10 s. The sample mixture was loaded onto an Oasis HLB cartridge (30 mg/1 mL) that was pre-conditioned with 1.0 mL of methanol followed by 1.0 mL of extraction buffer. The extraction cartridge was washed with 1.0 mL of extraction buffer followed by 1.0 mL of water. Analytes and IS were eluted with 1.0 mL of 0.5% ammonia in methanol and evaporated to dryness at 45°C under a stream of nitrogen. The dried extract was reconstituted in 1000 μL of mobile phase and transferred into injector vials. From these, a 15-μL aliquot was injected into the chromatographic system.
Pharmacokinetic study design
A pharmacokinetic study on the drug was performed in healthy male subjects (n=6). The Ethics Committee approved the protocol, and the volunteers provided informed written consent. Blood samples were collected following oral administration of 50-mg tablet of losartan at pre-dose and 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.33, 2.67, 3, 3.33, 3.67, 4, 4.5, 5, 6, 8, 10, 12, 24, and 36 h, in EDTA Vacutainer collection tubes (BD, Franklin, NJ, USA). The tubes were centrifuged at 3200 rpm for 10 min and the plasma was collected. The collected plasma samples were stored at –70°C until their use.
Method validation
The validation of the above method was carried out as per US FDA guidelines.[24] The parameters determined were selectivity, specificity, matrix effect, linearity, precision, accuracy, recovery, stability, and dilution integrity.
RESULTS AND DISCUSSION
Mass spectrometry
Mass parameters were tuned in both, positive and negative ionization modes for the analytes. Good response was found in positive ionization mode. The most sensitive mass transition for losartan was monitored from m/z 423.1 to 207.2, for losartan acid was monitored from m/z 437.1 to 235.2, for amlodipine was monitored from m/z 409.3 to 238.0, and for IS was monitored from m/z 429.2 to 206.9.
Method development
A mobile phase consisting of methanol and 0.1% formic acid (85:15, v/v) was found suitable as the analytes were protonated and well separated from endogenous components in this phase. Zorbax XDB-Phenyl column (75 mm × 4.6 mm; 3.5 micron particle size; Agilent Technologies, USA) gave a good peak shape and response, even at LLOQ level, for all the analytes and IS. The mobile phase was operated at a flow rate of 1.0 mL/min. The retention time of losartan, losartan acid, amlodipine, and IS are low enough (1.3, 1.4, 1.7 and 1.5 min), allowing a small run time of 2.5 min.
Specificity and selectivity
The specificity of the method was evaluated by injecting each analyte at the highest concentration in human plasma samples in the presence of other analytes. A typical chromatogram for the control human plasma (free of analyte and IS), human plasma spiked with IS, and human plasma spiked with analytes at LLOQ and IS is shown in Figures 2-4 (a–c). Results demonstrate the lack of chromatographic interference between each analyte and from endogenous components at the retention time of analyte and IS.
Figure 2.
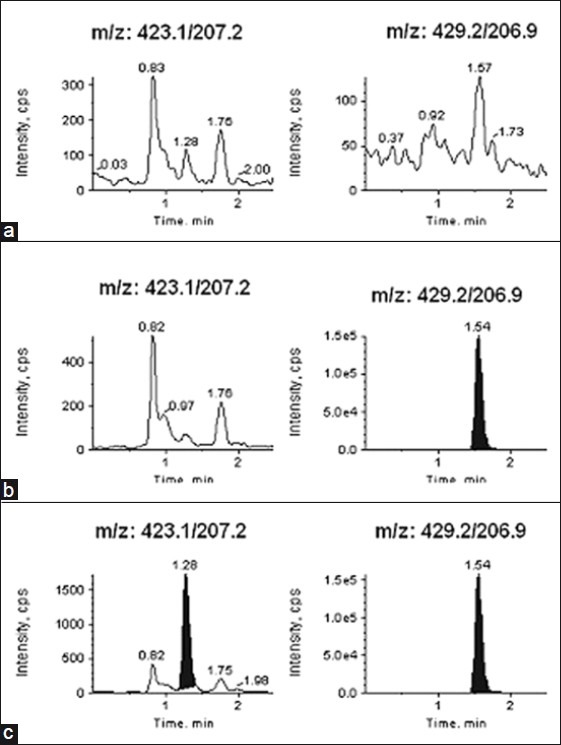
Typical MRM chromatograms of losartan (left panel) and internal standard [IS] (right panel) in (a) human blank plasma (b) human plasma spiked with IS (c) a LLOQ sample along with IS
Figure 4.
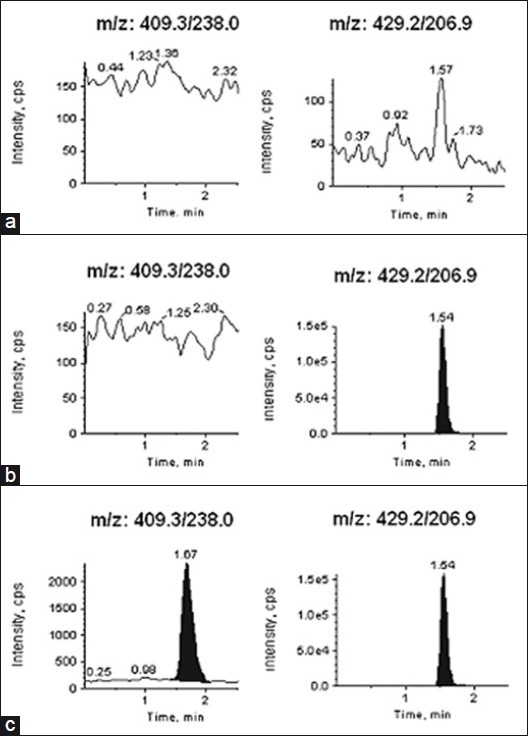
Typical MRM chromatograms of amlodipine (left panel) and internal standard [IS] (right panel) in (a) human blank plasma (b) human plasma spiked with IS (c) a LLOQ sample along with IS
Figure 3.
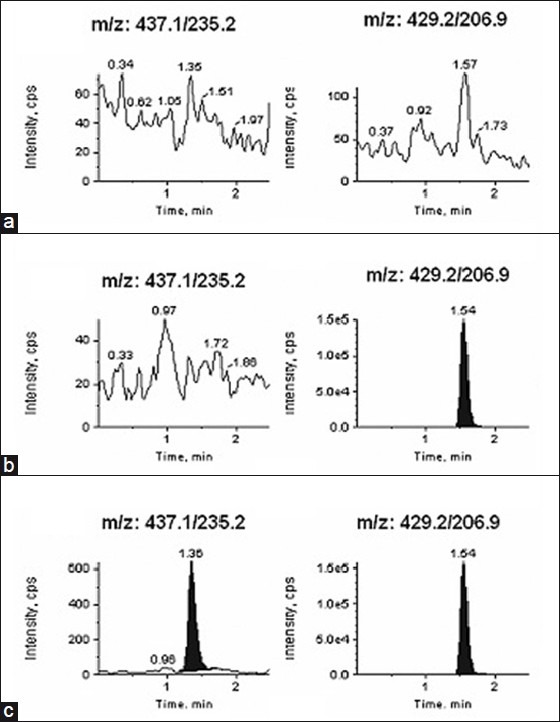
Typical MRM chromatograms of losartan acid (left panel) and internal standard [IS] (right panel) in (a) human blank plasma (b) human plasma spiked with IS (c) a LLOQ sample along with IS
Sensitivity
The lowest limit of reliable quantification for the analytes was set at the concentration of the LLOQ. The precision and accuracy at LLOQ concentration were found to be 5.31% and 103%; 5.36% and 109%; 6.88% and 105% for losartan, losartan acid, and amlodipine, respectively.
Extraction efficiency
Solid-phase extraction with HLB cartridge proved to be robust and provided the cleanest samples. The recoveries of analytes and IS were good and reproducible. The mean overall recoveries (with the precision range) of losartan, losartan acid, amlodipine, and IS are summarized in Table 1.
Table 1.
Mean overall recoveries of losartan, losartan acid, amlodipine and IS
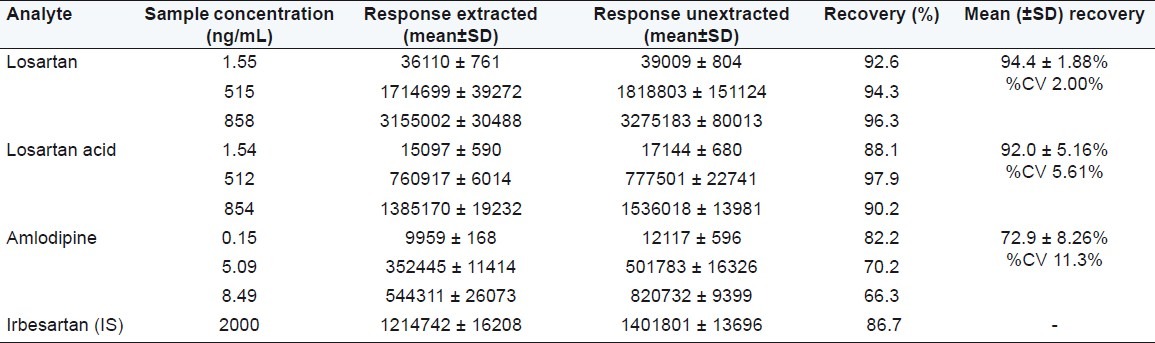
Matrix effect
No significant matrix effect was observed in all the six batches of human plasma for the analytes at LQC and HQC concentrations. The precision and accuracy for losartan, losartan acid, and amlodipine at LQC concentration were found to be 4.98% and 94.8%; 2.29% and 103%; 1.18% and 100%, respectively. Similarly, the precision and accuracy for losartan, losartan acid, and amlodipine at HQC concentration were found to be 1.65% and 108%; 1.43% and 102%; 0.56% and 102%, respectively.
Linearity
The nine-point calibration curve was found to be linear over the concentration range of 0.50–1000 ng/mL for losartan and for losartan acid and 0.05–10.1 ng/mL for amlodipine. Weighting factor of 1/x2 of the drug to the IS concentration was found to produce the best fit for the concentration-detector response relationship for both the analytes. The mean correlation coefficient of the weighted calibration curves generated during the validation was >0.99.
Precision and accuracy
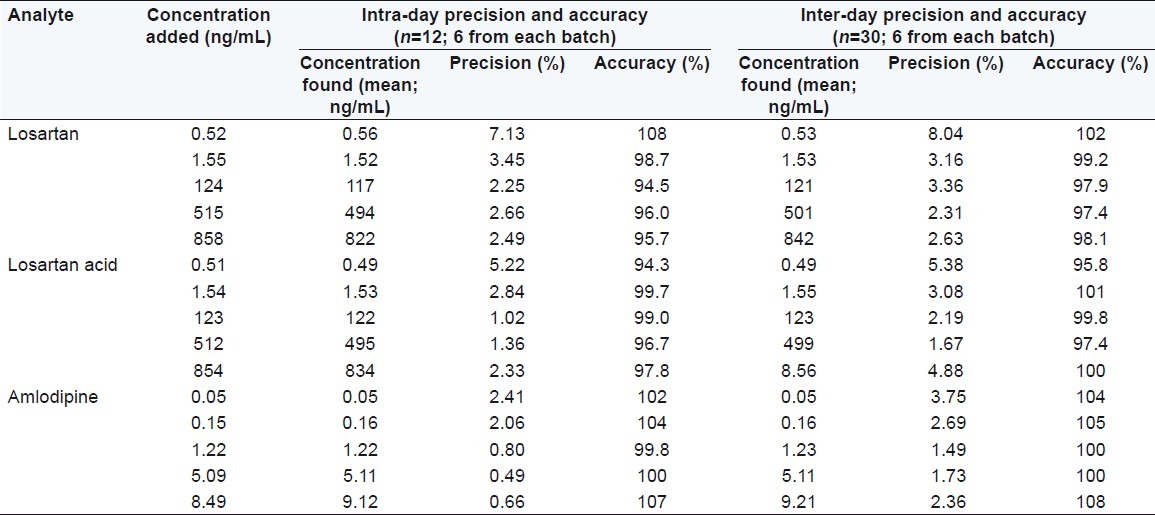
Precision and accuracy data for intra- and inter-day plasma samples for losartan, losartan acid, and amlodipine are presented in Table 2. The assay values on both the occasions (intra- and inter-day) were found to be within the accepted variable limits.
Table 2.
Precision and accuracy of the method for determining losartan, losartan acid and amlodipine
Dilution integrity
The upper concentration limits can be extended to 1700 ng/mL for losartan and for losartan acid and 17.2 ng/mL for amlodipine by 1/2 and 1/4 dilutions with screened human blank plasma. The mean back-calculated concentrations for 1/2 and 1/4 dilution samples were within 85-115% of their nominal value.
Stability studies
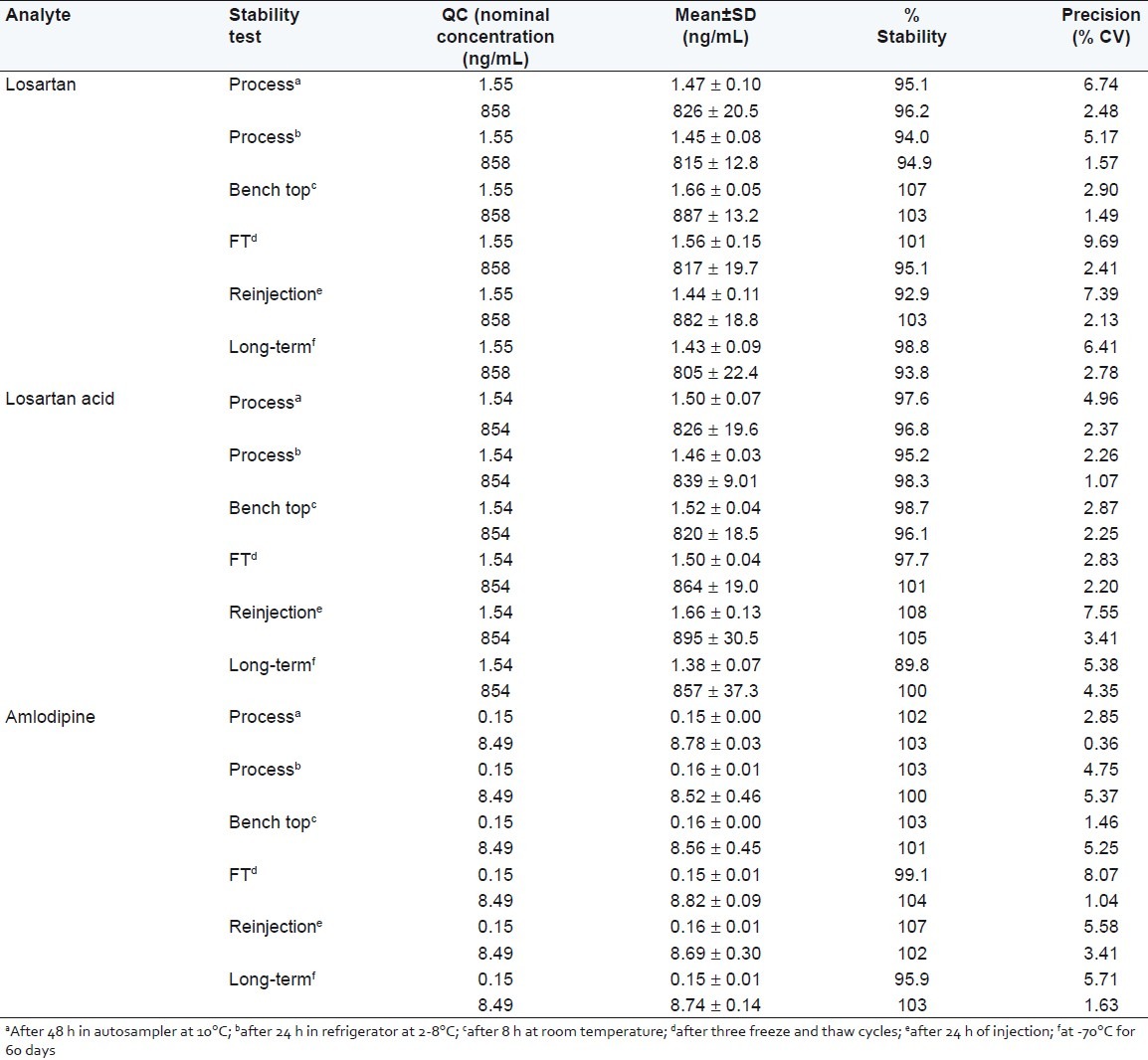
The predicted concentrations for each analyte at LQC and HQC samples deviated within ±15% of the nominal concentrations in a batter of stability tests viz. autosampler (48 h), bench-top (8 h), reinjection (24 h), and wet extract (24 h), repeated three freeze–thaw cycles and at –70 ± 10°C for at least 60 days [Table 3]. The results were found to be within the assay variability limits during the entire process.
Table 3.
Stability samples result for losartan, losartan acid and amlodipine (n=6)
Application to real human plasma samples
In order to verify the sensitivity and selectivity of this method in a real-time situation, the present method was used to test for losartan and its metabolite, losartan acid, in human plasma samples collected from healthy male volunteers (n=6). The mean plasma concentrations vs time profiles of losartan and losartan acid are shown in Figure 5. The maximum concentration in plasma (Cmax), time point of Cmax (tmax), half-life (t1/2), area under the plasma concentration time curve from zero hour to the last measurable concentration (AUC0-t), and area under the plasma concentration-time curve from zero hour to infinity (AUC0-inf) for losartan were 349 ± 46.2 ng/ mL, 1.88 ± 0.61 h, 7.79 ± 5.87 h, 1166 ± 292 ng.h/mL, and 1200 ± 318 ng.h/mL, respectively, and for losartan acid were 629 ± 180 ng/mL, 4.28 ± 0.48 h, 5.69 ± 0.37 h, 5559 ± 1831 ng h/mL, and 5651 ± 1852 ng h/mL, respectively. These values were in close proximity when compared with earlier reported values.[14]
Figure 5.
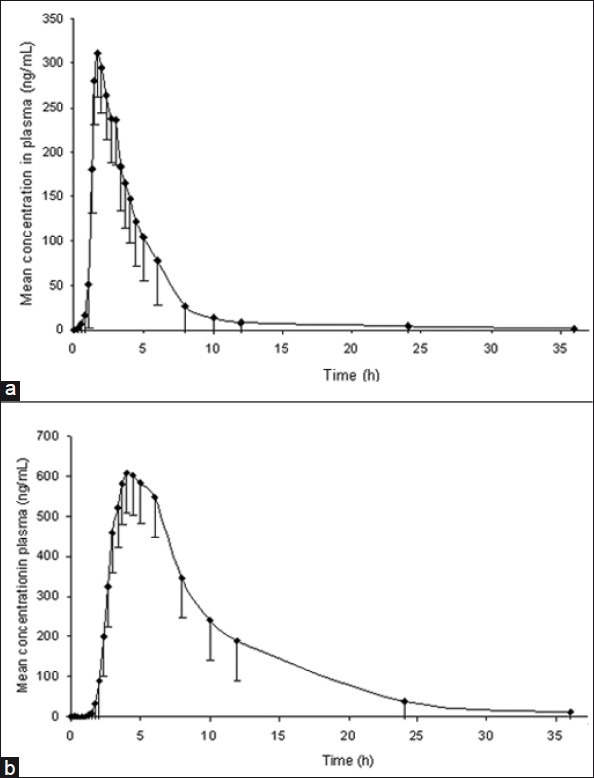
Mean plasma concentration-time profile of (a) losartan (b) losartan acid in human plasma following oral dosing of losartan 50 mg tablet to healthy volunteers (n=6)
CONCLUSIONS
The LC-MS/MS assay method described in this paper is rapid, simple, specific, and sensitive for quantification of losartan, losartan acid, and amlodipine in human plasma, and is fully validated as per the FDA guidelines. To the best of our knowledge, this is the first report on simultaneous assay of losartan, losartan acid, and amlodipine in human plasma without compromising on the reported sensitivity for each analyte. The method was found to be suitable for pharmacokinetic studies in humans. The SPE method gave consistent and reproducible recoveries for the analytes from plasma. The proposed method provided excellent specificity and reproducibility. A sample retention range of less than 2.5 min makes it an attractive procedure in high-throughput bioanalysis of losartan, losartan acid, and amlodipine.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Wellquest Clinical Research laboratories, Hyderabad, for providing necessary facilities for carrying out this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bernstein KE, Berk BC. The biology of angiotensin II receptors. Am J Kidney Dis. 1993;22:745–54. doi: 10.1016/s0272-6386(12)80441-0. [DOI] [PubMed] [Google Scholar]
- 2.Timmermans PB, Bensfield P, Chiu AT, Herblin WF, Wong PC, Smith RD. Angiotensin II receptors and functional correlates. Am J Hypertens. 1992;5:221S–35S. doi: 10.1093/ajh/5.12.221s. [DOI] [PubMed] [Google Scholar]
- 3.Tamimi JJ, Salem II, Mahmood Alam S, Zaman Q, Dham R. Comparative pharmacokinetics of two tablet formulations of losartan: Bioequivalence assessment. Biopharm Drug Dispos. 2005;26:205–10. doi: 10.1002/bdd.448. [DOI] [PubMed] [Google Scholar]
- 4.Oparil S, Barr E, Telkins M, Liss C, Vrecenak A, Edelman J. Efficacy, tolerability, and effects on quality of life of losartan, alone or with hydrochlorothiazide, versus amlodipine, alone or with hydrochlorothiazide, in patients with essential hypertension. Clinical Therapeutics. 1996;18:608–25. doi: 10.1016/s0149-2918(96)80212-8. [DOI] [PubMed] [Google Scholar]
- 5.Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44:797–814. doi: 10.2165/00003088-200544080-00003. [DOI] [PubMed] [Google Scholar]
- 6.Burnier M. Angiotensin II type 1 receptor blockers. Circulation. 2001;103:904–12. doi: 10.1161/01.cir.103.6.904. [DOI] [PubMed] [Google Scholar]
- 7.Abernethy DR. The pharmacokinetic profile of amlodipine. Am Heart J. 1989;118:1100–3. doi: 10.1016/0002-8703(89)90834-x. [DOI] [PubMed] [Google Scholar]
- 8.Kungys G, Naujoks H, Wanner C. Pharmacokinetics of amlodipine in hypertensive patients undergoing haemodialysis. Eur J Clin Pharmacol. 2003;59:291–5. doi: 10.1007/s00228-003-0620-4. [DOI] [PubMed] [Google Scholar]
- 9.Reid JL, Meredith PA, Donnelly R, Elliot HL. Pharmacokinetics of calcium antagonists. J Cardiovasc Pharmacol. 1988;12:22–6. doi: 10.1097/00005344-198812007-00005. [DOI] [PubMed] [Google Scholar]
- 10.Scholz H. Pharmacological aspects of calcium channel blockers. Cardiovasc Drugs Ther. 1997;10:869–72. doi: 10.1007/BF00051613. [DOI] [PubMed] [Google Scholar]
- 11.Murdoch D, Heel RC. Amlodipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cardiovascular disease. Drugs. 1991;41:478–505. doi: 10.2165/00003495-199141030-00009. [DOI] [PubMed] [Google Scholar]
- 12.Verdecchia P, Reboldi G, Angeli F, Gattobigio R, Bentivoglio M, Thijs L, et al. Angiotensin-converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention. Hypertension. 2005;46:386–92. doi: 10.1161/01.HYP.0000174591.42889.a2. [DOI] [PubMed] [Google Scholar]
- 13.Choi SM, Seo MJ, Kang KK, Kim JH, Ahn BO, Yoo M. Beneficial effects of the combination of amlodipine and losartan for lowering blood pressure in spontaneously hypertensive rats. Arch Pharm Res. 2009;32:353–8. doi: 10.1007/s12272-009-1307-x. [DOI] [PubMed] [Google Scholar]
- 14.Prasaja B, Sasongko L, Harahap Y, Lusthom W, Grigg M. Simultaneous quantification of losartan and active metabolite in human plasma by liquid chromatography-tandem mass spectrometry using irbesartan as internal standard. J Pharm Biomed Anal. 2009;49:862–7. doi: 10.1016/j.jpba.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Polinko M, Riffel K, Song H, Lo MW. Simultaneous determination of losartan and EXP3174 in human plasma and urine utilizing liquid chromatography/tandem mass spectrometry. J Pharm Biomed Anal. 2003;33:73–84. doi: 10.1016/s0731-7085(03)00348-0. [DOI] [PubMed] [Google Scholar]
- 16.Iwasa T, Takano T, Hara KI, Kamei T. Method for the simultaneous determination of losartan and its major metabolite, EXP-3174, in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr B. 1999;734:325–30. doi: 10.1016/s0378-4347(99)00358-8. [DOI] [PubMed] [Google Scholar]
- 17.Sarkar AK, Ghosh D, Das A, Selvan PS, Gowda KV, Mandal U, et al. Simultaneous determination of metoprolol succinate and amlodipine besylate in human plasma by liquid chromatography–tandem mass spectrometry method and its application in bioequivalence study. J Chromatogr B. 2008;873:77–85. doi: 10.1016/j.jchromb.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 18.Streel B, Laine C, Zimmer C, Sibenaler R, Ceccato A. Enantiomeric determination of amlodipine in human plasma by liquid chromatography coupled to tandem mass spectrometry. J Biochem Biophys Methods. 2002;54:357–68. doi: 10.1016/s0165-022x(02)00133-1. [DOI] [PubMed] [Google Scholar]
- 19.Suchanova B, Kostiainen R, Ketola RA. Characterization of the in vitro metabolic profile of amlodipine in rat using liquid chromatography-mass spectrometry. Eur J Pharm Sci. 2008;33:91–9. doi: 10.1016/j.ejps.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Sirikatitham A, Panrat K, Tanmanee N. Determination of amlodipine in human plasma by electrospray ionization LC-MS/MS method: Validation and its stability studies. Songklanakarin J Sci Technol. 2008;30:455–62. [Google Scholar]
- 21.Moses PF, Muralidharan S, Rajan S, Nagarajan, Suresh B. Simple and validated method for estimation of amlodipine by LC-MS (ESI) using healthy Indian human volunteers and evaluation of pharmacokinetic parameters. J Bioanal Biomed. 2010;2:69–74. [Google Scholar]
- 22.Nirogi RV, Kandikere VN, Mudigonda K, Shukla M, Maurya S. Sensitive and rapid liquid chromatography/tandem mass spectrometry assay for the quantification of amlodipine in human plasma. Biomed Chromatogr. 2006;20:833–42. doi: 10.1002/bmc.600. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt J, Singh S, Subbaiah G, Shah B, Kambli S, Ameta S. A rapid and sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the estimation of amlodipine in human plasma. Biomed Chromatogr. 2007;21:169–75. doi: 10.1002/bmc.730. [DOI] [PubMed] [Google Scholar]
- 24.US DHHS, FDA, CDER. Guidance for Industry: Bioanalytical Method Validation. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CV) 2001. [Last accessed on 2011 Nov 06]. Available from: http://www/fda.gov/cder/guidance/index.htm .


